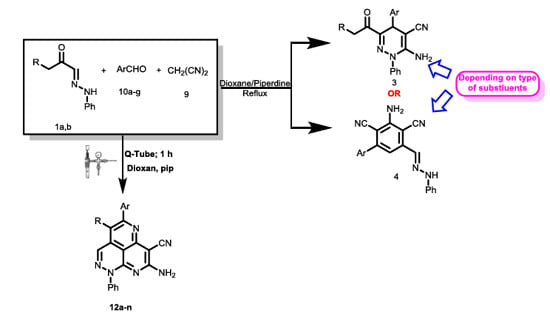
Multi Component Reactions under Increased Pressure: On the Mechanism of Formation of Pyridazino[5,4,3-de][1,6]naphthyridine Derivatives by the Reaction of Malononitrile, Aldehydes and 2-Oxoglyoxalarylhydrazones in Q-Tubes
Abstract
:1. Introduction
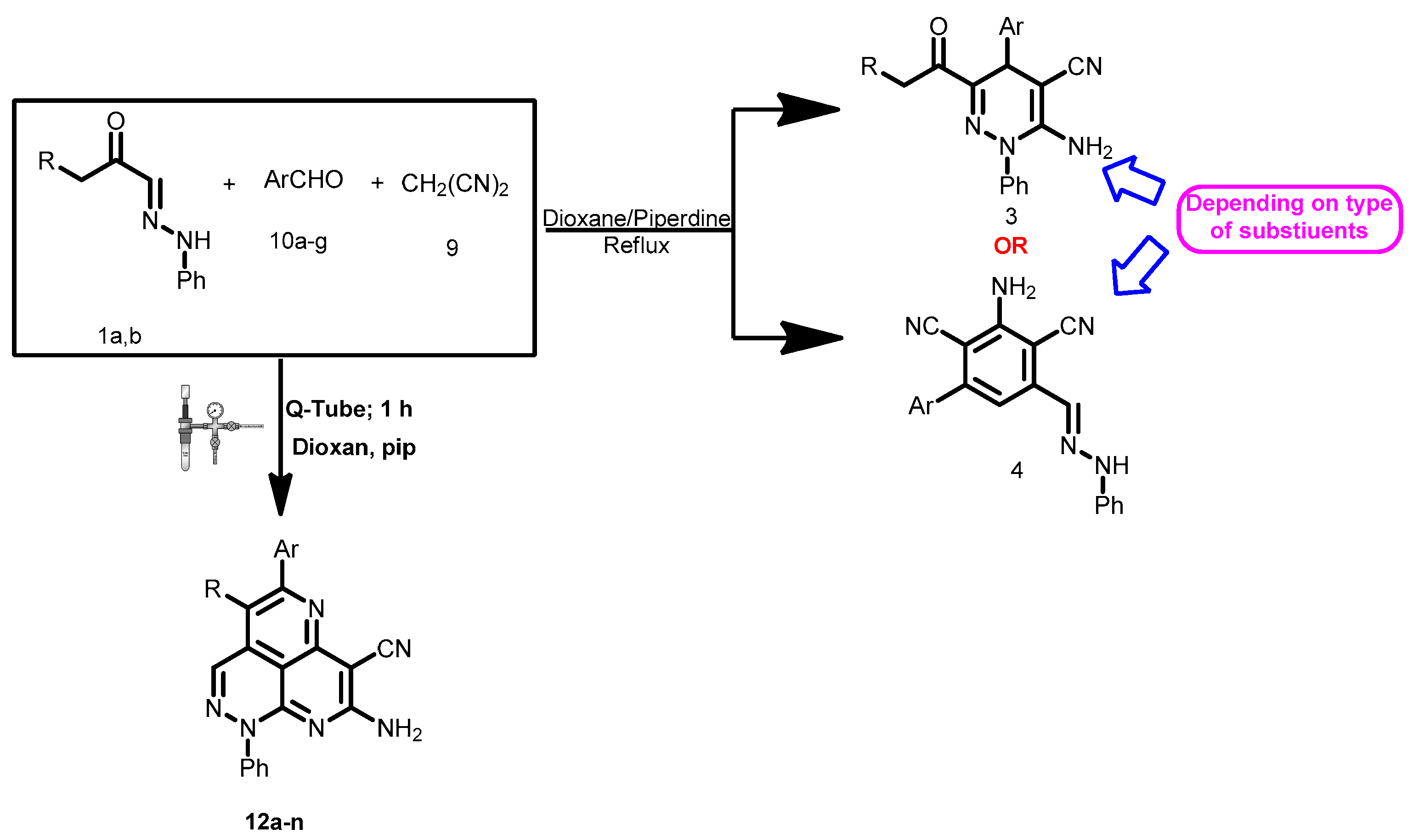
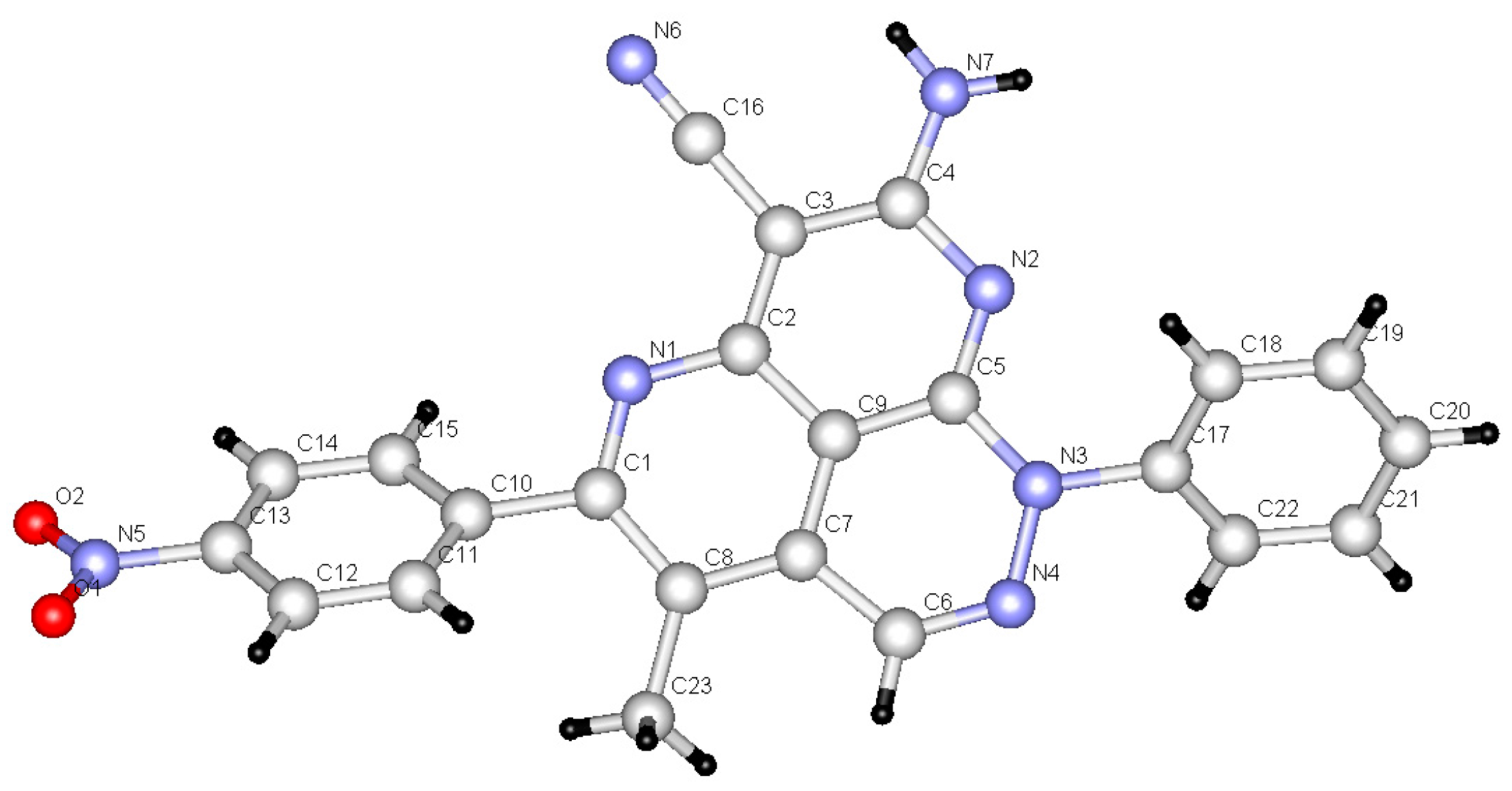
2. Results
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. General Procedures for Q-Tube-Assisted Synthesis of 12a–n
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Asif, M. Some Recent Approaches of Biologically Active Substituted Pyridazine and Phthalazine Drugs. Curr. Med. Chem. 2012, 19, 2984–2991. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Hegde, G.L.; Khanum, S.A.; Shashikanth, S. Synthesis and Pharmacological Activity of 4-Aryl-Thieno-(2,3-d)-Pyridazines. Indian J. Pharm. Sci. 2005, 67, 210–215. [Google Scholar]
- Tucaliuc, R.A.; Cotea, V.V.; Niculaua, M.; Tuchilus, C.; Mantu, D.; Mangalagiu, I.I. New pyridazine–fluorine derivatives: Synthesis, chemistry and biological activity. Eur. J. Med. Chem. 2013, 67, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhu, Y.; Lian, M.; Liu, M.; Yuan, J.; Yin, G.; Wu, A. Unexpected C–C Bond Cleavage: A Route to 3, 6-Diarylpyridazines and 6-Arylpyridazin-3-ones from 1,3-Dicarbonyl Compounds and Methyl Ketones. J. Org. Chem. 2012, 77, 9865–9870. [Google Scholar] [CrossRef] [PubMed]
- Kessler, S.N.; Wegner, H.A. One-Pot Synthesis of Phthalazines and Pyridazino-aromatics: A Novel Strategy for Substituted Naphthalenes. Org. Lett. 2012, 14, 3268–3271. [Google Scholar] [CrossRef] [PubMed]
