Design, Recombinant Fusion Expression and Biological Evaluation of Vasoactive Intestinal Peptide Analogue as Novel Antimicrobial Agent
Abstract
:1. Introduction
2. Results
2.1. Sequence and Structural Properties of VIP and the VIP Analogues
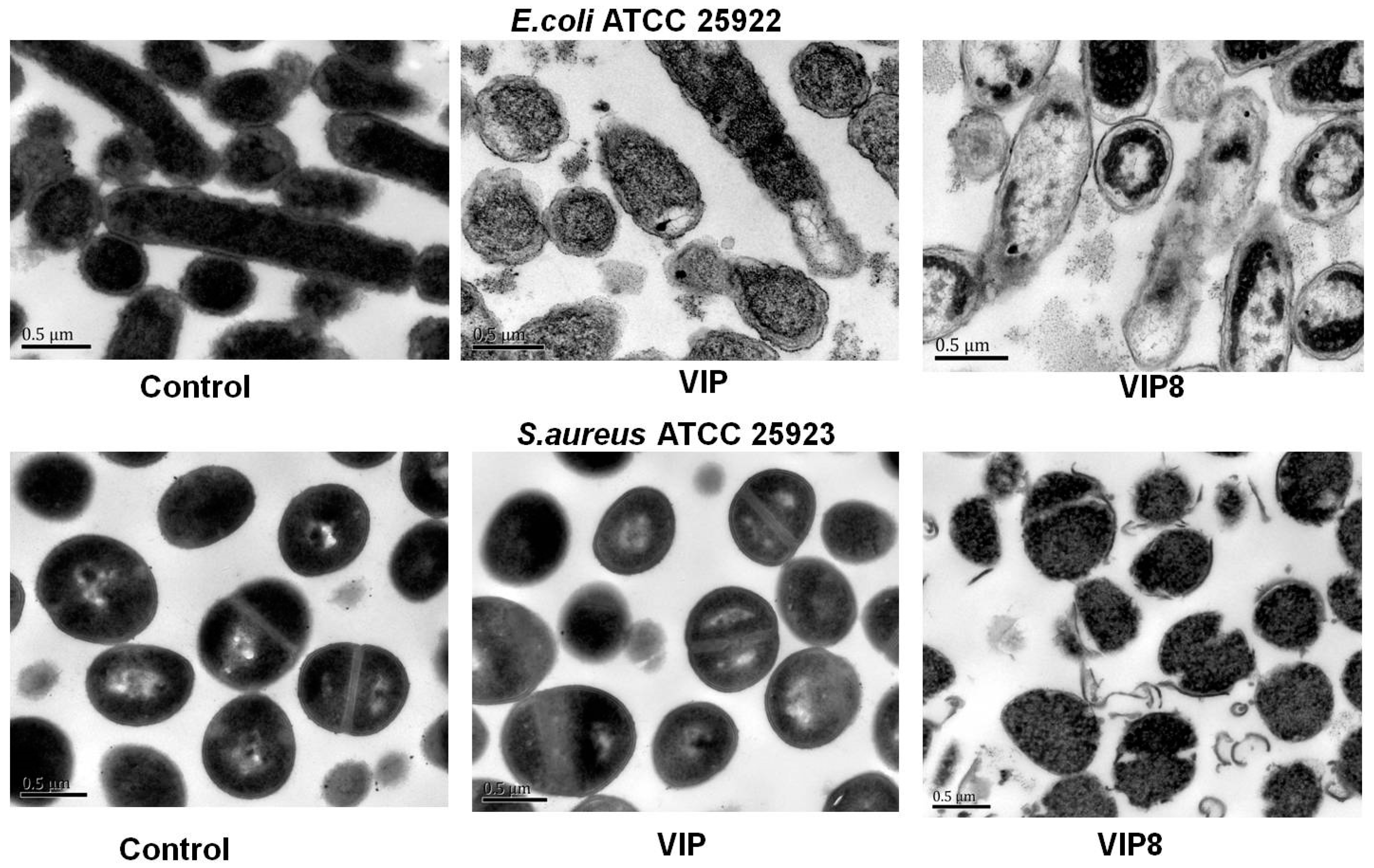
2.2. Antimicrobial Activity and Possible Mechanism of VIP and VIP Analogue 8
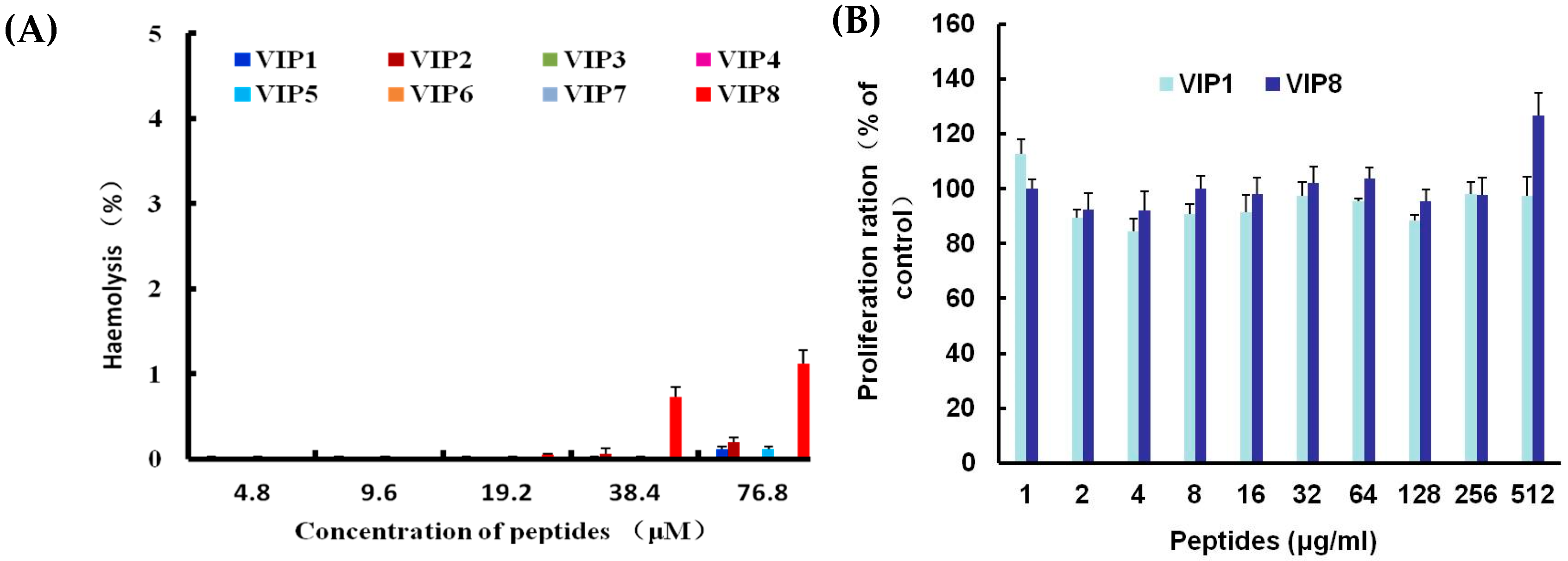
2.3. Haemolytic Activity and Cytotoxicity of VIP and VIP Analogue 8
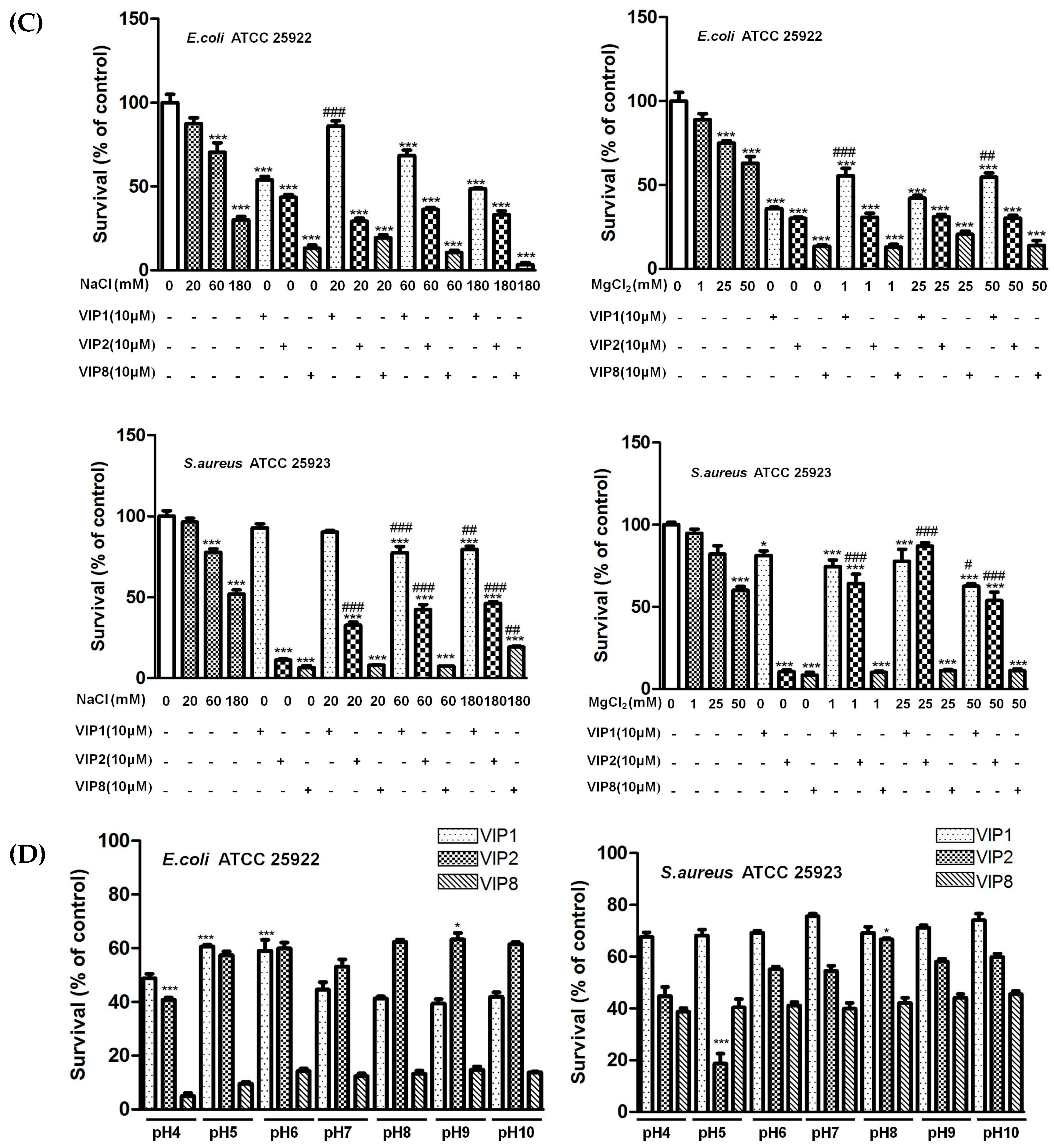
2.4. Ionic Strength and pH Influencing Antibacterial Activity of VIP and Its Analogues
2.5. Expression and Purification of Recombinant VIP Analogue 8
2.6. Antimicrobial Activity of Recombinant VIP Analogue 8
3. Discussion
4. Material and Methods
4.1. Peptides, Strains, Plasmids, Reagents, and Enzymes
4.2. VIP and Its Analogues Design
4.3. Antimicrobial, Hemolytic, Cytotoxicity Assay and Determination of Specific Factors Influencing Antimicrobial Activity
4.4. Construction of Recombinant pET32a-VIP8 Fusion Expression Vector
4.5. Expression and Purification of the Recombinant Fusion Protein Trx-VIP8
4.6. Enzymatic Cleavage of Trx-VIP8 and Release of VIP8
4.7. Antimicrobial Activity Assay of Recombinant VIP8
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of interest
References
- Czaplewski, L.; Bax, R.; Clokie, M.; Dawson, M.; Fairhead, H.; Fischetti, V.A.; Foster, S.; Gilmore, B.F.; Hancock, R.E.; Harper, D.; et al. Alternatives to antibiotics—A pipeline portfolio review. Lancet Infect. Dis. 2016, 16, 239–251. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Hakansson, J.; Ringstad, L.; Björn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Najeeb, S.; Mali, M.; Moin, S.F.; Raza, S.Q.; Zohaib, S.; Sefat, F.; Zafar, M.S. Histatin peptides: Pharmacological functions and their applications in dentistry. Saudi Pharm. J. 2017, 25, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Naseem, M.; Sheikh, Z.; Najeeb, S.; Shahab, S.; Zafar, M.S. Oral antimicrobial peptides: Types and role in the oral cavity. Saudi Pharm. J. 2016, 24, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- EI-Karim, I.A.; Linden, G.J.; Orr, D.F.; Lundy, F.T. Antimicrobial activity of neuropeptides against a range of micro-organisms from skin, oral, respiratory and gastrointestinal tract sites. J. Neuroimmunol. 2008, 200, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Gomariz, R.P.; Gutiérrez-Cañas, I.; Arranz, A.; Carrion, M.; Juarranz, Y.; Leceta, J.; Martinez, C. Peptides targeting toll-like receptor signaling pathway for novel immune therapeutics. Curr. Pharm. Des. 2010, 16, 1063–1080. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, D.; Nowak, J.; Lundy, F.T. Direct and indirect antimicrobial activities of neuropeptides and their therapeutic potential. Curr. Protein Pept. Sci. 2012, 13, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Mcclellan, S.A.; Barrett, R.P.; Zhang, Y.; Hazlett, L.D. Vasoactive intestinal peptide downregulates proinflammatory TLRs while upregulating anti-inflammatory TLRs in the infected cornea. J. Immunol. 2012, 189, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Ouchi, Y. Antimicrobial peptide defensin: Identification of novel isoforms and the characterization of their physiological roles and their significance in the pathogenesis of diseases. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2012, 88, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Li, B.; Jin, S.; Daniell, H. Expression and characterization of antimicrobial peptides Retrocyclin-101 and Protegrin-1 in chloroplasts to control viral and bacterial infections. Plant Biotechnol. J. 2011, 9, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Kim, C.; Seo, C.H.; Park, Y. The therapeutic applications of antimicrobial peptides (AMPs): A patent review. J. Micribiol. 