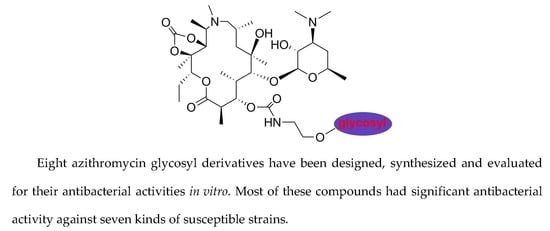
Synthesis and Antibacterial Evaluation of a Series of 11,12-Cyclic Carbonate Azithromycin-3-O-descladinosyl-3-O-carbamoyl Glycosyl Derivatives
Abstract
:1. Introduction
2. Results and Discussions
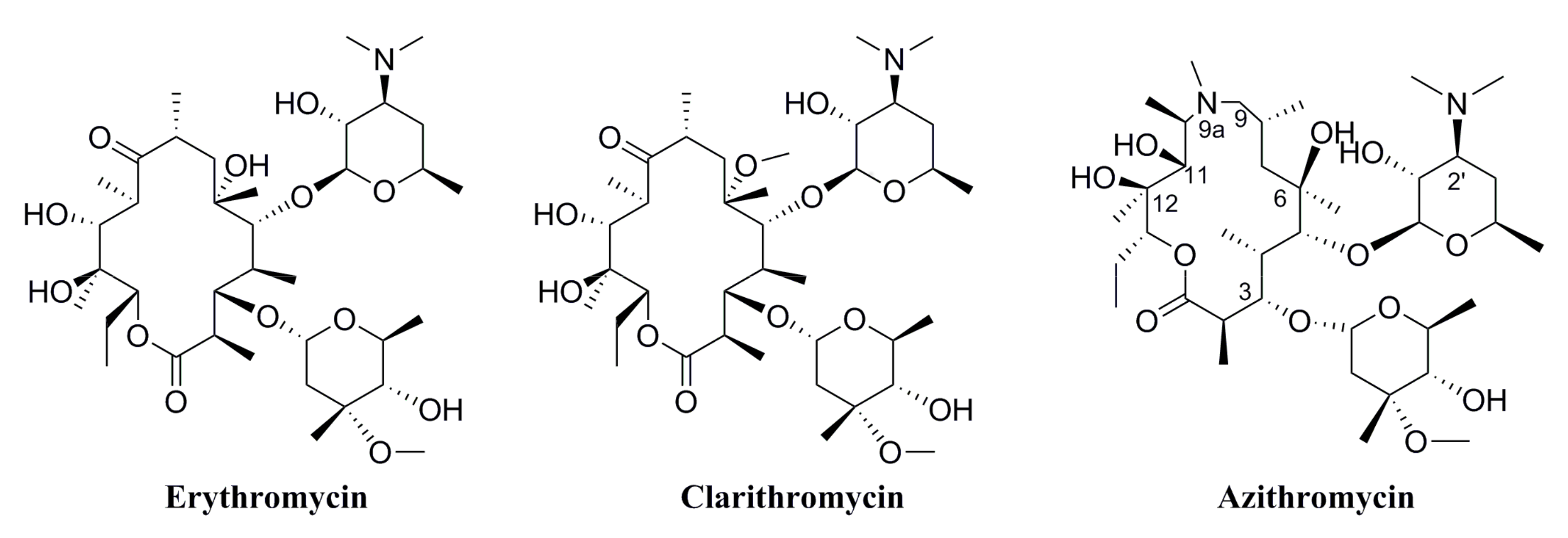
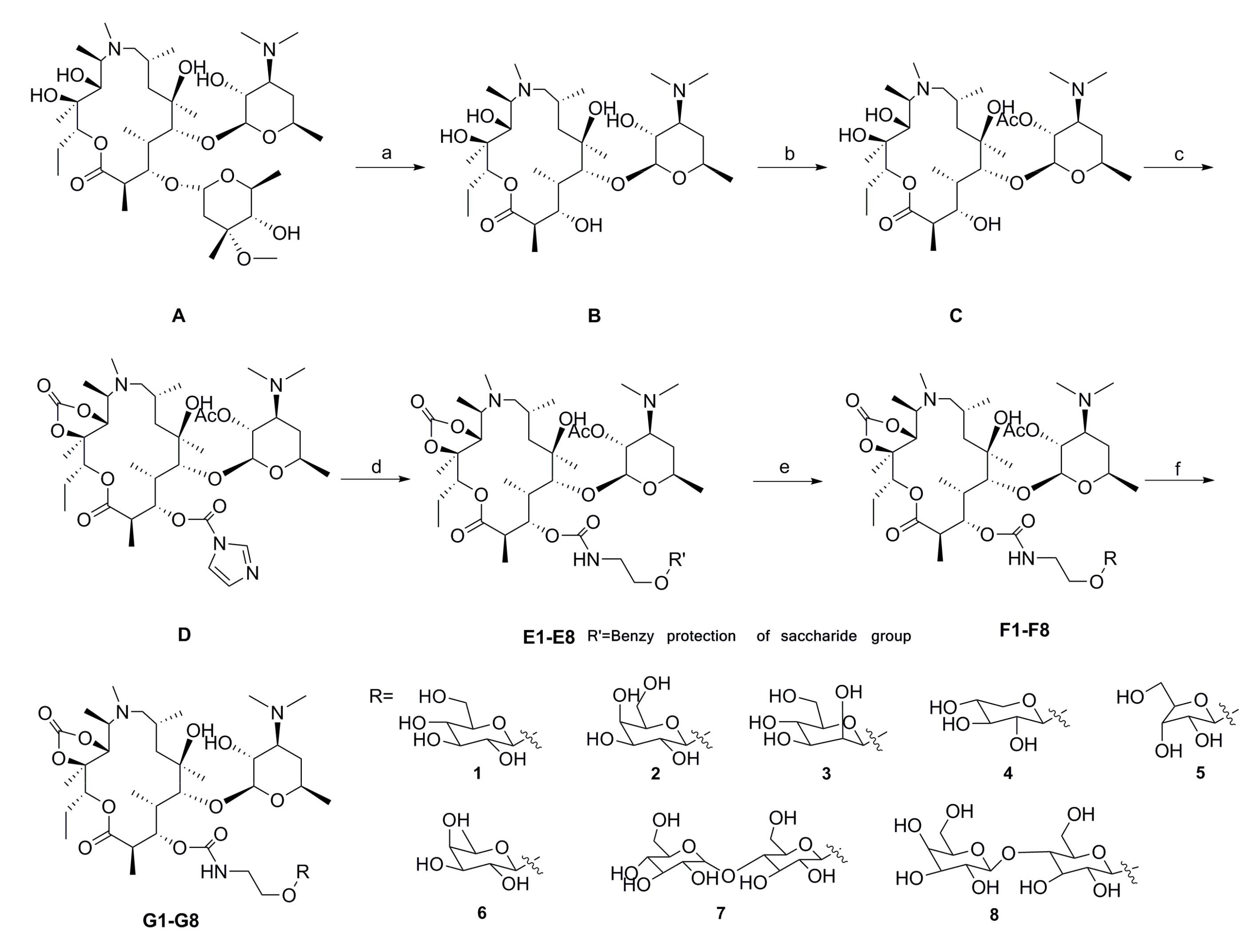
2.1. Chemistry
2.2. Antibacterial Activity
2.3. Biological Evaluation
3. Materials and Methods
3.1. Chemistry
3.1.1. 3-O-descladinosyl Azithromycin (B)
3.1.2. 2′-O-Acetyl-3-O-descladinosyl Azithromycin (C)
3.1.3. 2′-O-Acetyl-3-O-acylimidazolyl-3-O-descladinosyl Azithromycin-11,12-Cyclic Carbonate (D)
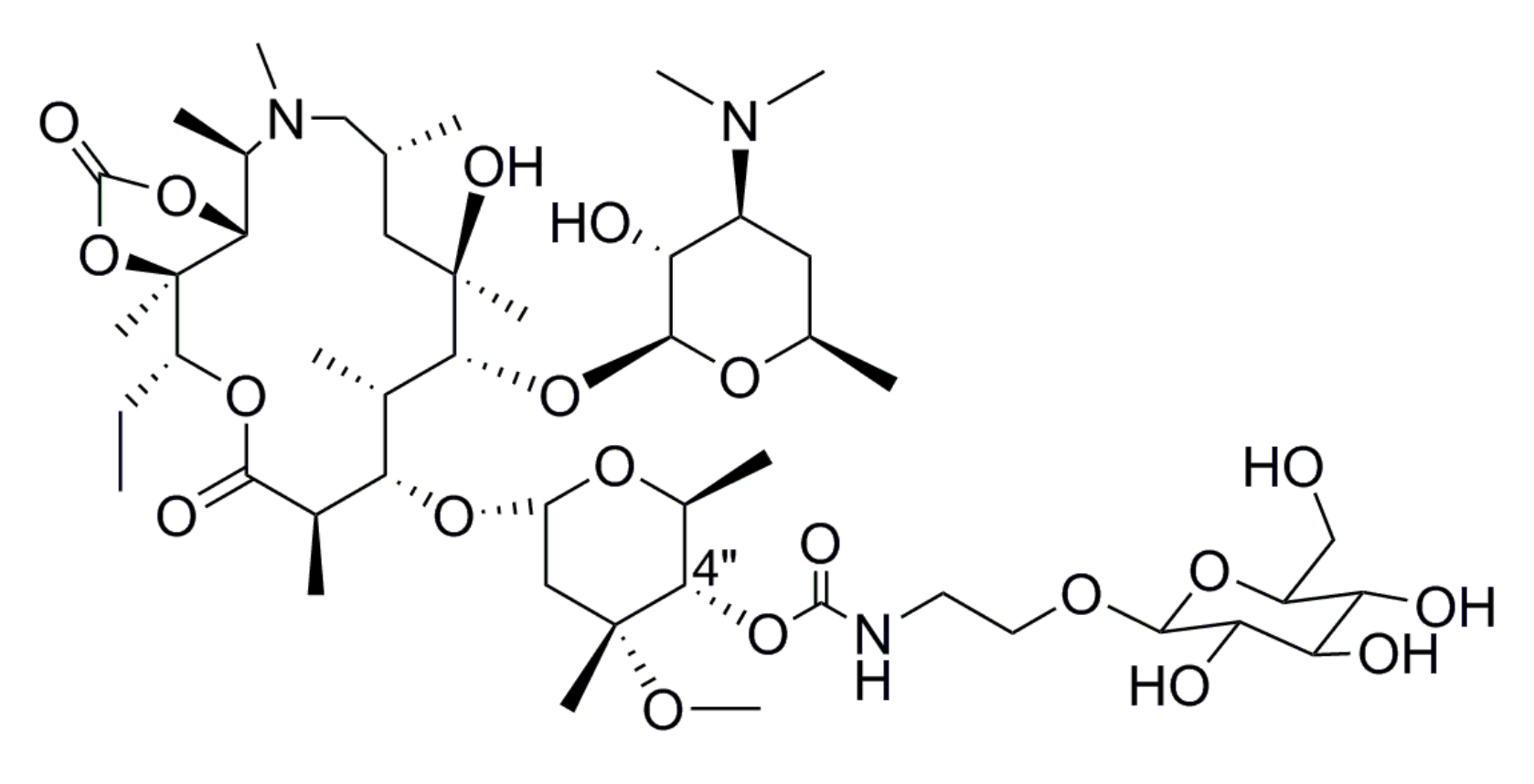
3.1.4. 2′-O-Acetyl-3-O-amido-[ethyl-2-(2,3,4,6-tetra-O-benzyl-β-d-glucopyranoside)]-3-O-descladinosyl Azithromycin-11,12-Cyclic Carbonate (E1)
3.1.5. 2′-O-Acetyl-3-O-amido-(ethyl-2-β-d-glucopyranoside)-3-O-descladinosyl Azithromycin-11,12-Cyclic Carbonate (F1)
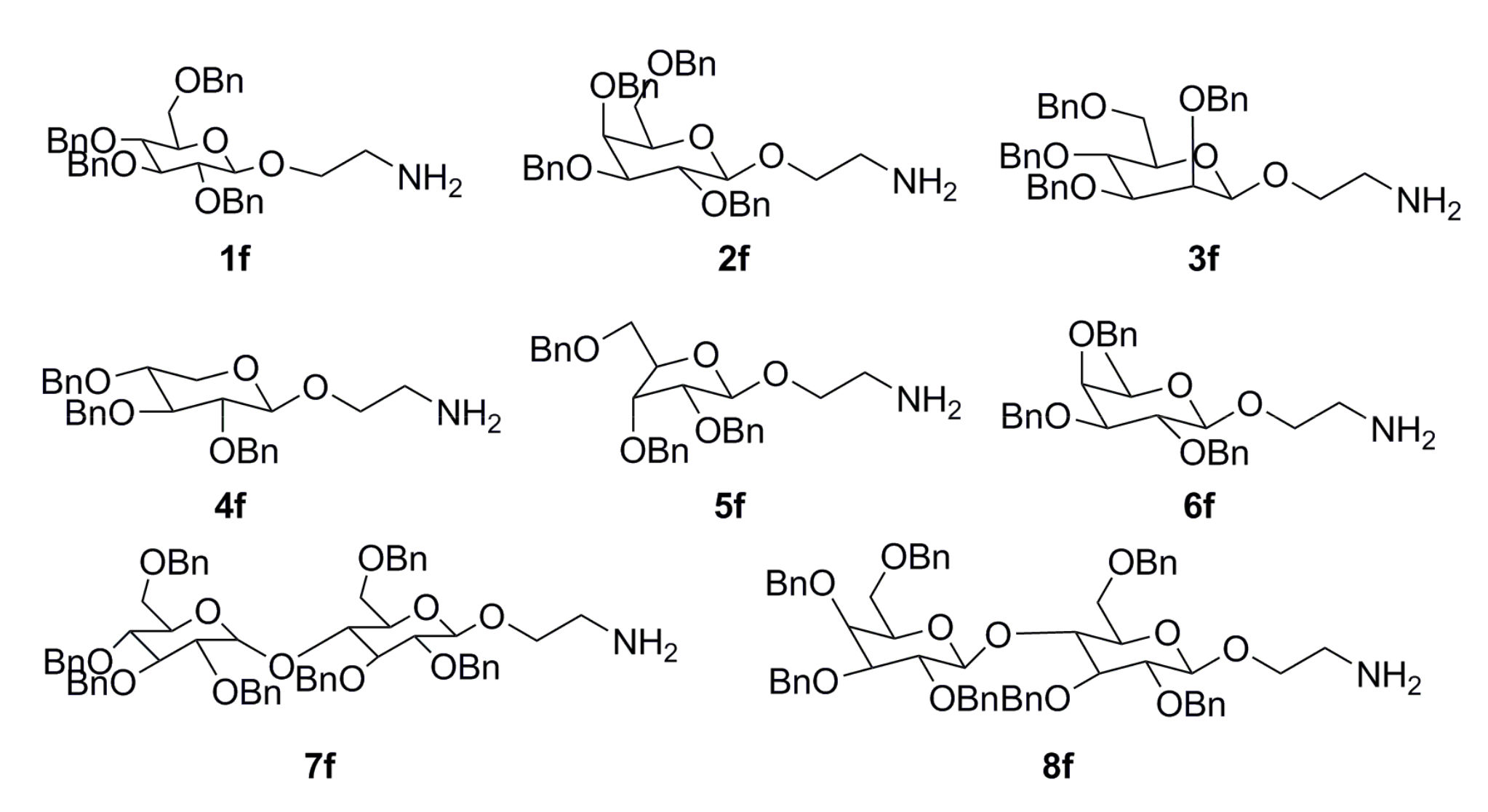
3.1.6. General Procedure for the Preparation of Azithromycin Derivatives G1–G8
3.2. Antibacterial Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fyfe, C.; Grossman, T.H.; Kerstein, K.; Sutcliffe, J. Resistance to Macrolide Antibiotics in Public Health Pathogens. Cold Spring Harb. Perspect. Med. 2016, 6, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Deane, C. Antibiotic discovery: Macrolides en masse. Nat. Chem. Biol. 2016, 12, 467. [Google Scholar] [CrossRef]
