Protective Effect of Prunus Cerasus (Sour Cherry) Seed Extract on the Recovery of Ischemia/Reperfusion-Induced Retinal Damage in Zucker Diabetic Fatty Rat
Abstract
:1. Introduction
2. Results
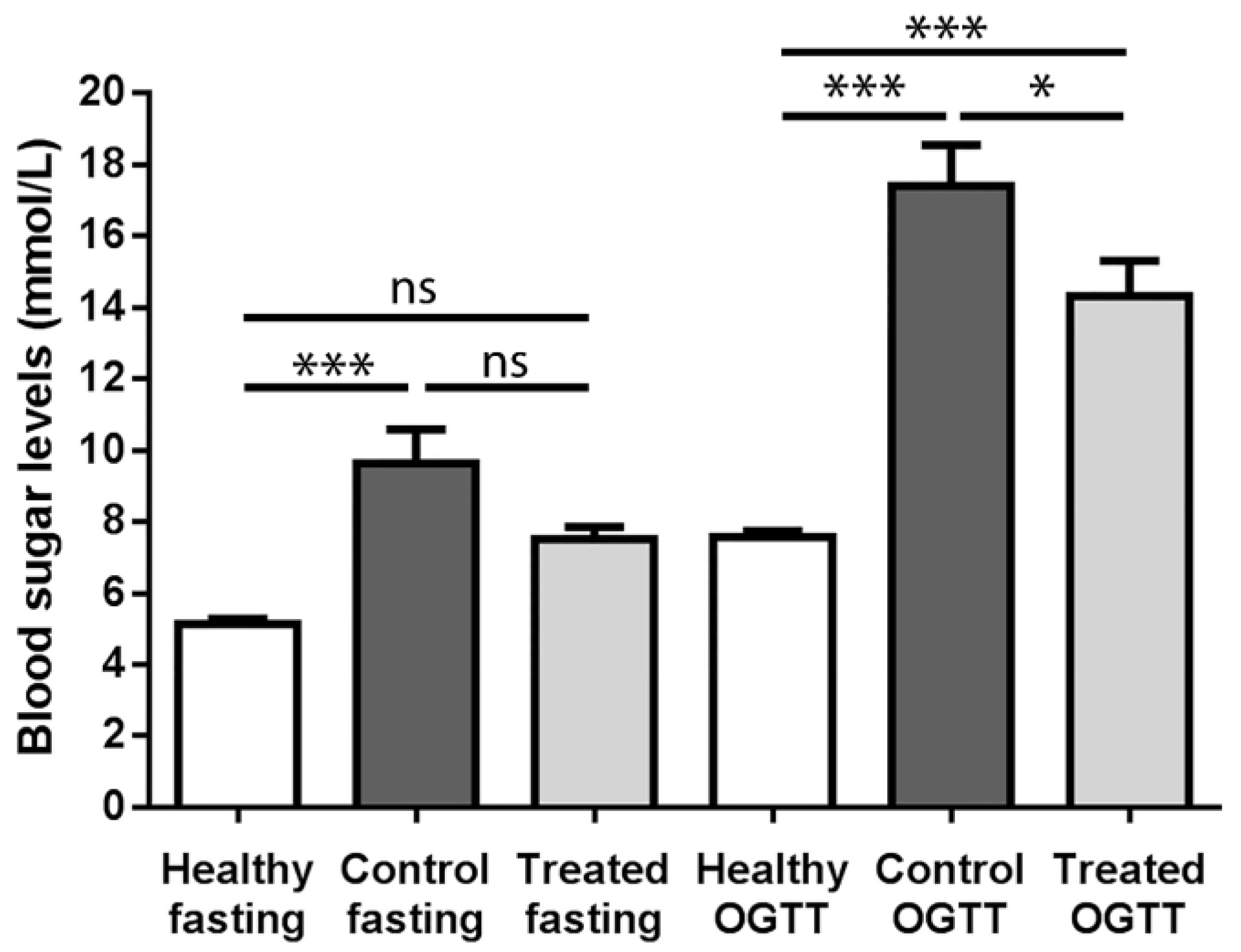
2.1. Fasting Blood Sugar Analysis and Oral Glucose Tolerance Test (OGTT) Results
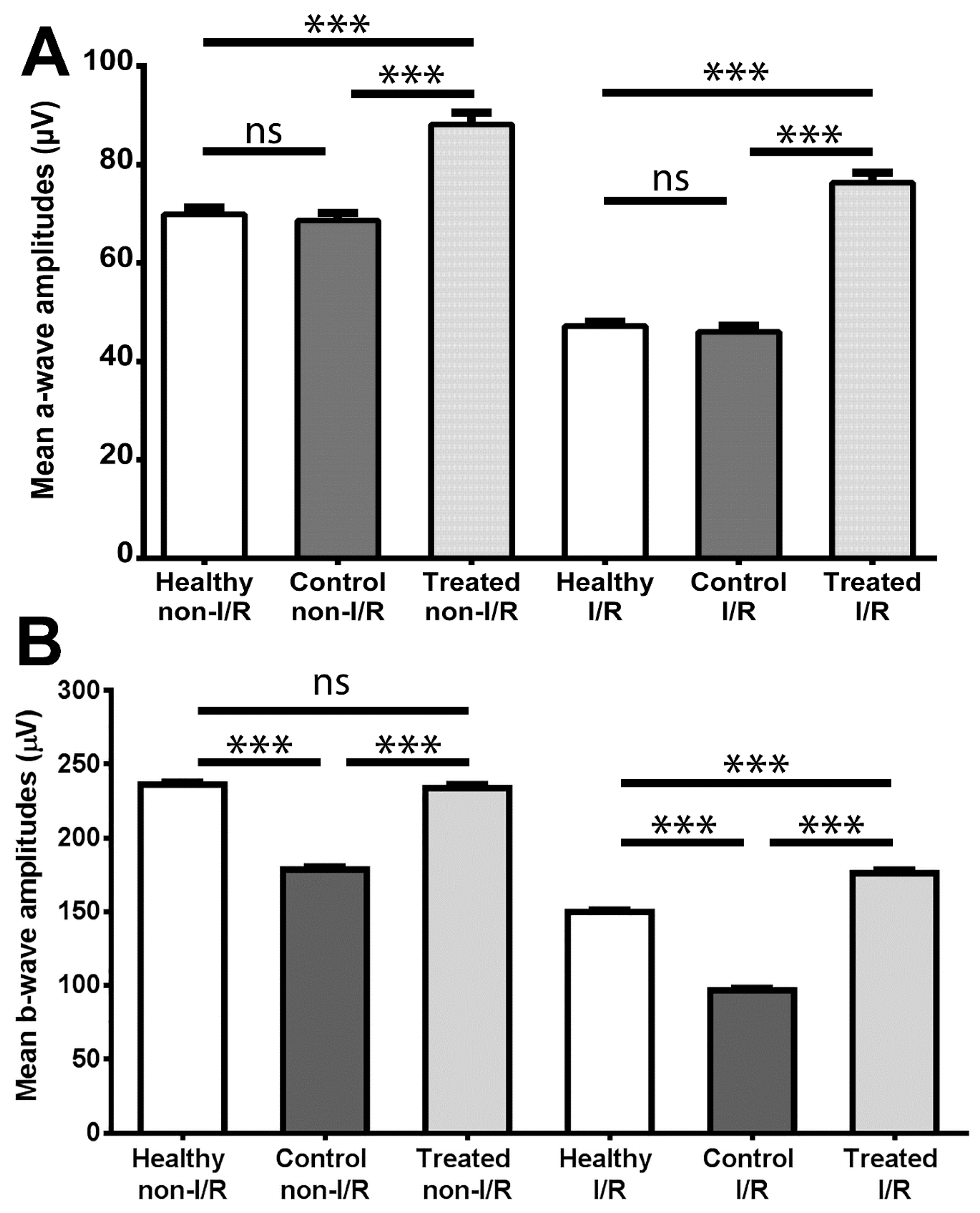
2.2. Electroretinography
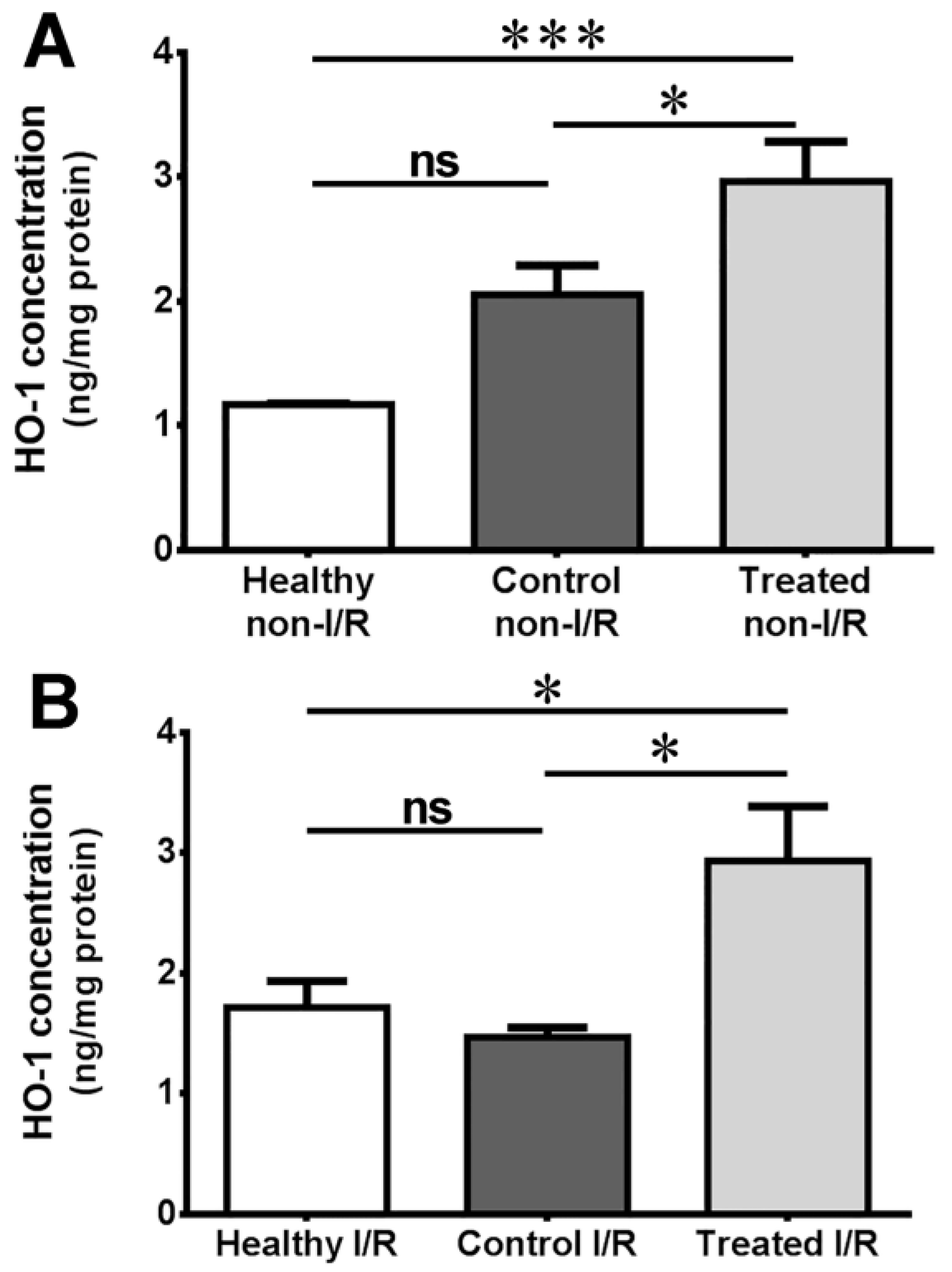
2.3. Measurement of Heme Oxygenase Concentration
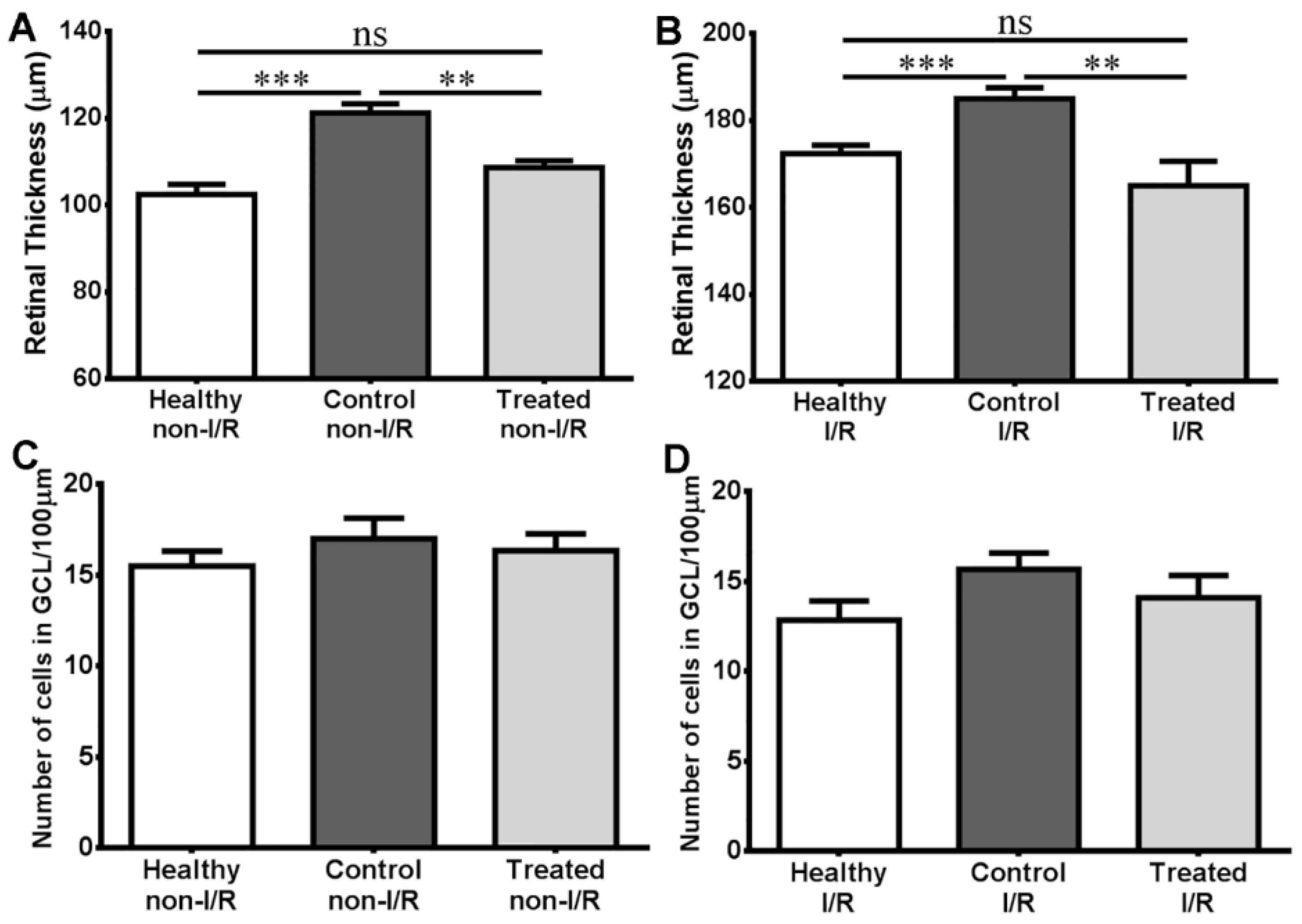
2.4. Histology Results
3. Discussion
4. Materials and Methods
4.1. Animals and Groups
4.2. Ocular Ischemia and Reperfusion (I/R)
4.3. Electroretinography (ERG)
4.4. Oral Glucose Tolerancy Test (OGTT)
4.5. Measurement of Heme Oxygenase Concentration
4.6. Processing for Examination by Light-Microscopy
4.7. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ZDF rats | Zucker Diabetic Fatty rats |
| SCSE | Sour cherry seed extract |
| ERG | Electroretinography |
| HO-1 | heme oxygenase-1 |
| I/R | Ischemia/reperfusion |
References
- Curtis, T.M.; Gardiner, T.A.; Stitt, A.W. Microvascular lesions of diabetic retinopathy: Clues towards understanding pathogenesis? Eye 2009, 23, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.; Paques, M.; Mones, J.; Glacet-Bernard, A. Retinal vein occlusions. Dev. Ophthalmol. 2010, 47, 111–135. [Google Scholar] [PubMed]
- Agarwal, R.; Gupta, S.K.; Agarwal, P.; Saxena, R.; Agrawal, S.S. Current concepts in the pathophysiology of glaucoma. Indian J. Ophthalmol. 2009, 57, 257–266. [Google Scholar] [PubMed]
- Juhasz, B.; Kertesz, A.; Balla, J.; Balla, G.; Szabo, Z.; Bombicz, M.; Priksz, D.; Gesztelyi, R.; Varga, B.; Haines, D.D.; et al. Cardioprotective effects of sour cherry seed extract (scse) on the hypercholesterolemic rabbit heart. Curr. Pharm. Des. 2013, 19, 6896–6905. [Google Scholar] [CrossRef] [PubMed]
