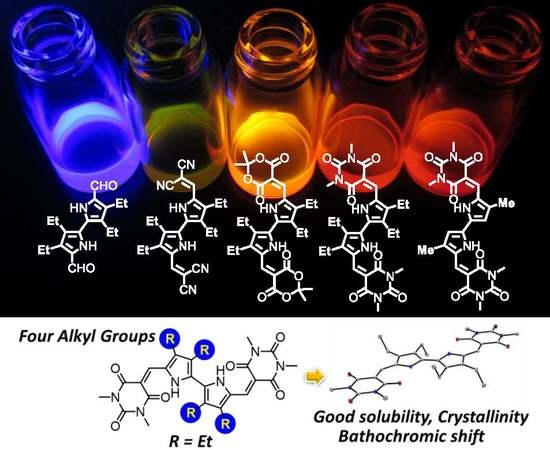
Synthesis, Crystal Structure, and Photoluminescent Properties of 3,3′,4,4′-Tetraethyl-5,5′-divinyl-2,2′-bipyrrole Derivatives
Abstract
:1. Introduction
2. Results and Discussion
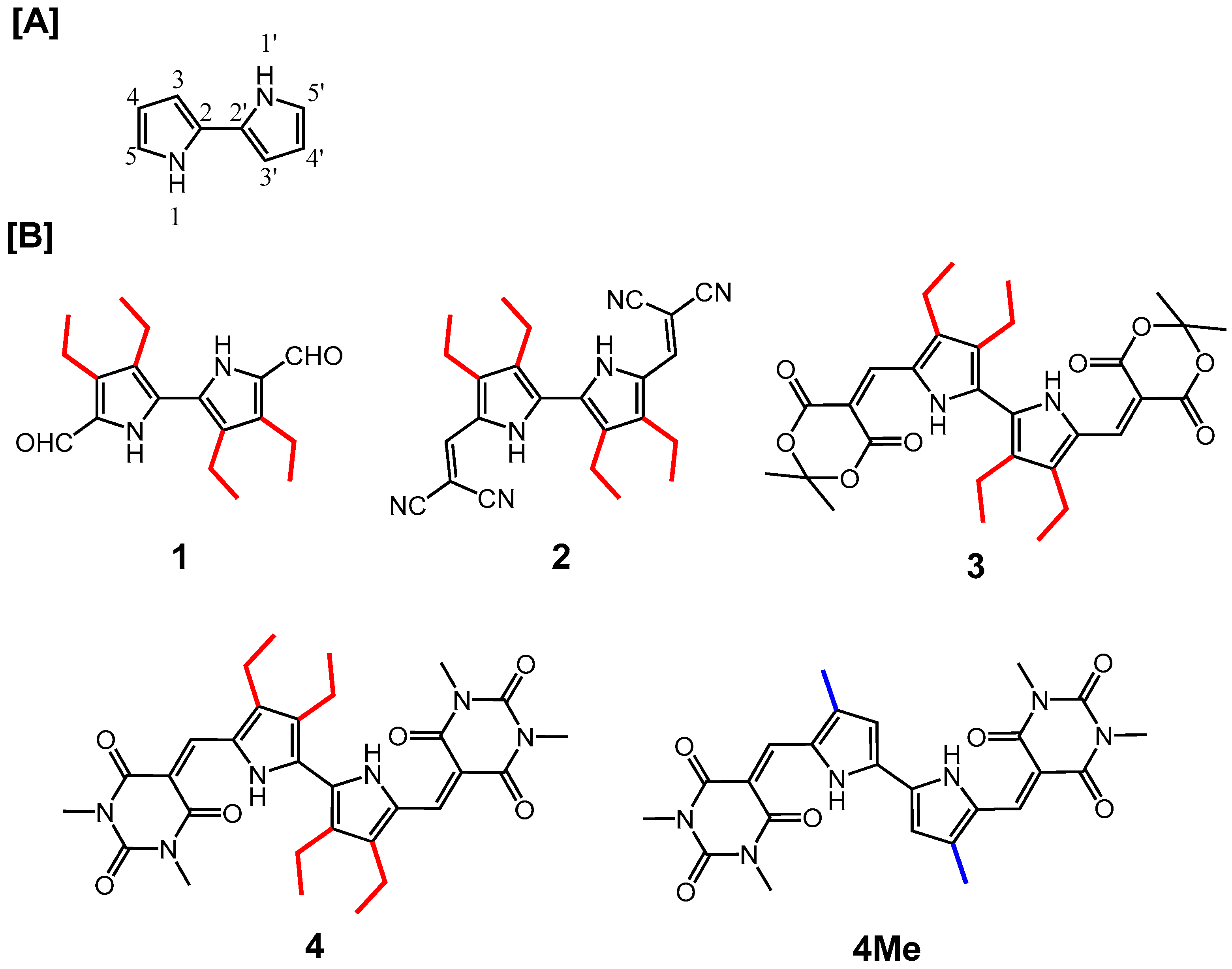
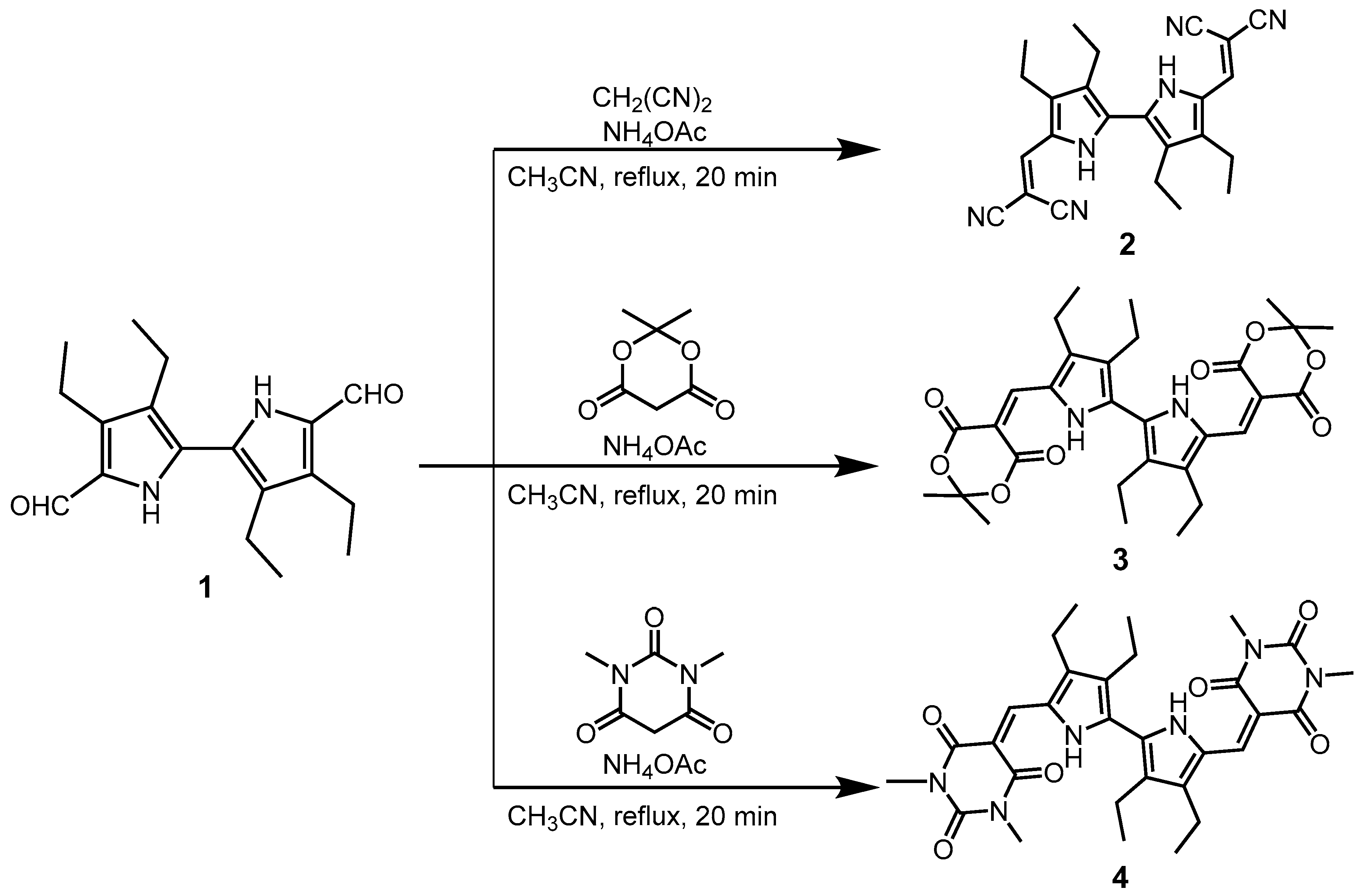
2.1. Synthesis
2.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
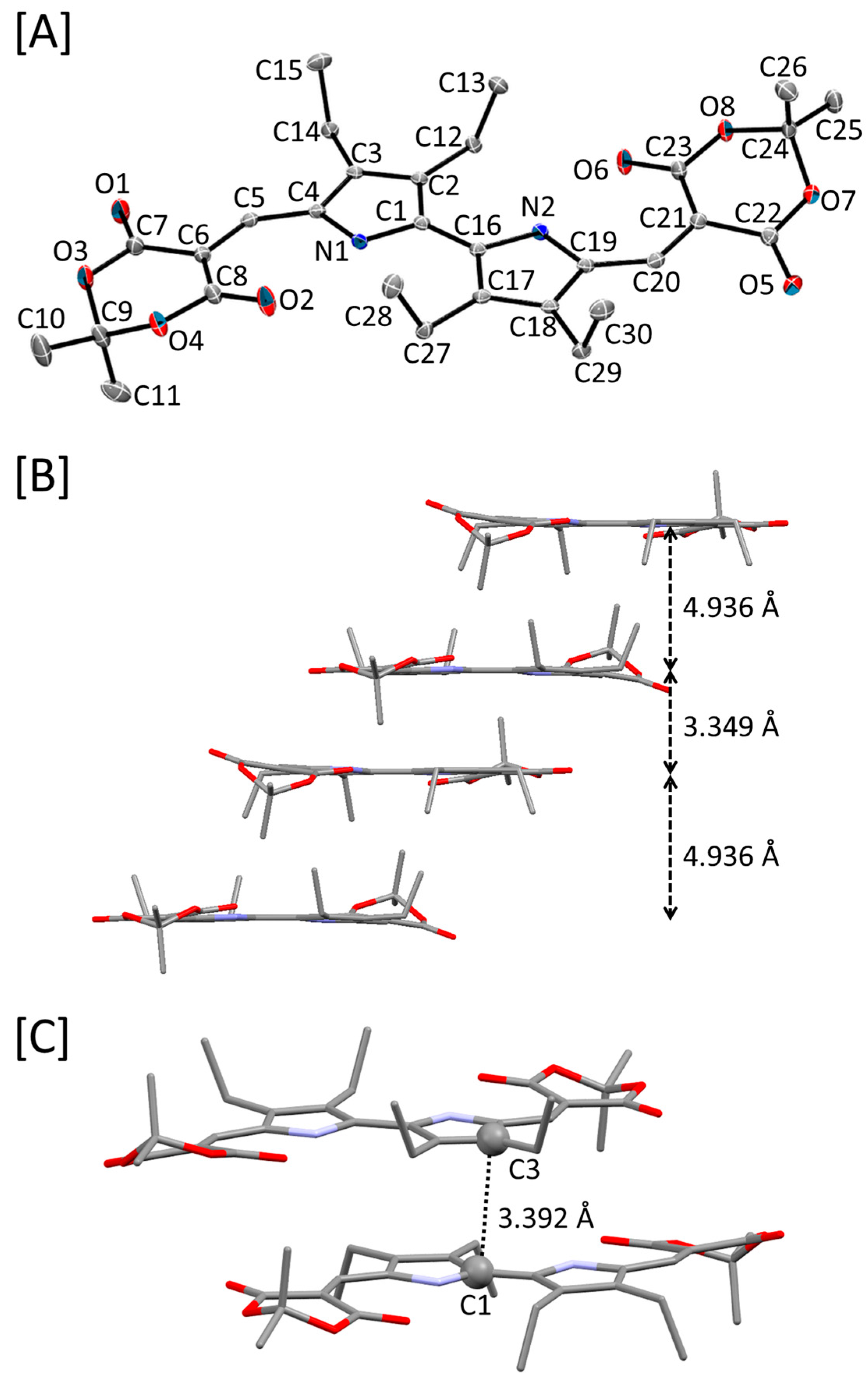
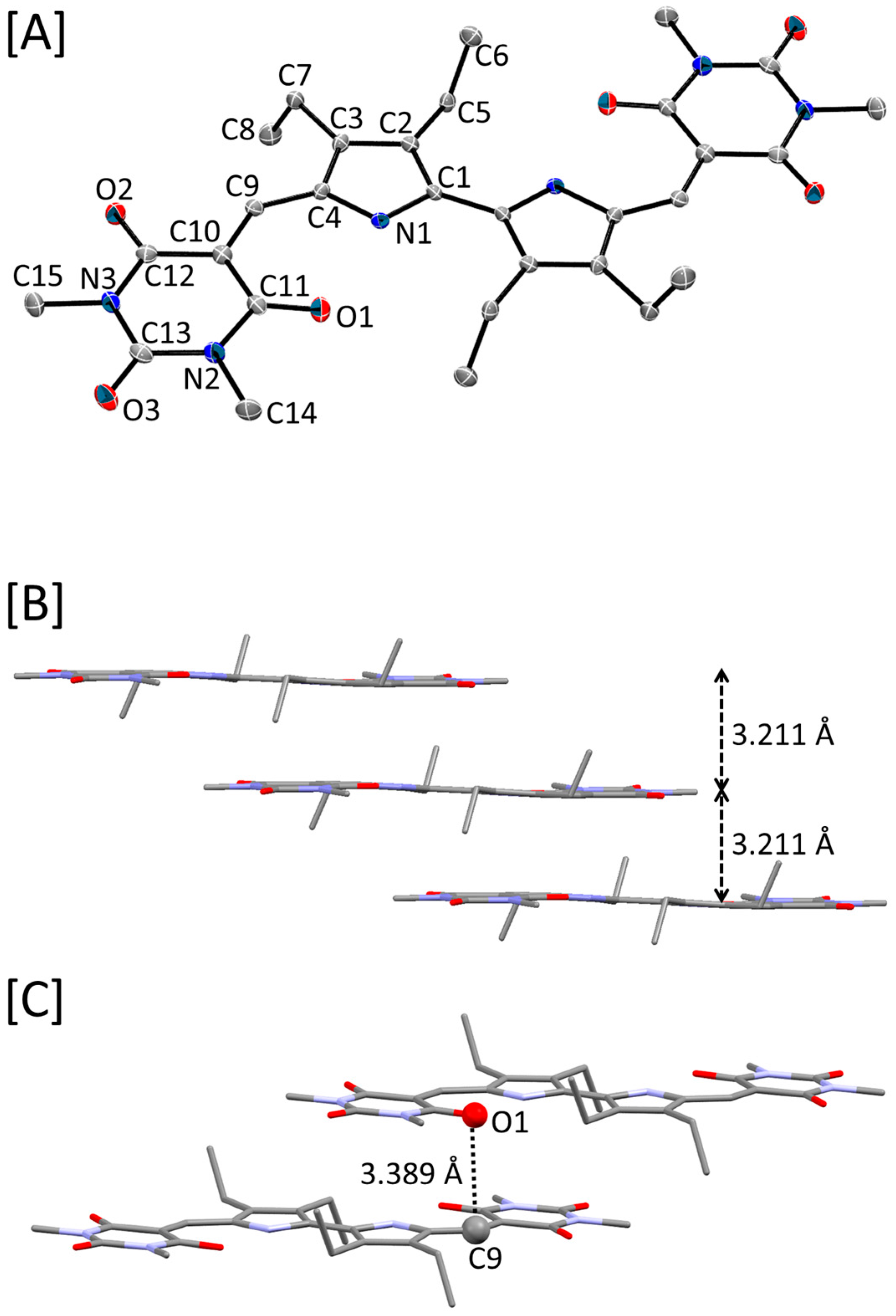
2.3. Crystal Structure
2.4. UV-Vis Absorption and Photoluminescence
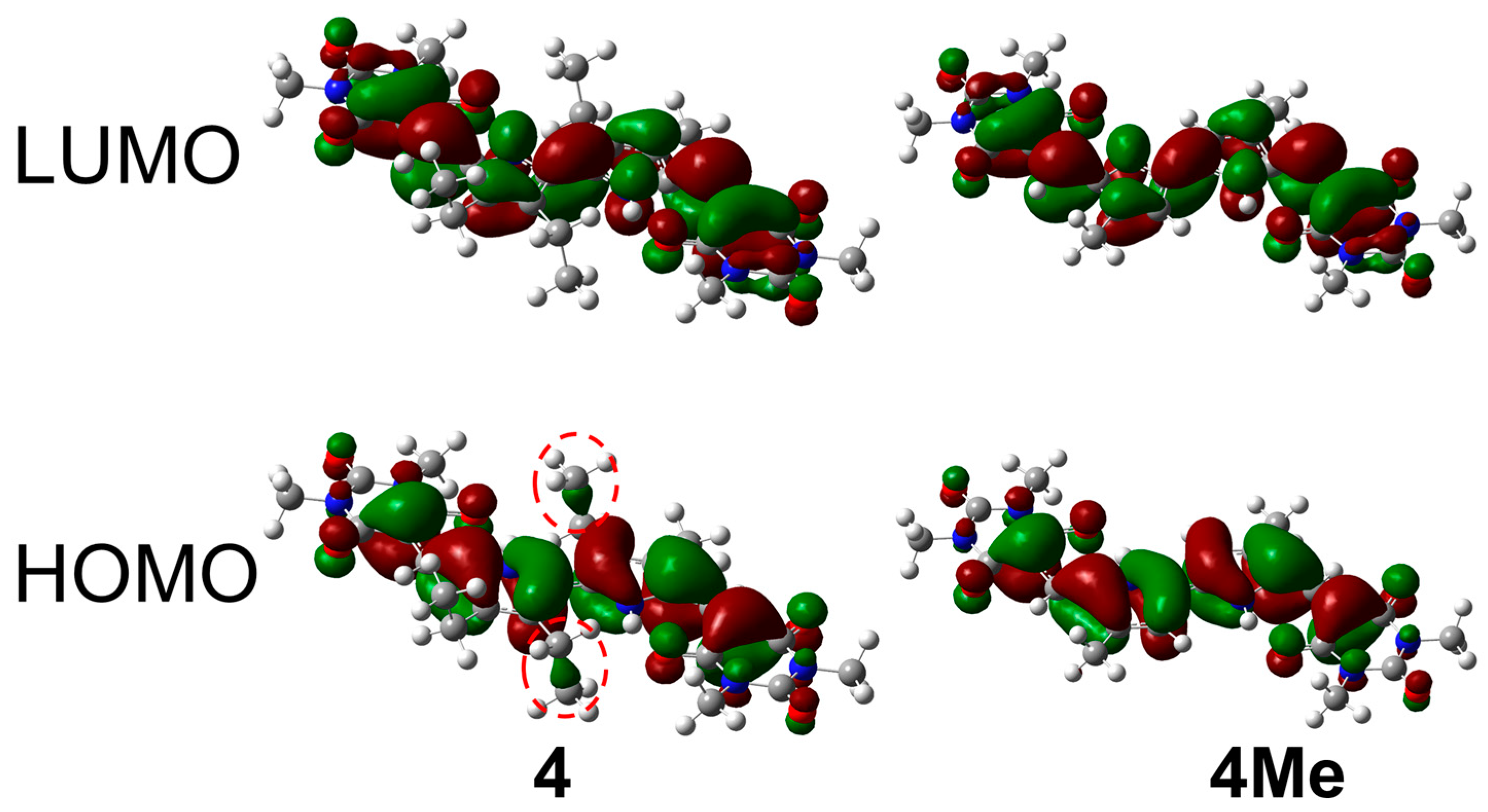
2.5. TD-DFT Calculations
3. Experimental
3.1. Materials and Instruments
3.2. X-ray Crystallography
3.3. Synthesis
3.4. Computational Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ma, D.; Tsuboi, T.; Qiu, Y.; Duan, L. Recent Progress in Ionic Iridium(III) Complexes for Organic Electronic Devices. Adv. Mater. 2017, 29, 1603253. [Google Scholar] [CrossRef] [PubMed]
- Niklaus, L.; Tansaz, S.; Dakhil, H.; Weber, K.T.; Pröschel, M.; Lang, M.; Kostrzewa, M.; Coto, P.B.; Detsch, R.; Sonnewald, U.; et al. Micropatterned Down-Converting Coating for White Bio-Hybrid Light-Emitting Diodes. Adv. Funct. Mater. 2017, 27, 1601792. [Google Scholar] [CrossRef]
- Lamansky, S.; Djurovich, P.; Murphy, D.; Abdel-Razzaq, F.; Lee, H.E.; Adachi, C.; Burrows, P.E.; Forrest, S.R.; Thompson, M.E. Highly Phosphorescent Bis-Cyclometalated Iridium Complexes: Synthesis, Photophysical Characterization, and Use in Organic Light Emitting Diodes. J. Am. Chem. Soc. 2001, 123, 4304–4312. [Google Scholar] [CrossRef] [PubMed]