- Poschenrieder, H.; Stachel, H.-D. Synthesis of pyrrolo[3,4-c]pyridazines. J. Heterocycl. Chem. 1995, 32, 1457–1460. [Google Scholar] [CrossRef]
- Behbehani, H.; Ibrahim, H.M. Microwave-Assisted Synthesis in Water: First One-Pot Synthesis of A Novel Class of Polysubstituted benzo[4,5]imidazo[1,2-b]pyridazines via Intramolecular SNAr. RSC Adv. 2015, 5, 89226–89237. [Google Scholar] [CrossRef]
- Elnagdi, M.H.; Moustafa, M.S.; Sadek, K.U. Green Synthesis of Biologically Relevant Azoles and Azines Derivatives, 1st ed.; Lap Lambert Acadimic Publishing: Saarbrücken, Germany, 2014; p. 64. ISBN 9783659551086. [Google Scholar]
- Ghozlan, S.A.S.; Abdelhamid, I.A.; Hassaneen, H.M.; Elnagdi, M.H. Studies with Enamines and Azaenamines: A novel Efficient Route to 6-amino-1,4-dihydropyridazines and their Condensed Derivatives. J. Heterocycl. Chem. 2007, 44, 105–108. [Google Scholar] [CrossRef]
- Al-Mousawi, S.M.; Moustafa, M.S.; Abdelhamid, I.A.; Elnagdi, M.H. Reassignment of the Structures of Condensation Products of α-keto α′-formylarylhydrazones with Ethyl Cyanoacetate: A Novel Route to ethyl 5-arylazo-2-hydroxynicotinates. Tetrahedron Lett. 2011, 52, 202–204. [Google Scholar] [CrossRef]
- Ackermann, L.; Gunnoe, T.B.; Habgood, L.G. Catalytic Hydroarylation of Carbon-Carbon Multiple Bonds; Wiley-VCH Verlag Gmbh & Co. KGaA: Weinheim, Germany, 2017; pp. 51–58. ISBN 9783527340132. [Google Scholar]
- Ghozlan, S.A.S.; Abdelmoniem, A.M.; Butenschon, H.; Abdelhamid, I.A. Discrepancies in The Reactivity Pattern of Azaenamines towards Cinnamonitriles: Synthesis of Novel aza-steroid Analogues. Tetrahedron 2015, 71, 1413–1418. [Google Scholar] [CrossRef]