- Doern, G.V.; Heilmann, K.P.; Huynh, H.K.; Rhomberg, P.R.; Coffman, S.L.; Brueggemann, A.B. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999–2000, including a comparison of resistance rates since 1994–1995. Antimicrob. Agents Chemother. 2001, 45, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Kamruzzaman, M.; Shoma, S.; Thomas, C.M.; Partridge, S.R.; Iredell, J.R. Plasmid interference for curing antibiotic resistance plasmids in vivo. PLoS ONE 2017, 12, e0172913. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.; Martens, E.; Pereira, D. Nature nurtures the design of new semi-synthetic macrolide antibiotics. J. Antibiot. 2017, 70, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Jung, S.I.; Ko, K.S.; Kim, N.Y.; Son, J.S.; Chang, H.H.; Ki, H.K.; Oh, W.S.; Suh, J.Y.; Peck, K.R.; et al. High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ANSORP study). Antimicrob. Agents Chemother. 2004, 48, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Dueck, M.; Hoban, D.J.; Vercaigne, L.M.; Embil, J.M.; Gin, A.S.; Karlowsky, J.A. Review of macrolides and ketolides: Focus on respiratory tract infections. Drugs 2001, 61, 443–498. [Google Scholar] [CrossRef] [PubMed]
- Seiple, I.B.; Zhang, Z.Y.; Jakubec, P.; Mercier, A.L.; Wright, P.M.; Hog, D.T.; Yabu, K.; Allu, S.R.; Fukuzaki, T.; Carlsen, P.N.; et al. A platform for the discovery of new macrolide antibiotics. Nature 2016, 533, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Krajačić, M.B.; Perić, M.; Smith, K.S.; Schönfeld, Z.I.; Žiher, D.; Fajdetić, A.; Kujundžić, N.; Schönfeld, W.; Landek, G.; Padovan, J.; et al. Synthesis, structure-activity relationship, and antimalarial activity of ureas and thioureas of 15-membered azalides. J. Med. Chem. 2011, 54, 3595–3605. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Wada, H.; Rossios, C.; Takagi, D.; Higaki, M.; Mikura, S.; Goto, H.; Barnes, P.J.; Ito, K. A novel macrolide solithromycin exerts superior anti-inflammatory effect via NF-κB inhibition. J. Pharmacol. Exp. Ther. 2013, 345, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Champney, W.S.; Tober, C.L. Structure-activity relationships for six ketolide antibiotics. Curr. Microbiol. 2001, 42, 203–210. [Google Scholar] [PubMed]
- Heggelund, A.; Rømming, C.; Undheim, K. Preparation and antibacterial activity of cyclic 2′,3′-carbamate derivatives of azithromycin. Eur. J. Med. Chem. 2008, 43, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, L.; Liu, Z.; Li, H.; Lu, Y.; Li, Z.; Ma, S. Synthesis and antibacterial activity of novel 3-O-carbamoyl derivatives of clarithromycin and 11,12-cyclic carbonate azithromycin. Eur. J. Med. Chem. 2010, 45, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Bukvić, K.M.; Novak, P.; Dumić, M.; Cindrić, M.; Paljetak, H.C.; Kujundzić, N. Novel ureas and thioureas of 15-membered azalides with antibacterial activity against key respiratory pathogens. Eur. J. Med. Chem. 2009, 44, 3459–3470. [Google Scholar] [CrossRef] [PubMed]