- Reiss, P.; Carrière, M.; Lincheneau, C.; Vaure, L.; Tamang, S. Synthesis of Semiconductor Nanocrystals, Focusing on Nontoxic and Earth-Abundant Materials. Chem. Rev. 2016, 116, 10731–10819. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Yuan, K.; Chen, T.; Xu, P.; Li, H.; Chen, R.; Zheng, C.; Zhang, L.; Huang, W. Thermally Activated Delayed Fluorescence Materials Towards the Breakthrough of Organoelectronics. Adv. Mater. 2014, 26, 7931–7958. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Yuan, K.; Chen, T.; Xu, P.; Li, H.; Chen, R.; Zheng, C.; Zhang, L.; Huang, W. Hydrogen-Bond and Supramolecular-Contact Mediated Fluorescence Enhancement of Electrochromic Azomethines. Chem. Eur. J. 2016, 22, 11382–11393. [Google Scholar] [CrossRef]
- Samanta, S.; Goswami, S.; Hoque, M.N.; Ramesh, A.; Das, G. An aggregation-induced emission (AIE) active probe renders Al(III) sensing and tracking of subsequent interaction with DNA. Chem. Commun. 2014, 50, 11833–11836. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.A.; Tomas, R.P.; Quesada, R. Anion Transporters and Biological Systems. Acc. Chem. Res. 2013, 46, 2801–2813. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, H.; Holden, K.G. The Synthesis of Prodigiosin. J. Am. Chem. Soc. 1962, 84, 635–642. [Google Scholar] [CrossRef]
- Hong, T.; Song, H.; Li, X.; Zhanga, W.; Xie, Y. Syntheses of mono- and diacylated bipyrroles with rich substitution modes and development of a prodigiosin derivative as a fluorescent Zn(II) probe. RSC Adv. 2014, 4, 6133–6140. [Google Scholar] [CrossRef]
- Sessler, J.L.; Eller, L.R.; Cho, W.S.; Nicolaou, S.; Aguilar, A.; Lee, J.T.; Lynch, V.M.; Magda, D.J. Synthesis, Anion-Binding Properties, and In Vitro Anticancer Activity of Prodigiosin Analogues. Angew. Chem. Int. Ed. 2005, 44, 5989–5992. [Google Scholar] [CrossRef] [PubMed]