2017, 55, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Campos-Salinas, J.; Cavazzuti, A.; O’Valle, F.; Forte-Lago, I.; Caro, M.; Beverley, S.M.; Delgado, M.; Gonzalez-Rey, E. Therapeutic efficacy of stable analogues of vasoactive intestinal peptide against pathogens. J. Biol. Chem. 2014, 289, 14583–14599. [Google Scholar] [CrossRef] [PubMed]
- Aleinein, R.A.; Hamoud, R.; Schafer, H.; Wink, M. Molecular cloning and expression of ranalexin, a bioactive antimicrobial peptide from Rana catesbeiana in Escherichia coli and assessments of its biological activities. Appl. Microbiol. Biotechnol. 2013, 97, 3535–3543. [Google Scholar] [CrossRef] [PubMed]
- Zorko, M.; Jerala, R. Production of recombinant antimicrobial peptides in bacteria. Methods Mol. Biol. 2010, 618, 61–76. [Google Scholar] [PubMed]
- LaVallie, E.R.; Diblasio, E.A.; Kovacic, S.; Grant, K.L.; Schendel, P.F.; McCoy, J.M. A thioredoxin gene fusion expression system that circumvents inclusion body formation in E. coli cytoplasm. Nat. Biotechnol. 1993, 11, 187–193. [Google Scholar] [CrossRef]
- Dockray, G.J. Vasoactive intestinal polypeptide and related peptides. In Gut Hormones: Biochemistry and Physiology, 1st ed.; Walsh, J.H., Dockray, G.J., Eds.; Raven Press: New York, NY, USA, 1994; p. 447. [Google Scholar]
- Du, B.H.; Eng, J.; Hulmes, J.D.; Chang, M.; Pan, Y.C.; Yalow, R.S. Guinea pig has a unique mammalian VIP. Biochem. Biophys. Res. Commun. 1985, 128, 1093–1098. [Google Scholar] [CrossRef]
- Stiuso, P.; Marabotti, A.; Facchiano, A.; Lepretti, M.; Dicitore, A.; Ferranti, P.; Carteni, M. Assessment of the conformational features of vasoactive intestinal peptide in solution by limited proteolysis experiments. Biopolymers 2006, 81, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Onoue, S.; Matsumoto, A.; Nagano, Y.; Ohshima, K.; Ohmori, Y.; Yamada, S.; Kimura, R.; Yajima, T.; Kashimoto, K. α-Helical structure in the C-terminus of vasoactive intestinal peptide: Functional and structural consequences. Eur. J. Pharmacol. 