Pyrene-Based Blue AIEgen: Enhanced Hole Mobility and Good EL Performance in Solution-Processed OLEDs
Abstract
:1. Introduction
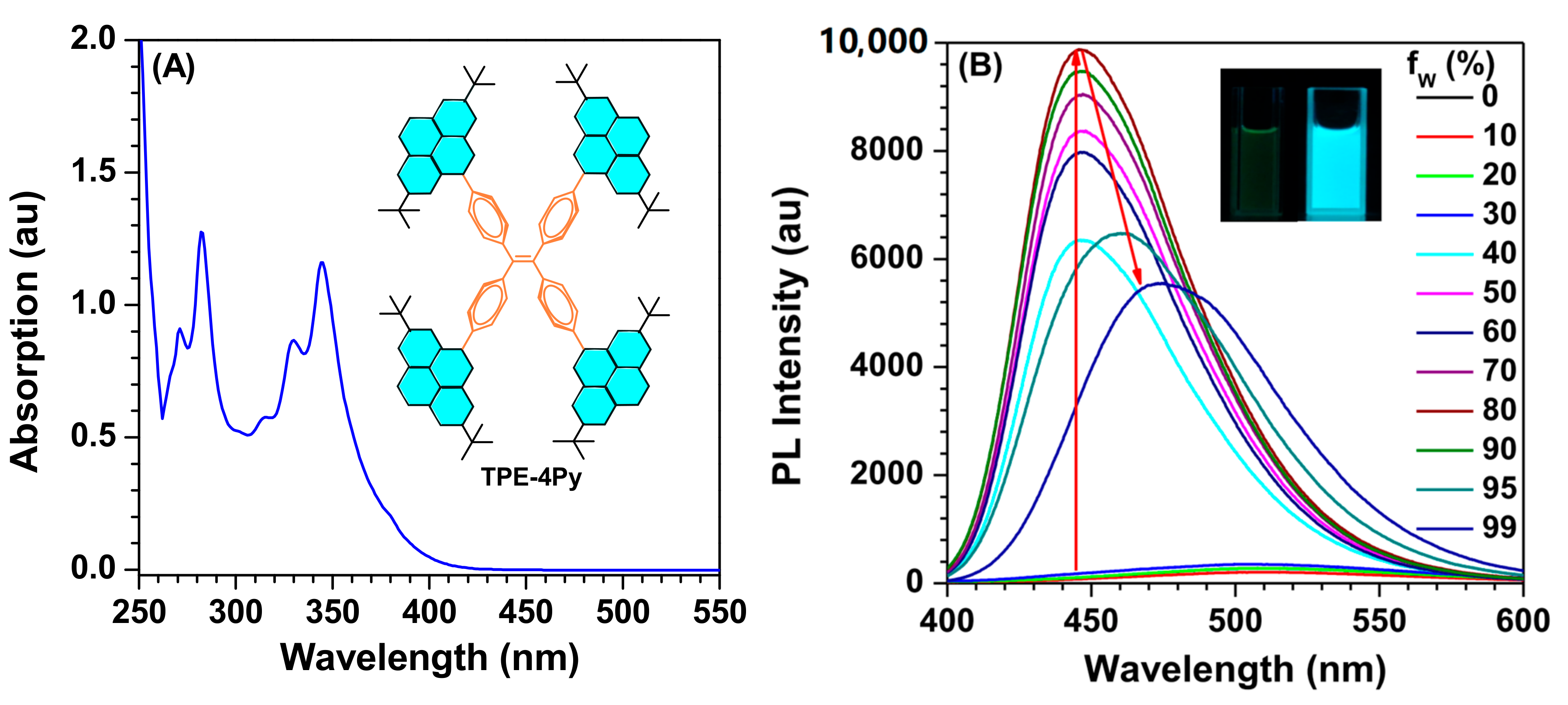
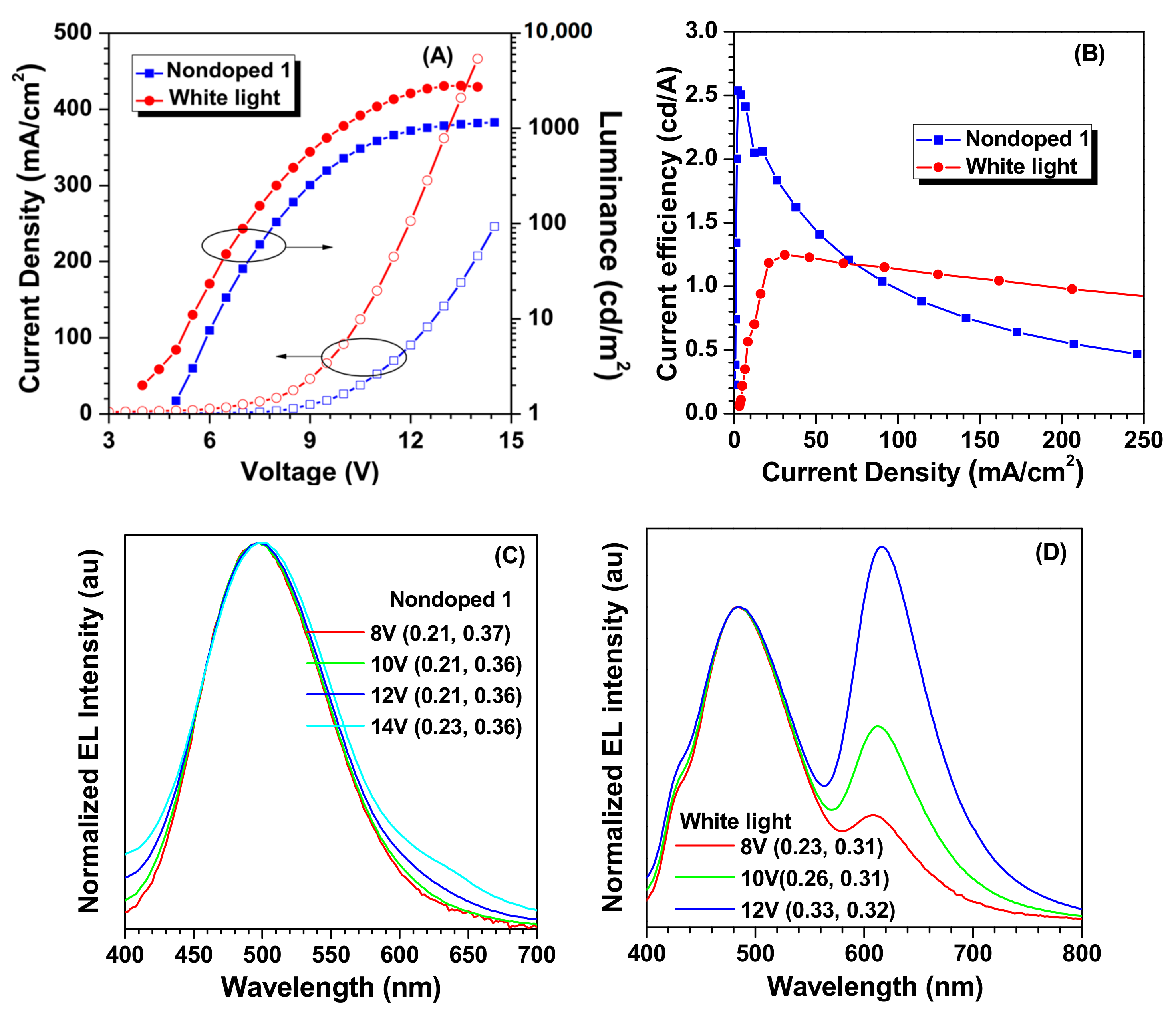
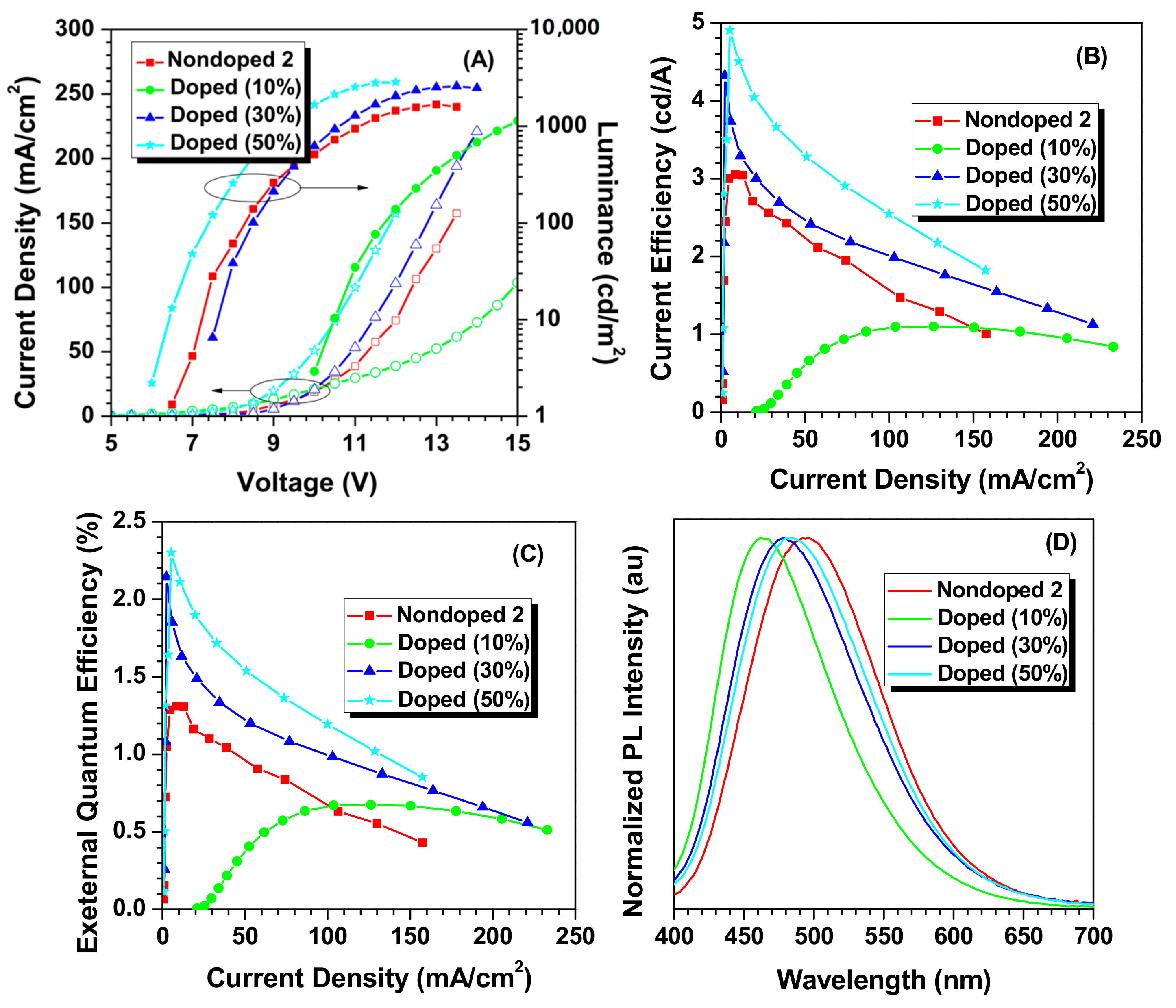
2. Results and Discussion
3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baldo, M.A.; Thompson, M.E.; Forrest, S.R. High-efficiency fluorescent organic light-emitting devices using a phosphorescent sensitizer. Nature 2000, 403, 750–753. [Google Scholar] [CrossRef] [PubMed]
- D’Andrade, B.W.; Forrest, S.R. White organic light-emitting devices for solid-state lighting. Adv. Mater. 2004, 16, 1585–1595. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, C. Blue fluorescent emitters: Design tactics and applications in organic light-emitting diodes. Chem. Soc. Rev. 2013, 42, 4963–4976. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, X.; Zhou, G. Recent advances of the emitters for high performance deep-blue organic light-emitting diodes. J. Mater. Chem. C 2015, 3, 913–944. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.; Kwok, R.; Lam, J.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hang, J.; Li, Q.; Li, Z. Blue AIEgens: Approaches to control the intramolecular conjugation and the optimized performance of OLED devices. J. Mater. Chem. C 2016, 4, 2663–2684. [Google Scholar] [CrossRef]
- Duarte, T.; Müllen, K. Pyrene-based materials for organic electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Peng, Z.; Zhang, X.; Wang, P.; Lee, C.; Lee, S. Highly Efficient Non-Doped Blue Organic Light-Emitting Diodes Based on Fluorene Derivatives with High Thermal Stability. Adv. Funct. Mater. 2005, 15, 1716–1721. [Google Scholar] [CrossRef]