- Vogel, E.; Koch, P.; Hou, X.L.; Lex, J.; Lausmann, M.; Kisters, D.C.M.; Aukauloo, M.A.; Richard, P.; Guilard, R. New Porphycene Ligands: Octaethyl- and Etioporphycene (OEPc and EtioPc)—Tetra- and Pentacoordinated Zinc Complexes of OEPc. Angew. Chem. Int. Ed. Engl. 1993, 32, 1600–1604. [Google Scholar] [CrossRef]
- Vogel, E.; Balci, M.; Pramod, K.; Koch, P.; Lex, J.; Ermer, O. 2,7,12,17-Tetrapropylporphycene—Counterpart of Octaethylporphyrin in the Porphycene Series. Angew. Chem. Int. Ed. Engl. 1987, 26, 928–931. [Google Scholar] [CrossRef]
- Jiao, L.J.; Hao, E.H.; Fronczek, F.R.; Vicente, M.G.H.; Smith, K.M. Palladium(0) catalyzed 2,2′-bipyrrole syntheses. J. Porphyrins Phthalocyanines 2011, 15, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Czerski, I.; Listkowski, A.; Nawrocki, J.; Urbańska, N.; Piwoński, H.; Sokołowski, A.; Pietraszkiewicz, O.; Pietraszkiewicz, M.; Waluk, J. The long and winding road to new porphycenes. J. Porphyrins Phthalocyanines 2012, 16, 589–602. [Google Scholar] [CrossRef]
- Kumagai, T.; Hanke, F.; Gawinkowski, S.; Sharp, J.; Kotsis, K.; Waluk, J.; Persson, M.; Grill, L. Controlling intramolecular hydrogen transfer in a porphycene molecule with single atoms or molecules located nearby. Nat. Chem. 2014, 6, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Sessler, J.L.; An, D.; Cho, W.S.; Lynch, V. Calix[2]bipyrrole[2]furan and Calix[2]bipyrrole[2]thiophene: New Pyrrolic Receptors Exhibiting a Preference for Carboxylate Anions. J. Am. Chem. Soc. 2003, 125, 13646–13647. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Lee, J.; Williams, N.J.; Lynch, V.M.; Hay, B.P.; Moyer, B.A.; Sessler, J.L. Bipyrrole-Strapped Calix[4]pyrroles: Strong Anion Receptors That Extract the Sulfate Anion. J. Am. Chem. Soc. 2014, 136, 15079–15085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lv, G.; Wang, G.; Zhu, K.; Yu, D.; Shao, J.; Wang, Y.; Liu, Y. Facile preparation and fluorescence properties of a soluble oligopyrrole derivative. J. Photochem. Photobiol. A Chem. 2015, 309, 30–36. [Google Scholar] [CrossRef]