2004, 485, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Sugishita, K.; Harada, M.; Fujii, N.; Miyajima, K. Interactions of an antimicrobial peptide, magainin 2, with outer and inner membranes of Gram-negative bacteria. Biochim. Biophys. Acta 1997, 1327, 119–130. [Google Scholar] [CrossRef]
- Aoki, W.; Ueda, M. Characterization of antimicrobial peptides toward the development of novel antibiotics. Pharmaceuticals 2013, 6, 1055–1081. [Google Scholar] [CrossRef] [PubMed]
- Kondejewski, L.H.; Jelokhani-Niaraki, M.; Farmer, S.W.; Lix, B.; Kay, C.M.; Sykes, B.D.; Hancock, R.E.; Hodges, R.S. Dissociation of antimicrobial and hemolytic activities in cyclic peptide diastereomers by systematic alterations in amphipathicity. J. Biol. Chem. 1999, 274, 13181–13192. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.J.; Anderson, G.M.; Stolzenberg, E.D.; Kari, U.P.; Zasloff, M.; Wilson, J.M. Human-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 1997, 88, 553–560. [Google Scholar] [CrossRef]
- Sharma, H.; Nagaraj, R. Human β-defensin 4 with non-native disulfide bridges exhibit antimicrobial activity. PLoS ONE 2015, 10, e0119525. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Kajiya, M.; Zhu, T.; Nishi, H.; Mawardi, H.; Shin, J.; Elbadawi, L.; Kamata, N.; Komatsuzawa, H.; Kawai, T. Additive effects of orexin B and vasoactive intestinal polypeptide on LL-37-mediated antimicrobial activities. J. Neuroimmunol. 2011, 233, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Leong, S.S.; Jiang, R. Soluble fusion expression and characterization of bioactive human beta-defensin 26 and 27. Appl. Microbiol. Biotechnol. 2009, 84, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Harper, S.; Speicher, D.W. Purification of proteins fused to glutathione S-transferase. Methods Mol. Biol. 2011, 681, 259–280. [Google Scholar] [PubMed]