- Bombicz, M.; Priksz, D.; Varga, B.; Gesztelyi, R.; Kertesz, A.; Lengyel, P.; Balogh, P.; Csupor, D.; Hohmann, J.; Bhattoa, H.P.; et al. Anti-atherogenic properties of allium ursinum liophylisate: Impact on lipoprotein homeostasis and cardiac biomarkers in hypercholesterolemic rabbits. Inter. J. Mol. Sci. 2016, 17, 1284. [Google Scholar] [CrossRef] [PubMed]
- Bombicz, M.; Priksz, D.; Varga, B.; Kurucz, A.; Kertesz, A.; Takacs, A.; Posa, A.; Kiss, R.; Szilvassy, Z.; Juhasz, B. A novel therapeutic approach in the treatment of pulmonary arterial hypertension: Allium ursinum liophylisate alleviates symptoms comparably to sildenafil. Inter. J. Mol. Sci. 2017, 18, 1436. [Google Scholar] [CrossRef] [PubMed]
- Feher, P.; Ujhelyi, Z.; Varadi, J.; Fenyvesi, F.; Roka, E.; Juhasz, B.; Varga, B.; Bombicz, M.; Priksz, D.; Bacskay, I.; et al. Efficacy of pre- and post-treatment by topical formulations containing dissolved and suspended silybum marianum against uvb-induced oxidative stress in guinea pig and on hacat keratinocytes. Molecules 2016, 21, 1269. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Huang, L.; Yu, J. Effects of blueberry anthocyanins on retinal oxidative stress and inflammation in diabetes through nrf2/ho-1 signaling. J. Neuroimmunol. 2016, 301, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bak, I.; Czompa, A.; Csepanyi, E.; Juhasz, B.; Kalantari, H.; Najm, K.; Aghel, N.; Varga, B.; Haines, D.D.; Tosaki, A. Evaluation of systemic and dermal toxicity and dermal photoprotection by sour cherry kernels. Phytother. Res. 2011, 25, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Bak, I.; Lekli, I.; Juhasz, B.; Varga, E.; Varga, B.; Gesztelyi, R.; Szendrei, L.; Tosaki, A. Isolation and analysis of bioactive constituents of sour cherry (prunus cerasus) seed kernel: An emerging functional