- Che, C.M.; Wan, C.W.; Lin, W.Z.; Yu, W.Y.; Zhou, Z.Y.; Lai, W.Y.; Lee, S.T. Highly luminous substituted bipyrroles. Chem. Commun. 2001, 721–722. [Google Scholar] [CrossRef]
- Okawara, T.; Doi, A.; Ono, T.; Abe, M.; Takehara, K.; Hisaeda, Y.; Matsushima, S. Synthesis and X-ray crystallography of bipyrroles: Impacts of a CO–π interaction on their structure. Tetrahedron Lett. 2015, 56, 1407–1410. [Google Scholar] [CrossRef]
- Kawano, R.; Kato, T.; Fukuda, R.; Okawara, T.; Takehara, K.; Nagamura, T. Synthesis, Structure, and Spectroscopy of Green to Yellow Fluorescent Divinylbipyrroles. ChemistrySelect 2016, 1, 4144–4151. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, X.; Chen, Y. One-pot synthesis of a near-infrared fluorophore for living cells imaging. Dyes Pigm. 2017, 140, 56–61. [Google Scholar] [CrossRef]
- Wu, A.; Kolanowski, J.L.; Boumelhem, B.B.; Yang, K.; Lee, R.; Kaur, A.; Fraser, S.T.; New, E.J.; Rendina, L.M. A Carborane-Containing Fluorophore as a Stain of Cellular Lipid Droplets. Chem. Asian J. 2017, 12, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Paine, J.B., III; Dolphin, D. 5-Unsubstituted 2-pyrrolecarboxaldehydes for porphyrin synthesis and the cyanovinyl protecting group. J. Org. Chem. 1988, 53, 2787–2795. [Google Scholar] [CrossRef]
- Bauer, V.J.; Clive, D.L.J.; Dolphin, D.; Paine, J.B., III; Harris, F.L.; King, M.M.; Loder, J.; Wang, S.W.C.; Woodward, R.B. Sapphyrins: Novel aromatic pentapyrrolic macrocycles. J. Am. Chem. Soc. 1983, 105, 6429–6436. [Google Scholar] [CrossRef]
- Jiao, L.; Yu, C.; Li, J.; Wang, Z.; Wu, M.; Hao, E. β-Formyl-BODIPYs from the Vilsmeier-Haack Reaction. J. Org. Chem. 2009, 74, 7525–7528. [Google Scholar] [CrossRef] [PubMed]