- Wu, K.; Ku, P.; Lin, C.; Shih, H.; Wu, F.; Huang, M.; Lin, J.; Chen, I.; Cheng, C. The Photophysical Properties of Dipyrenylbenzenes and Their Application as Exceedingly Efficient Blue Emitters for Electroluminescent Devices. Adv. Funct. Mater. 2008, 18, 67–75. [Google Scholar] [CrossRef]
- Liang, Z.; Li, Y.; Yang, J.; Ren, Y.; Tao, X. Suppression of aggregation-induced fluorescence quenching in pyrene derivatives: Photophysical properties and crystal structures. Tetrahedron Lett. 2011, 52, 1329–1333. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.; Sun, N.; Peng, Q.; Li, Q.; Ma, D.; Li, Z. Twist versus linkage mode: Which one is better for the construction of blue luminogens with AIE properties? Chem. Eur. J. 2015, 21, 6862–6868. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, L.; Yu, Y.; Ren, Z.; Peng, Q.; Ye, S.; Li, Q.; Li, Z. Blue pyrene-based AIEgens: Inhibited intermolecular π–π stacking through the introduction of substituents with controllable intramolecular conjugation, and high external quantum efficiencies up to 3.46% in non-doped OLEDs. Mater. Chem. Front. 2017, 1, 91–99. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Q.; Wen, X.; Gao, X.; Peng, Q.; Li, Q.; Ma, D.; Li, Z. Pyrene-based blue AIEgens: Tunable intramolecular conjugation, good hole mobility and reversible mechanochromism. J. Mater. Chem. C 2016, 4, 8506–8513. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Ma, Z.; Ding, J.; Wang, L.; Jing, X.; Wang, F. Solution-Processible 2,2′-Dimethyl-biphenyl Cored Carbazole Dendrimers as Universal Hosts for Efficient Blue, Green, and Red Phosphorescent OLEDs. Adv. Funct. Mater. 2014, 24, 3413–3421. [Google Scholar] [CrossRef]
- Li, Y.; Xie, G.; Gong, S.; Wu, K.; Yang, C. Dendronized delayed fluorescence emitters for non-doped, solution-processed organic light-emitting diodes with high efficiency and low efficiency roll-off simultaneously: Two parallel emissive channels. Chem. Sci. 2016, 7, 5441–5447. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, S.; Shen, X.; Mahtab, F.; Yu, Y.; Lu, P.; Lam, J.; Kwok, H.; Tang, B. Aggregation-induced emission, self-assembly, and electroluminescence of 4,4′-bis(1,2,2-triphenylvinyl)biphenyl. Chem. Commun. 2010, 46, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, G.; Zhao, L.; Yuan, W.; Zhang, Y.; Tang, B. Diethylamino functionalized tetraphenylethenes: Structural and electronic modulation of photophysical properties, implication for the CIE mechanism and application to cell imaging. J. Mater. Chem. C 2015, 3, 112–120. [Google Scholar] [CrossRef]
- Luo, X.; Li, J.; Li, C.; Heng, L.; Dong, Y.; Liu, Z.; Bo, Z.; Tang, B.Z. Reversible switching of the emission of diphenyldibenzofulvenes by thermal and mechanical stimuli. Adv. Mater. 2011, 23, 3261–3265. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wu, Y.; Chen, Y.; Zhu, Z.; Yang, X.; Yang, C.; Wang, K.; Tan, W. Pyrene-Excimer Probes Based on the Hybridization Chain Reaction for the Detection of Nucleic Acids in Complex Biological Fluids. Angew. Chem. Int. Ed. 2011, 50, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Lu, P.; Chen, S.; Lam, J.; Wang, Z.; Liu, Y.; Kwok, H.; Ma, Y.; Tang, B. Changing the Behavior of Chromophores from Aggregation-Caused Quenching to Aggregation-Induced Emission: Development of Highly Efficient Light Emitters in the Solid State. Adv. Mater. 2010, 22, 2159–2163. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yasuda, T.; Yang, Y.; Adachi, C. Bifunctional Star-Burst Amorphous Molecular Materials for OLEDs: Achieving Highly Efficient Solid State Luminescence and Carrier Transport Induced by Spontaneous Molecular Orientation. Adv. Mater. 2013, 25, 2666–2671. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yang, J.; Ren, Z.; Xie, G.; Li, Q.; Li, Z. Synthesis of Solution Processable Blue AIEgens and the Device Performance. Acta Chim. Sin. 2016, 74, 865–870. [Google Scholar] [CrossRef]
- Xue, S.; Yao, L.; Liu, S.; Gu, C.; Shen, F.; Li, W.; Zheng, H.; Wu, H.; Ma, Y. Simultaneous enhancement of the carrier mobility and luminous efficiency through thermal annealing a molecular glass material and device. J. Mater. Chem. 2012, 22, 21502–21506. [Google Scholar] [CrossRef]
- Aldred, M.P.; Li, C.; Zhang, G.; Gong, W.; Li, A.; Dai, Y.; Ma, D.; Zhu, M. Fluorescence quenching and enhancement of vitrifiable oligofluorenes end-capped with tetraphenylethene. J. Mater. Chem. 2012, 22, 7515–7528. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z. Molecular conformation and packing: their critical roles in the emission performance of mechanochromic fluorescence materials. Mater. Chem. Front. 2017, 1, 2174–2194. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are no available from the authors. |