food. J. Med. Food 2010, 13, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Csonka, C.; Varga, E.; Kovacs, P.; Ferdinandy, P.; Blasig, I.E.; Szilvassy, Z.; Tosaki, A. Heme oxygenase and cardiac function in ischemic/reperfused rat hearts. Free Radic. Biol. Med. 1999, 27, 119–126. [Google Scholar] [CrossRef]
- Juhasz, B.; Varga, B.; Czompa, A.; Bak, I.; Lekli, I.; Gesztelyi, R.; Zsuga, J.; Kemeny-Beke, A.; Antal, M.; Szendrei, L.; et al. Postischemic cardiac recovery in heme oxygenase-1 transgenic ischemic/reperfused mouse myocardium. J. Cell Mol. Med. 2011, 15, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.D.; Lekli, I.; Teissier, P.; Bak, I.; Tosaki, A. Role of haeme oxygenase-1 in resolution of oxidative stress-related pathologies: Focus on cardiovascular, lung, neurological and kidney disorders. Acta Physiol. 2012, 204, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Varga, B.; Gesztelyi, R.; Bombicz, M.; Haines, D.; Szabo, A.M.; Kemeny-Beke, A.; Antal, M.; Vecsernyes, M.; Juhasz, B.; Tosaki, A. Protective effect of alpha-melanocyte-stimulating hormone (alpha-msh) on the recovery of ischemia/reperfusion (i/r)-induced retinal damage in a rat model. J. Mol. Neurosci. 2013, 50, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, F.F.; Al-Awadhi, R.; Haines, D.D.; Dashti, A.; Dashti, H.; Al-Ozairi, E.; Bak, I.; Tosaki, A. Sour cherry seed kernel extract increases heme oxygenase-1 expression and decreases representation of cd3 + tnf-alpha+ and cd3 + il-8+ subpopulations in peripheral blood leukocyte cultures from type 2 diabetes patients. Phytother. Res. 2013, 27, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, F.; Haines, D.; Al-Awadhi, R.; Dashti, A.A.; Al-Awadhi, A.; Ibrahim, B.; Al-Zayer, B.; Juhasz, B.; Tosaki, A. Sour cherry (prunus cerasus) seed extract increases heme oxygenase-1 expression and decreases proinflammatory signaling in peripheral blood human leukocytes from rheumatoid arthritis patients. Int. Immunopharmacol. 