- Polander, L.E.; Barlow, S.; Seifried, B.M.; Marder, S.R. A 2,6-Diformylnaphthalene-1,8:4,5-bis(dicarboximide): Synthesis and Knoevenagel Condensation with Malononitrile. J. Org. Chem. 2012, 77, 9426–9428. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Ma, Y.; Xiong, Y.; Cao, X.; Li, Y.; Chen, L. Enhanced AIE and different stimuli-responses in red fluorescent (1,3-dimethyl)barbituric acid-functionalized anthracenes. J. Mater. Chem. C 2016, 4, 751–757. [Google Scholar] [CrossRef]
- Xu, D.; Li, Z.; Peng, Y.X.; Geng, J.; Qian, H.F.; Huang, W. Post-modification of 2-formylthiophene based heterocyclic azo dyes. Dyes Pigm. 2016, 133, 143–152. [Google Scholar] [CrossRef]
- Gupta, A.; Kelson, M.M.A.; Armel, V.; Bilic, A.; Bhosale, S.V. N-Alkyl- and N-aryl-dithieno[3,2-b:2′,3′-d]pyrrole-containing organic dyes for efficient dye-sensitized solar cells. Tetrahedron 2014, 70, 2141–2150. [Google Scholar] [CrossRef]
- Falk, H.; Flodl, H. Beiträge zur Chemie der Pyrrolpigmente, 76. Mitt.: Die Synthese von symmetrisch substituierten 2,2′-Bipyrrolen durch oxidative Kupplung. Monatsh. Chem. 1988, 119, 247–252. [Google Scholar] [CrossRef]
- Sullivan, P.; Collis, G.E.; Rochford, L.A.; Arantes, J.F.; Kemppinen, P.; Jones, T.S.; Winzenberg, K.N. An N-ethylated barbituric acid end-capped bithiophene as an electron-acceptor material in fullerene-free organic photovoltaics. Chem. Commun. 2015, 51, 6222–6225. [Google Scholar] [CrossRef] [PubMed]
- Merz, A.; Kronberger, J.; Dunsch, L.; Neudeck, A.; Petr, A.; Parkanyi, L. Radical Dimerization of 5,5′-Diphenyl-3,3′,4,4′-tetramethoxy-2,2′-bipyrrole: π Dimer in the Crystal, σ Dimer in Solution. Angew. Chem. Int. Ed. 1999, 38, 1442–1446. [Google Scholar] [CrossRef]