- Al-Zaydi, K.M. Microwave Assisted Synthesis, Part 1: Rapid Solventless Synthesis of 3-Substituted Coumarins and Benzocoumarins by Microwave Irradiation of the Corresponding Enaminones. Molecules 2003, 8, 541–555. [Google Scholar] [CrossRef]
- Al-Zaydi, K.M.; Borik, R.M.; Elnagdi, M.H. Arylhydrazononitriles as Precursors to 2-Substituted 1,2,3-triazoles and 4-amino-5-cyano-pyrazole Derivatives Utilizing Microwave and Ultrasound Irradiation. Green Chem. Lett. Rev. 2012, 5, 241–250. [Google Scholar] [CrossRef]
- Al-Zaydi, K.M.; Nhari, L.M.; Borik, R.M.; Elnagdi, M.H. Green Technologies in Organic Synthesis: Self-Condensation of Enamines, Enaminones and EnaminoestersUnder Microwave Irradiation in Ionic Liquid. Green Chem. Lett. Rev. 2010, 3, 93–99. [Google Scholar] [CrossRef]
- Al-Zaydi, K.M.; Borik, R.M.; Elnagdi, M.H. 2-Arylhydrazonopropanals as Building Blocks in Heterocyclic Chemistry: Microwave Assisted Condensation of 2-Aryl-hydrazonopropanals with Amines and Active Methylene Reagents. Molecules 2003, 8, 910–923. [Google Scholar] [CrossRef]
- Kappe, C.O.; Stadler, A. Microwaves in Organic and Medicinal Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 3–5. ISBN 9783527606559. [Google Scholar] [CrossRef]
- Kappe, C.O.; Dallinger, D.; Murphree, S.S. Practical Microwave Synthesis for Organic Chemists—Strategies; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 2–4. ISBN 9783527623907. [Google Scholar] [CrossRef]
- Moustafa, M.S.; Al-Mousawi, S.M.; Abdelhamid, I.A.; Elnagdi, M.H. Use of A Novel Multicomponent Reaction Under High Pressure for The Efficient Construction of A New Pyridazino[5,4,3-de][1,6]naphthyridine Tricyclic System. RSC Adv. 2016, 93, 90840–90845. [Google Scholar] [CrossRef]