2014, 20, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Bak, I.; Lekli, I.; Juhasz, B.; Nagy, N.; Varga, E.; Varadi, J.; Gesztelyi, R.; Szabo, G.; Szendrei, L.; Bacskay, I.; et al. Cardioprotective mechanisms of prunus cerasus (sour cherry) seed extract against ischemia-reperfusion-induced damage in isolated rat hearts. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1329–H1336. [Google Scholar] [CrossRef] [PubMed]
- Czompa, A.; Gyongyosi, A.; Czegledi, A.; Csepanyi, E.; Bak, I.; Haines, D.D.; Tosaki, A.; Lekli, I. Cardioprotection afforded by sour cherry seed kernel: The role of heme oxygenase-1. J. Cardiovasc. Pharmacol. 2014, 64, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Szabo, M.E.; Gallyas, E.; Bak, I.; Rakotovao, A.; Boucher, F.; de Leiris, J.; Nagy, N.; Varga, E.; Tosaki, A. Heme oxygenase-1-related carbon monoxide and flavonoids in ischemic/reperfused rat retina. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3727–3732. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Motaharitabar, E.; Goudarzi, M.; Rezaie, A.; Kalantari, H. Toxicity evaluation of microemulsion (nano size) of sour cherry kernel extract for the oral bioavailability enhancement. Jundishapur J. Nat. Pharm. Prod. 2014, 9, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Csiki, Z.; Papp-Bata, A.; Czompa, A.; Nagy, A.; Bak, I.; Lekli, I.; Javor, A.; Haines, D.D.; Balla, G.; Tosaki, A. Orally delivered sour cherry seed extract (scse) affects cardiovascular and hematological parameters in humans. Phytother. Res. 2015, 29, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, F.F.; Al-Awadhi, A.M.; Haines, D.D. Amelioration of human osteoarthritis symptoms with topical ‘biotherapeutics’: A phase i