- Luan, C.; Zhang, H.W.; Song, D.G.; Xie, Y.G.; Feng, J.; Wang, Y.Z. Expressing antimicrobial peptide cathelicidin-BF in Bacillus subtilis using SUMO technology. Appl. Microbiol. Biotechnol. 2014, 98, 3651–3658. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.G.; Luan, C.; Zhang, H.W.; Han, F.F.; Feng, J.; Choi, Y.J.; Groleau, D.; Wang, Y.Z. Effects of thioredoxin, sumo and intein on soluble fusion expression of an antimicrobial peptide OG2 in Escherichia coli. Protein Pept. Lett. 2013, 20, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Wani, N.A.; Singh, G.; Shankar, S.; Sharma, A.; Katoch, M.; Rai, R. Short hybrid peptides incorporating β- and γ-amino acids as antimicrobial agents. Peptides 2017, 97, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Liu, Y.; Xie, Y.; Gao, Y.; Luan, C.; Wang, Y. Antimicrobial peptides derived from different animals: Comparative studies of antimicrobial properties, cytotoxicity and mechanism of action. World J. Microbiol. Biotechnol. 2011, 27, 1847–1857. [Google Scholar] [CrossRef]
- Kayaci, F.; Umu, O.C.O.; Tekinay, T.; Uyar, T. Antibacterial electrospun poly(lactic acid) (PLA) nanofibrous webs incorporating triclosan/cyclodextrin inclusion complexes. J. Agric. Food Chem. 2013, 61, 3901–3908. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds vasoactive intestinal peptide analogues are available from the authors. |

| Name | Amino Acides Sequence | Charge | GRAVY a | Molecular Weight |
|---|---|---|---|---|
| VIP1 b | HSDAVFTDNYTRLRKQMAVKKYLNSILN | +3 | −0.639 | 3326.8 |
| VIP2 | HSKAVFTKNYTRLRKQMAVKKYLNSILN | +7 | −0.668 | 3352.9 |