- Perić, M.; Fajdetić, A.; Rupčić, R.; Alihodžić, S.; Žiher, D.; Krajačić, M.B.; Smith, K.S.; Schönfeld, Z.I.; Padovan, J.; Landek, G.; et al. Antimalarial activity of 9a-N substituted 15-membered azalides with improved in vitro and in vivo activity over azithromycin. J. Med. Chem. 2012, 55, 1389–1401. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Choi, J.Y.; Fu, H.; Harvey, C.; Ravindran, S.; Roush, W.R.; Boothroyd, J.C.; Khosla, C. Chemistry and biology of macrolide antiparasitic agents. J. Med. Chem. 2011, 54, 2792–2804. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, D.; Mutak, S. Discovery of 4″-ether linked azithromycin-quinolone hybrid series: Influence of the central linker on the antibacterial activity. ACS Med. Chem. Lett. 2011, 2, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Stimac, V.; Škugor, M.M.; Jakopović, I.P.; Vinter, A.; Ilijaš, M.; Alihodžić, S.; Mutak, S. Initial Scale-Up and Process Improvements for the preparation of a lead antibacterial macrolone compound. Org. Process Res. Dev. 2010, 14, 1401–1409. [Google Scholar] [CrossRef]
- Zhang, L.; Chai, X.; Wang, B.; Yu, S.; Hu, H.; Zou, Y.; Zhao, Q.; Meng, Q.; Wu, Q. Design, synthesis and biological evaluation of azithromycin glycosyl derivatives as potential antibacterial agents. Bioorg. Med. Chem. Lett. 2013, 23, 5057–5060. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, S.T.; Yan, M.; Wang, Y.Z.; Ma, S.T. Synthesis and antibacterial evaluation of novel 11,4″-disubstituted azithromycin analogs with greatly improved activity against erythromycin-resistant bacteria. Eur. J. Med. Chem. 2013, 59, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.T.; Ma, R.X.; Xian, R.Q.; Hao, B. Synthesis of novel 15-membered macrolide dimers. Chin. Chem. Lett. 2009, 20, 931–934. [Google Scholar] [CrossRef]
- Tanikawa, T.; Asaka, T.; Kashimura, M.; Suzuki, K.; Sugiyama, H.; Sato, M.; Kameo, K.; Morimoto, S.; Nishida, A. Synthesis and antibacterial activity of a novel series of acylides: 3-O-(3-pyridyl)acetylerythromycin A derivatives. J. Med. Chem. 2003, 46, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Ma, R.X.; Jia, L.; Venter, H.; Ma, S.T. Synthesis and antibacterial activity of novel 3-O-descladinosylazithromycin derivatives. Eur. J. Med. Chem. 2017, 127, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard-Eight Edition: CLSI Document M07-A8; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009. [Google Scholar]
Sample Availability: Samples of the compounds G1–G8 are available from the authors. |
| Compd. | S. aureus MSSA-1 | S. aureus MRSA-1 | S. pneumoniae 943 | S. pneumoniae 746 | S. pyogenes 447 | E. coli 236 | E. coli ATCC 25922 |