human trial. Cell Stress Chaperones 2015, 20, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Hungarian Ministry of Health. Professional protocol of ministry of health: Therapy for ocular complications in diabetes mellitus. Hung. Bull. Health 2009, 21, 3577–3585. [Google Scholar]
- Literati-Nagy, B.; Tory, K.; Peitl, B.; Bajza, A.; Koranyi, L.; Literati-Nagy, Z.; Hooper, P.L.; Vigh, L.; Szilvassy, Z. Improvement of insulin sensitivity by a novel drug candidate, bgp-15, in different animal studies. Metab. Syndr. Relat. Disord. 2014, 12, 125–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry antioxidants: From farm to table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef] [PubMed]
- Lachin, T. Effect of antioxidant extract from cherries on diabetes. Recent Pat. Endocr. Metab. Immune Drug Discov. 2014, 8, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Yang, N.; Wang, D.; Li, S.; Ming, J.; Wang, J.; Yu, X.; Song, Y.; Zhou, X.; Yang, Y. Resveratrol prevents retinal dysfunction by regulating glutamate transporters, glutamine synthetase expression and activity in diabetic retina. Neurochem. Res. 2016, 41, 1050–1064. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, K.; Wang, Q.; Ruan, Y.; Zhang, Y.; Ye, W. Exendin-4 protects retinal cells from early diabetes in goto-kakizaki rats by increasing the bcl-2/bax and bcl-xl/bax ratios and reducing reactive gliosis. Mol. Vis. 2014, 20, 1557–1568. [Google Scholar] [PubMed]
- Hernandez, C.; Bogdanov, P.; Corraliza, L.; Garcia-Ramirez, M.; Sola-Adell, C.; Arranz, J.A.; Arroba, A.I.; Valverde, A.M.; Simo, R. Topical administration of glp-1 receptor agonists prevents retinal neurodegeneration in experimental diabetes. Diabetes 2016, 65, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.N.; Casson, R.J.; Wood, J.P.; Chidlow, G.; Graham, M.; Melena, J. Retinal ischemia: Mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res. 2004, 23, 91–147. [Google Scholar] [CrossRef] [PubMed]