| TPE-4Py | λEL | Von | Lmax | ηP, max | ηC, max | ηext, max | CIE |
|---|---|---|---|---|---|---|---|
| (nm) | (V) | (cd m−2) | (lm W−1) | (cd A−1) | (%) | (x, y) | |
| Nondoped 1 | 498 | 5.0 | 1153 | 1.06 | 2.54 | 1.08 | 0.21, 0.36 |
| White light | / | 4.0 | 2800 | 0.46 | 1.25 | 0.70 | 0.33, 0.32 |
| Nondoped 2 | 496 | 6.5 | 1678 | 1.00 | 3.05 | 1.31 | 0.21, 0.36 |
| Doped 10 | 462 | 10.0 | 1955 | 0.23 | 1.10 | 0.67 | 0.18, 0.22 |
| Doped 30 | 480 | 7.5 | 2581 | 1.60 | 4.32 | 2.14 | 0.20, 0.29 |
| Doped 50 | 484 | 6.0 | 2862 | 1.93 | 4.90 | 2.30 | 0.20, 0.32 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Qin, J.; Ren, Z.; Peng, Q.; Xie, G.; Li, Z. Pyrene-Based Blue AIEgen: Enhanced Hole Mobility and Good EL Performance in Solution-Processed OLEDs. Molecules 2017, 22, 2144. https://doi.org/10.3390/molecules22122144
Yang J, Qin J, Ren Z, Peng Q, Xie G, Li Z. Pyrene-Based Blue AIEgen: Enhanced Hole Mobility and Good EL Performance in Solution-Processed OLEDs. Molecules. 2017; 22(12):2144. https://doi.org/10.3390/molecules22122144
Chicago/Turabian StyleYang, Jie, Jianwen Qin, Zichun Ren, Qian Peng, Guohua Xie, and Zhen Li. 2017. "Pyrene-Based Blue AIEgen: Enhanced Hole Mobility and Good EL Performance in Solution-Processed OLEDs" Molecules 22, no. 12: 2144. https://doi.org/10.3390/molecules22122144