| VIP3 | HSDAVFTDNSTRLRKQMAVKKSLNSILN | +3 | −0.604 | 3174.6 |
| VIP4 | HSDAVFTDNYTRLRKQMAVKKYLNSILT | +3 | −0.539 | 3313.8 |
| VIP5 | HSKAVFTKNYTRLRKQMAVKKYLNSILT | +7 | −0.568 | 3339.9 |
| VIP6 | HSDAVFTDNSTRLRKQMAVKKSLNSILT | +3 | −0.504 | 3161.6 |
| VIP7 c | HSDAVFTANYTRLRRQLAVRRYLAAILGRR | +6 | −0.350 | 3545.2 |
| VIP8 c | FTANYTRLRRQLAVRRYLAAILGRR | +7 | −0.360 | 3035.6 |
| Pathogen | E. coli ATCC 25922 | S. aureus ATCC 25923 | ||
|---|---|---|---|---|
| Compound | MIC a | MBC b | MIC | MBC |
| VIP1 c | 64 | 128 | >256 | >256 |
| VIP2 | 16 | 32 | 64 | 128 |
| VIP3 | >256 | >256 | >256 | >256 |
| VIP4 | >256 | >256 | >256 | >256 |
| VIP5 | >256 | >256 | >256 | >256 |
| VIP6 | >256 | >256 | >256 | >256 |
| VIP7 | 8 | 8 | 16 | 32 |
| VIP8 | 2 | 4 | 2 | 4 |
| Cecropin P1 | 2 | 4 | 8 | 8 |
| Microorganism | Cecropin P1 | Natural VIP | Recombinant VIP8 | |
|---|---|---|---|---|
| MIC (μM) | MIC (μM) | MIC (μM) | ||
| G+ | S. aureus ATCC 25923 | 8 | >256 | 2 |
| G− | E. coli ATCC 25922 | 2 | 64 | 2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Guo, Y.; Qiao, X.; Shang, X.; Niu, W.; Jin, M. Design, Recombinant Fusion Expression and Biological Evaluation of Vasoactive Intestinal Peptide Analogue as Novel Antimicrobial Agent. Molecules 2017, 22, 1963. https://doi.org/10.3390/molecules22111963
Xu C, Guo Y, Qiao X, Shang X, Niu W, Jin M. Design, Recombinant Fusion Expression and Biological Evaluation of Vasoactive Intestinal Peptide Analogue as Novel Antimicrobial Agent. Molecules. 2017; 22(11):1963. https://doi.org/10.3390/molecules22111963
Chicago/Turabian StyleXu, Chunlan, Yu Guo, Xiangjin Qiao, Xiaoya Shang, Weining Niu, and Mingliang Jin. 2017. "Design, Recombinant Fusion Expression and Biological Evaluation of Vasoactive Intestinal Peptide Analogue as Novel Antimicrobial Agent" Molecules 22, no. 11: 1963. https://doi.org/10.3390/molecules22111963