- Varga, B.; Szabadfi, K.; Kiss, P.; Fabian, E.; Tamas, A.; Griecs, M.; Gabriel, R.; Reglodi, D.; Kemeny-Beke, A.; Pamer, Z.; et al. Pacap improves functional outcome in excitotoxic retinal lesion: An electroretinographic study. J. Mol. Neurosci. 2011, 43, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.; Lin, C.M.; Muthusamy, A.; Fontes-Ribeiro, C.; Ambrosio, A.F.; Abcouwer, S.F.; Fernandes, R.; Antonetti, D.A. Protective effect of a glp-1 analog on ischemia-reperfusion induced blood-retinal barrier breakdown and inflammation. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2584–2592. [Google Scholar] [CrossRef] [PubMed]
- Otterbein, L.E.; Soares, M.P.; Yamashita, K.; Bach, F.H. Heme oxygenase-1: Unleashing the protective properties of heme. Trends Immunol. 2003, 24, 449–455. [Google Scholar] [CrossRef]
- Szabo, M.E.; Droy-Lefaix, M.T.; Doly, M.; Carre, C.; Braquet, P. Ischemia and reperfusion-induced histologic changes in the rat retina. Demonstration of a free radical-mediated mechanism. Investig. Ophthalmol. Vis. Sci. 1991, 32, 1471–1478. [Google Scholar] [PubMed]
- Li, S.Y.; Yang, D.; Yeung, C.M.; Yu, W.Y.; Chang, R.C.; So, K.F.; Wong, D.; Lo, A.C. Lycium barbarum polysaccharides reduce neuronal damage, blood-retinal barrier disruption and oxidative stress in retinal ischemia/reperfusion injury. PLoS ONE 2011, 6, e16380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, X.S.; Feng, Q.; Lo, A.C.; Chang, R.C.; Lin, B.; Chung, S.K.; So, K.F. Protection of retinal ganglion cells and retinal vasculature by lycium barbarum polysaccharides in a mouse model of acute ocular hypertension. PLoS ONE 2012, 7, e45469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Yin, J.; Deng, Y.; Yang, M.; Xu, L.; Teng, F.; Li, D.; Cheng, Y.; Liu, S.; Wang, D.; et