- Sanchez-García, D.; Borrell, J.I.; Nonell, S. One-Pot Synthesis of Substituted 2,2′-Bipyrroles. A Straightforward Route to Aryl Porphycenes. Org. Lett. 2009, 11, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.-L.; Jian, F.-F. 5-(2-Fluorobenzylidene)-2,2-dimethyl-1,3-dioxane-4,6-dione. Acta Cryst. 2009, E65, o2587. [Google Scholar] [CrossRef] [PubMed]
- García-Álvarez, F.; Romero, N.; Lobato-García, C.E.; Terána, J.L.; Mendoza, A. 2,2-Dimethyl-5-(2-nitrobenzylidene)-1,3-dioxane-4,6-dione. Acta Cryst. 2013, E69, o50. [Google Scholar] [CrossRef]
- Sabino, J.R.; Damasceno, F.; Cunha, S. 2,2-Dimethyl-5-(pyrrolidin-2-ylidene)-1,3-dioxane-4,6-dione. Acta Cryst. 2007, E63, o1913. [Google Scholar] [CrossRef]
- Egli, M.; Sarkhel, S. Lone Pair-Aromatic Interactions: To Stabilize or Not to Stabilize. Acc. Chem. Res. 2007, 40, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Caracelli, I.; Zukerman-Schpector, J.; Haiducc, I.; Tiekink, E.R.T. Main group metal lone-pair⋯π(arene) interactions: A new bonding mode for supramolecular associations. CrystEngComm. 2016, 18, 6960–6978. [Google Scholar] [CrossRef]
- Anguera, G.; Kauffmann, B.; Borrell, J.I.; Borros, S.; Sanchez-García, D. Stable 5,5′-Substituted 2,2′-Bipyrroles: Building Blocks for Macrocyclic and Materials Chemistry. J. Org. Chem. 2017, 82, 6904–6912. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Yanai, T.; Tew, D.; Handy, N. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- JTomasi, J.; Mennucci, B.; Cancès, E. The IEF version of the PCM solvation method: An overview of a new method addressed to study molecular solutes at the QM ab initio level. J. Mol. Struct. Theochem 1999, 464, 211–226. [Google Scholar] [CrossRef]
- SAINT, V8.34A, Bruker AXS; Bruker AXS Inc.: Madison, WI, USA, 2013.