- Sadek, K.U.; Selim, M.A.; .Alnajjar, A.; Atallah, M.; Elnagdi, M.H. Multicomponent Reactions under Increased Pressure: On the Reaction of Arylhydrazonals, Aromatic Aldehydes and Malononitrile in Q-Tube. Chem. Eur. J. 2016, 7, 468–472. [Google Scholar] [CrossRef]
- Almansour, A.I.; Kumar, R.S.; Arumugam, R.; Basiri, A.; Kia, Y.; Ali, M.A. An Expedient Synthesis, Acetylcholinesterase Inhibitory Activity, and Molecular Modeling Study of Highly Functionalized Hexahydro-1,6-naphthyridines. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Raboisson, P.; Desjarlais, R.L.; Reed, R.; Lattanze, J.; Chaikin, M.; Manthey, C.L.; Tomczuk, B.E.; Marugan, J.J. Identification of novel short chain 4-substituted indoles as potent alphavbeta3 antagonist using structure-based drug design. Eur. J. Med. Chem. 2007, 42, 334. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, D.; Wu, N.; Zhang, A.; Jia, Z.; Li, X. The synthesis and biological evaluation of a novel series of C7 non-basic substituted fluoroquinolones as antibacterial agents. Bioorg. Med. Chem. Lett. 2009, 19, 4130. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Wang, Y.; Kazemi, R.; Xu, S.; Xu, Z.; Sanchez, T.W.; Yang, L.; Debnath, B.; Odde, S.; Xie, H. Repositioning HIV-1 Integrase Inhibitors for Cancer Therapeutics: 1,6-Naphthyridine-7-carboxamide as a Promising Scaffold with Drug-like Properties. J. Med. Chem. 2012, 55, 9492–9509. [Google Scholar] [CrossRef] [PubMed]
- CCDC 1434604 Contains the X-ray Structure Crystallographic Data for This Paper. Available online: http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-1223-336033; E-mail: [email protected]).