al. The protective effects of ginsenoside rg1 against hypertension target-organ damage in spontaneously hypertensive rats. BMC Complement. Altern. Med. 2012, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, F.; Cheng, H.; Liu, C.; Sun, M.; Wu, K.; Ai, M. Protective effects of total flavonoids from flos puerariae on retinal neuronal damage in diabetic mice. Mol. Vis. 2013, 19, 1999–2010. [Google Scholar] [PubMed]
- McCulloch, D.L.; Marmor, M.F.; Brigell, M.G.; Hamilton, R.; Holder, G.E.; Tzekov, R.; Bach, M. Iscev standard for full-field clinical electroretinography (2015 update). Doc. Ophthalmol. 2015, 130, 1–12. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varga, B.; Priksz, D.; Lampé, N.; Bombicz, M.; Kurucz, A.; Szabó, A.M.; Pósa, A.; Szabó, R.; Kemény-Beke, Á.; Remenyik, J.; et al. Protective Effect of Prunus Cerasus (Sour Cherry) Seed Extract on the Recovery of Ischemia/Reperfusion-Induced Retinal Damage in Zucker Diabetic Fatty Rat. Molecules 2017, 22, 1782. https://doi.org/10.3390/molecules22101782
Varga B, Priksz D, Lampé N, Bombicz M, Kurucz A, Szabó AM, Pósa A, Szabó R, Kemény-Beke Á, Remenyik J, et al. Protective Effect of Prunus Cerasus (Sour Cherry) Seed Extract on the Recovery of Ischemia/Reperfusion-Induced Retinal Damage in Zucker Diabetic Fatty Rat. Molecules. 2017; 22(10):1782. https://doi.org/10.3390/molecules22101782
Chicago/Turabian StyleVarga, Balázs, Dániel Priksz, Nóra Lampé, Mariann Bombicz, Andrea Kurucz, Adrienn Mónika Szabó, Anikó Pósa, Renáta Szabó, Ádám Kemény-Beke, Judit Remenyik, and et al. 2017. "Protective Effect of Prunus Cerasus (Sour Cherry) Seed Extract on the Recovery of Ischemia/Reperfusion-Induced Retinal Damage in Zucker Diabetic Fatty Rat" Molecules 22, no. 10: 1782. https://doi.org/10.3390/molecules22101782