- SADABS, 2014/5, Bruker AXS; Bruker AXS Inc.: Madison, WI, USA, 2014.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
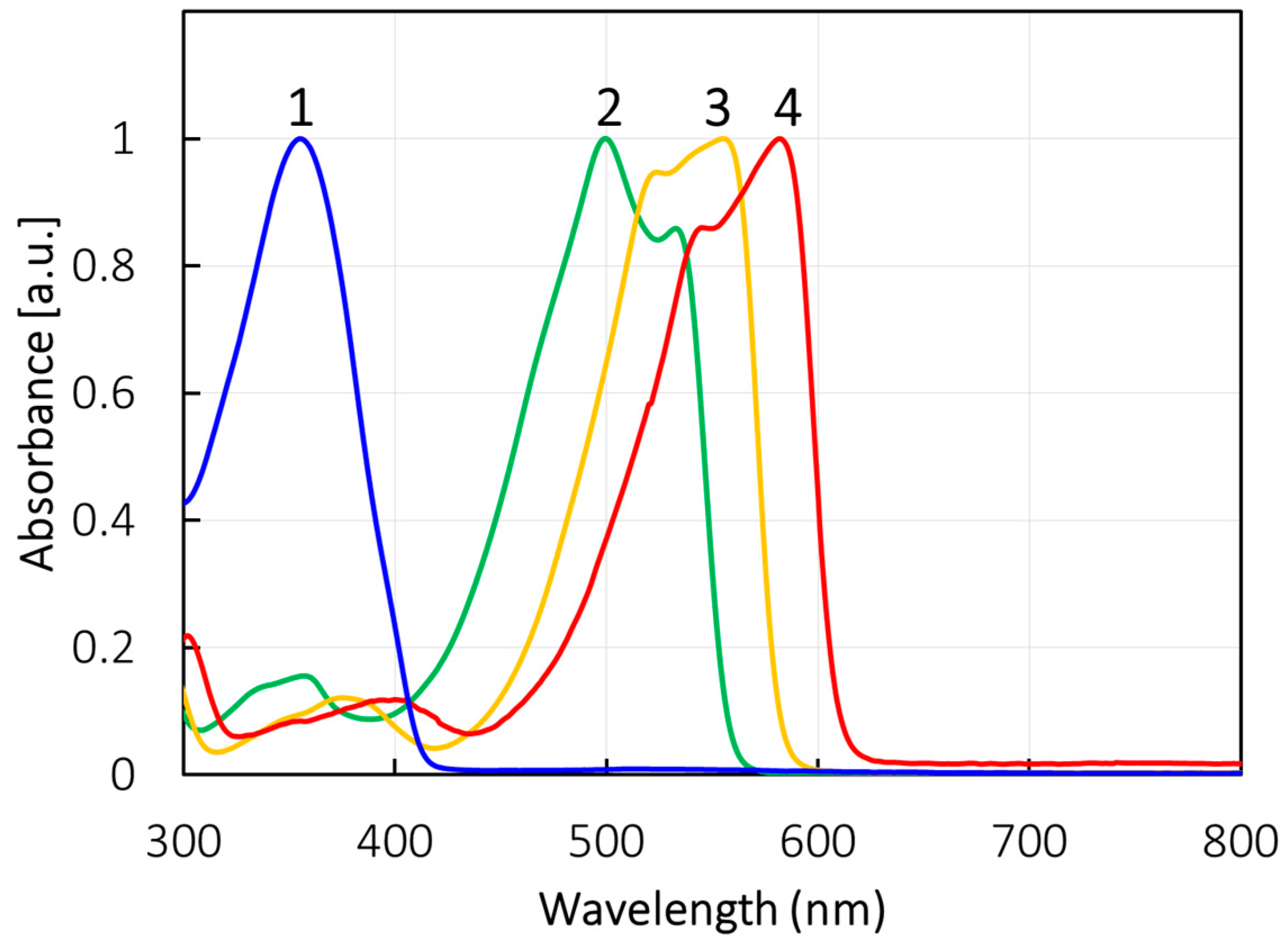
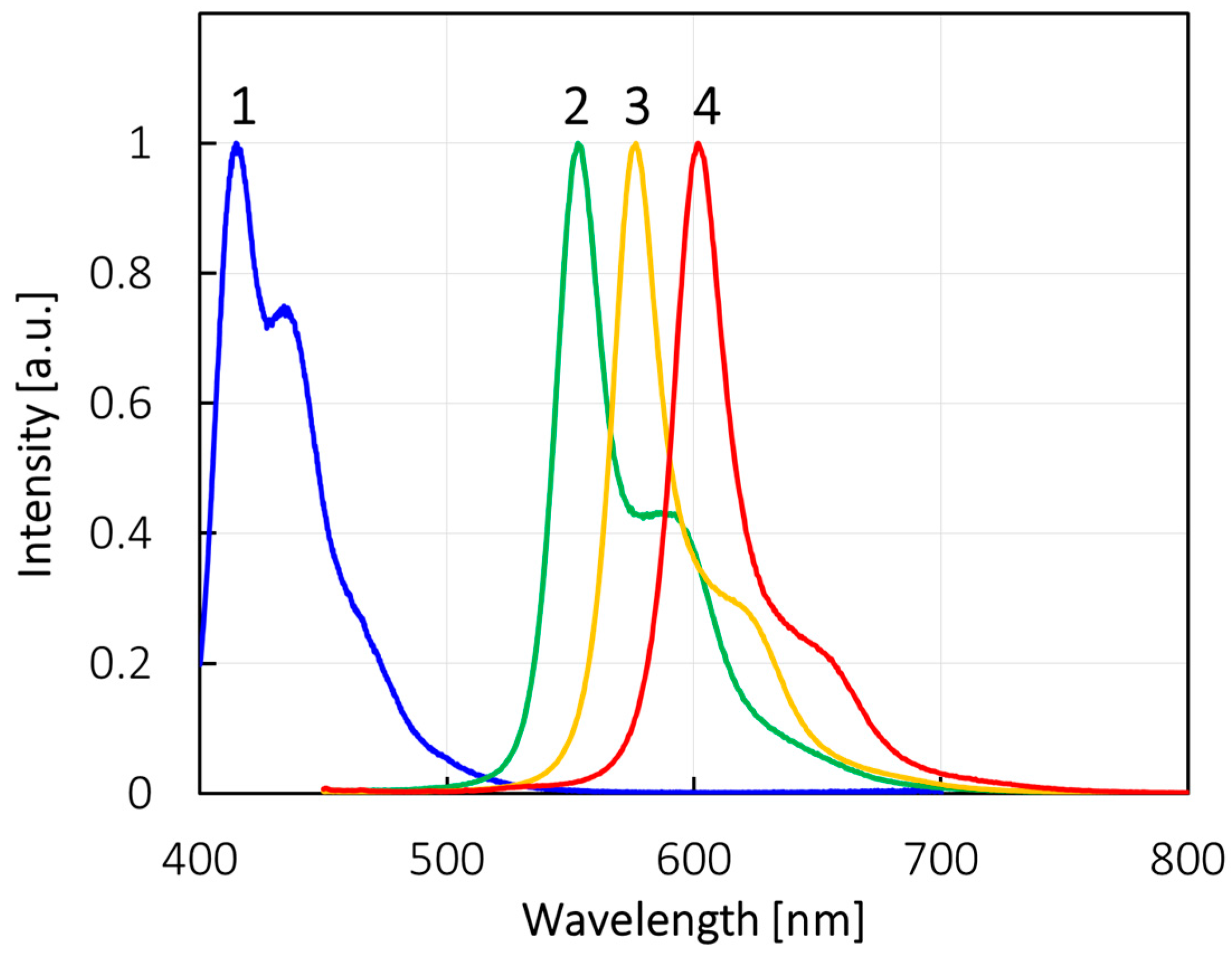
| Compounds | λAbs (nm) | λFL (nm) | ΦFL (−) |
|---|---|---|---|
| 1 | 355 | 414 | NA a |
| 2 | 499, 531 | 553 | 0.05 |
| 3 | 555 | 576 | 0.81 |
| 4 | 582 | 602 | 0.93 |
| 4Me [22] | 569 | 578 | 0.88 |
| Compounds | λAbs (Obs.) (nm) | λAbs (Calcd.) (nm) | Transitions | Oscillator Strength |
|---|---|---|---|---|
| 1 | 355 | 403.31 | 81(HOMO)‒82(LUMO) | 1.0825 |
| 2 | 499, 531 | 556.11 | 104(HOMO−1)‒107(LUMO+1) 105(HOMO)‒106(LUMO) | 1.5891 |
| 3 | 555 | 553.40 | 146(HOMO−1)‒149(LUMO+1) 147(HOMO)‒148(LUMO) | 1.7047 |
| 4 | 582 | 574.31 | 152(HOMO−1)‒155 (LUMO+1) 153(HOMO)‒154(LUMO) | 1.8560 |
| 4Me | 569 | 560.00 | 128(HOMO−1)‒131(LUMO+1) 129(HOMO)‒130(LUMO) | 1.9396 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okawara, T.; Kawano, R.; Morita, H.; Finkelstein, A.; Toyofuku, R.; Matsumoto, K.; Takehara, K.; Nagamura, T.; Iwasa, S.; Kumar, S. Synthesis, Crystal Structure, and Photoluminescent Properties of 3,3′,4,4′-Tetraethyl-5,5′-divinyl-2,2′-bipyrrole Derivatives. Molecules 2017, 22, 1816. https://doi.org/10.3390/molecules22111816