- CCDC 1434605 Contains the X-ray Structure Crystallographic Data for This Paper. Available online: http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-1223-336033; E-mail: [email protected]).
- CCDC 1493165 Contains the X-ray Structure Crystallographic Data for This Paper. Available online: http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-1223-336033; E-mail: [email protected]).
- Mittelbach, M. An improved and facile synthesis of 2-Amino-1,1,3-trycyanopropene. Monatsh. Chem. 1985, 116, 689–691. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 12a–n are available from the authors. |





| Entry | R | Ar | Yield % | Time (min) |
|---|---|---|---|---|
| 12a | H | Ph | 85 | 60 |
| 12b | H | 4-ClC6H4 | 75 | 60 |
| 12c | H | 2-ClC6H4 | 80 | 120 |
| 12d | H | 4-CH3C6H4 | 77 | 60 |
| 12e | H | 2-CH3C6H4 | 73 | 120 |
| 12f | H | 4-O2NC6H4 | 82 | 60 |
| 12g | H | 2-furyl | 86 | 120 |
| 12h | CH3 | Ph | 83 | 60 |
| 12j | CH3 | 4-ClC6H4 | 78 | 60 |
| 12k | CH3 | 4-CH3C6H4 | 80 | 60 |
| 12l | CH3 | 2-CH3C6H4 | 72 | 120 |
| 12m | CH3 | 4-O2NC6H4 | 82 | 60 |
| 12n | CH3 | 2-furyl | 86 | 120 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
AL-Johani, M.A.; Al-Zaydi, K.M.; Mousally, S.M.; Alqahtani, N.F.; Elnagdi, N.H.; Elnagdi, M.H. Multi Component Reactions under Increased Pressure: On the Mechanism of Formation of Pyridazino[5,4,3-de][1,6]naphthyridine Derivatives by the Reaction of Malononitrile, Aldehydes and 2-Oxoglyoxalarylhydrazones in Q-Tubes. Molecules 2017, 22, 2114. https://doi.org/10.3390/molecules22122114
AL-Johani MA, Al-Zaydi KM, Mousally SM, Alqahtani NF, Elnagdi NH, Elnagdi MH. Multi Component Reactions under Increased Pressure: On the Mechanism of Formation of Pyridazino[5,4,3-de][1,6]naphthyridine Derivatives by the Reaction of Malononitrile, Aldehydes and 2-Oxoglyoxalarylhydrazones in Q-Tubes. Molecules. 2017; 22(12):2114. https://doi.org/10.3390/molecules22122114
Chicago/Turabian StyleAL-Johani, Majdah A., Khadijah M. Al-Zaydi, Sameera M. Mousally, Norah F. Alqahtani, Noha Hilmy Elnagdi, and Mohamed H. Elnagdi. 2017. "Multi Component Reactions under Increased Pressure: On the Mechanism of Formation of Pyridazino[5,4,3-de][1,6]naphthyridine Derivatives by the Reaction of Malononitrile, Aldehydes and 2-Oxoglyoxalarylhydrazones in Q-Tubes" Molecules 22, no. 12: 2114. https://doi.org/10.3390/molecules22122114