|---|---|---|---|---|---|---|---|
| G1 | 2 | 8 | 1 | 2 | 8 | 32 | 128 |
| G2 | 2 | 8 | 2 | 2 | 32 | 32 | 128 |
| G3 | 4 | 8 | 8 | 4 | 32 | 32 | 128 |
| G4 | 8 | 8 | 4 | 8 | 32 | 64 | 256 |
| G5 | 4 | 8 | 4 | 16 | 32 | 64 | 128 |
| G6 | 2 | 4 | 8 | 16 | 64 | 64 | 128 |
| G7 | 8 | 16 | 32 | 32 | 128 | 128 | 256 |
| G8 | 16 | 16 | 16 | 16 | 128 | 256 | 256 |
| Azi | 2 | 16 | 4 | 8 | 32 | 256 | 256 |
| Ery | 16 | 16 | 8 | 16 | 32 | 256 | 256 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-M.; Zhao, F.-L.; Zhang, L.; Chai, X.-Y.; Meng, Q.-G. Synthesis and Antibacterial Evaluation of a Series of 11,12-Cyclic Carbonate Azithromycin-3-O-descladinosyl-3-O-carbamoyl Glycosyl Derivatives. Molecules 2017, 22, 2146. https://doi.org/10.3390/molecules22122146
Wang C-M, Zhao F-L, Zhang L, Chai X-Y, Meng Q-G. Synthesis and Antibacterial Evaluation of a Series of 11,12-Cyclic Carbonate Azithromycin-3-O-descladinosyl-3-O-carbamoyl Glycosyl Derivatives. Molecules. 2017; 22(12):2146. https://doi.org/10.3390/molecules22122146
Chicago/Turabian StyleWang, Chao-Ming, Feng-Lan Zhao, Lei Zhang, Xiao-Yun Chai, and Qing-Guo Meng. 2017. "Synthesis and Antibacterial Evaluation of a Series of 11,12-Cyclic Carbonate Azithromycin-3-O-descladinosyl-3-O-carbamoyl Glycosyl Derivatives" Molecules 22, no. 12: 2146. https://doi.org/10.3390/molecules22122146