Okawara T, Kawano R, Morita H, Finkelstein A, Toyofuku R, Matsumoto K, Takehara K, Nagamura T, Iwasa S, Kumar S. Synthesis, Crystal Structure, and Photoluminescent Properties of 3,3′,4,4′-Tetraethyl-5,5′-divinyl-2,2′-bipyrrole Derivatives. Molecules. 2017; 22(11):1816. https://doi.org/10.3390/molecules22111816
Chicago/Turabian StyleOkawara, Toru, Reo Kawano, Hiroya Morita, Alan Finkelstein, Renjiro Toyofuku, Kanako Matsumoto, Kenji Takehara, Toshihiko Nagamura, Seiji Iwasa, and Sanjai Kumar. 2017. "Synthesis, Crystal Structure, and Photoluminescent Properties of 3,3′,4,4′-Tetraethyl-5,5′-divinyl-2,2′-bipyrrole Derivatives" Molecules 22, no. 11: 1816. https://doi.org/10.3390/molecules22111816
APA StyleOkawara, T., Kawano, R., Morita, H., Finkelstein, A., Toyofuku, R., Matsumoto, K., Takehara, K., Nagamura, T., Iwasa, S., & Kumar, S. (2017). Synthesis, Crystal Structure, and Photoluminescent Properties of 3,3′,4,4′-Tetraethyl-5,5′-divinyl-2,2′-bipyrrole Derivatives. Molecules, 22(11), 1816. https://doi.org/10.3390/molecules22111816