Genistein Binding to Copper(II)—Solvent Dependence and Effects on Radical Scavenging
Abstract
:1. Introduction
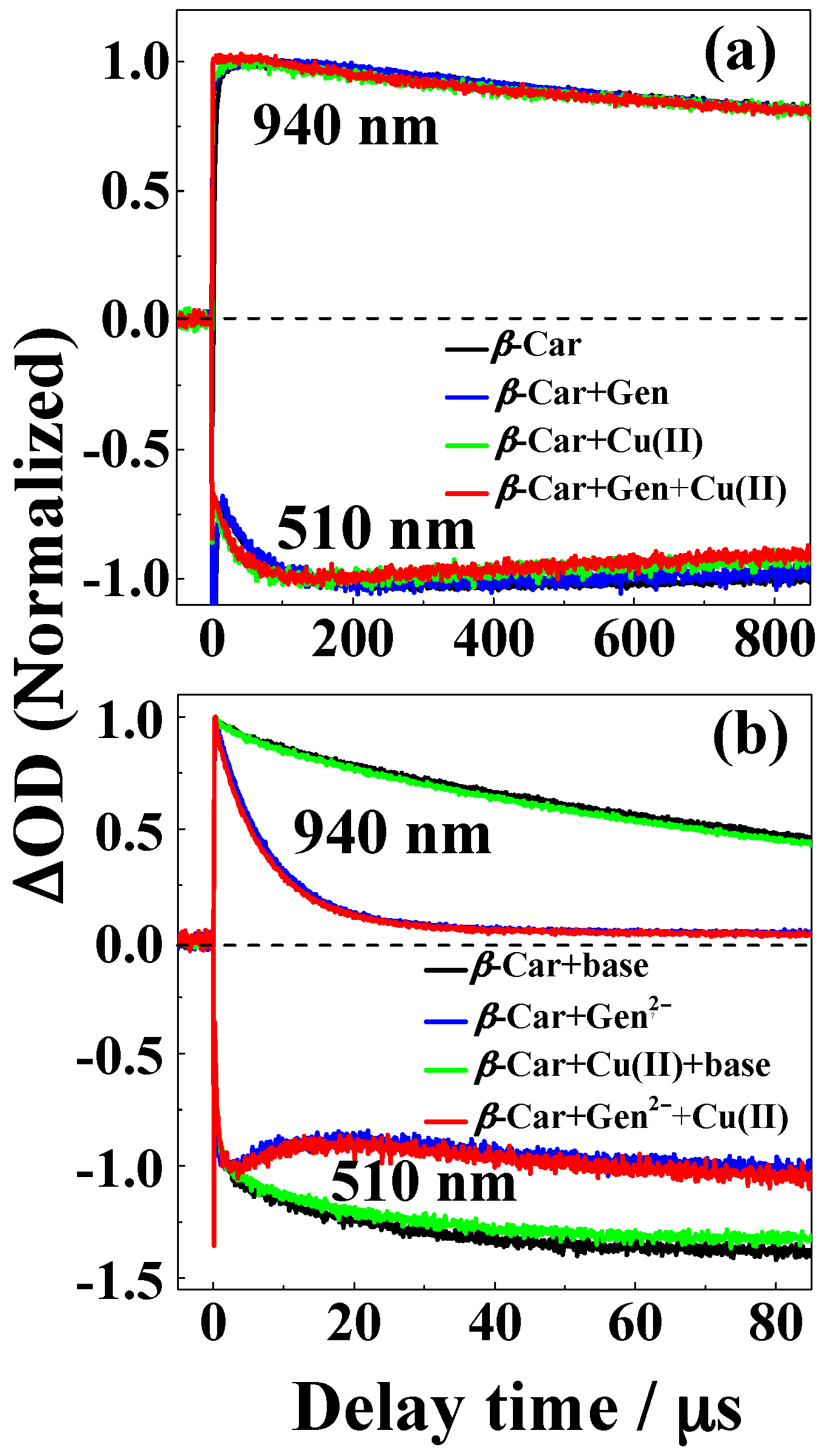
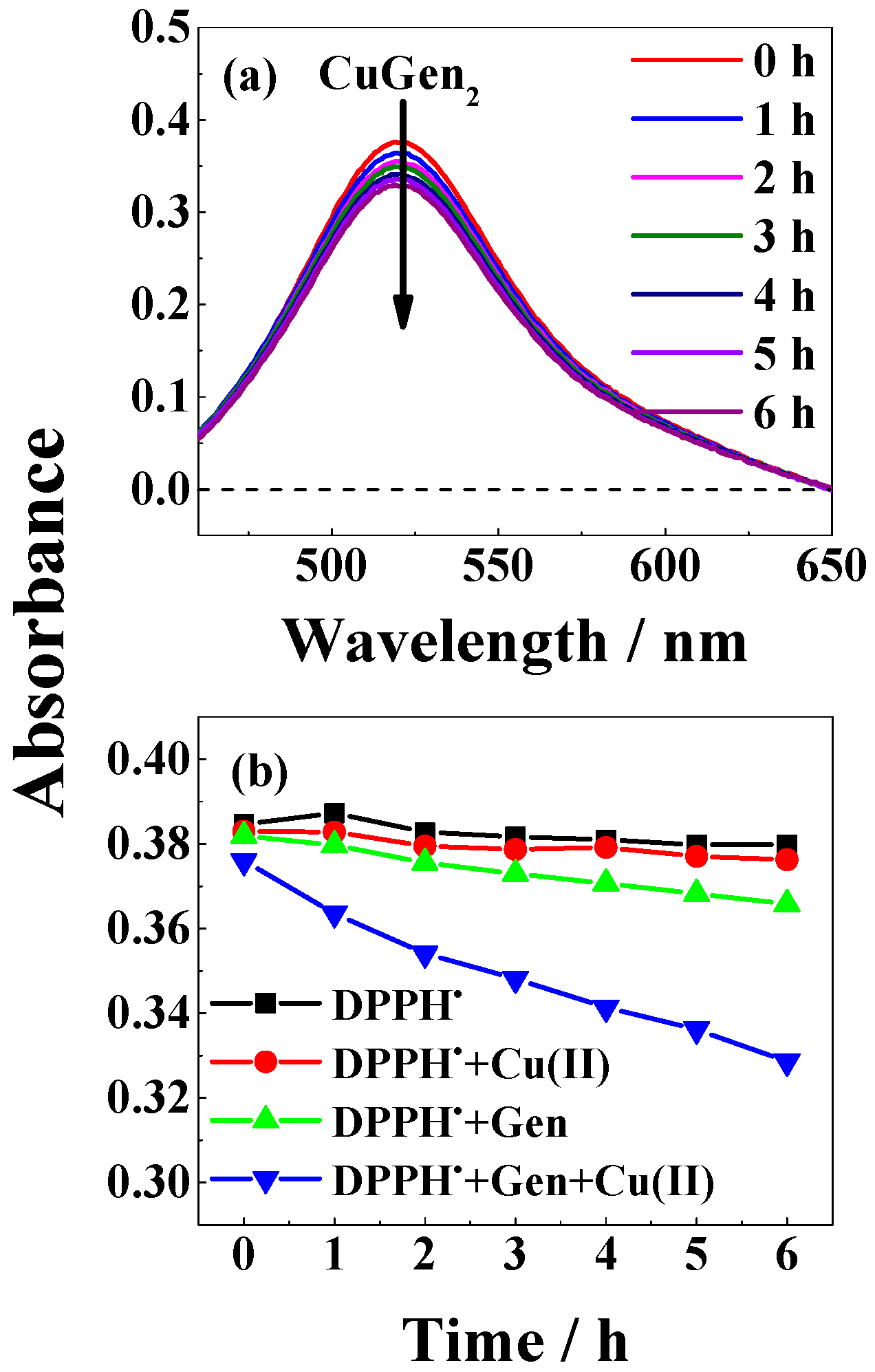
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Reaction of Cu(II) with Genistein
4.3. NMR and Mass Spectra Characterization
4.4. Determination of Oxidation Potentials
4.5. β-Carotene Bleaching Assay and β-Carotene Radical Cation Quenching Kinetics
4.6. DPPH Radical Scavenging
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F. Silymarin as a natural antioxidant: An overview of the current evidence and perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef] [PubMed]
- Han, R.M.; Tian, Y.X.; Becker, E.M.; Andersen, M.L.; Zhang, J.P.; Skibsted, L.H. Puerarin and conjugate bases as radical scavengers and antioxidants: Molecular mechanism and synergism with β-carotene. J. Agric. Food Chem. 2007, 55, 2384–2391. [Google Scholar] [CrossRef] [PubMed]
- Han, R.M.; Tian, Y.X.; Liu, Y.; Chen, C.H.; Ai, X.C.; Zhang, J.P.; Skibsted, L.H. Comparison of flavonoids and isoflavonoids as antioxidants. J. Agric. Food Chem. 2009, 57, 3780–3785. [Google Scholar] [CrossRef] [PubMed]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: Updated review of mechanisms and catalyzing metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Marković, J.M.D.; Marković, Z.S.; Brdarić, T.P.; Pavelkić, V.M.; Jadranin, M.B. Iron complexes of dietary flavonoids: Combined spectroscopic and mechanistic study of their free radical scavenging activity. Food Chem. 2011, 129, 1567–1577. [Google Scholar] [CrossRef]
- Ryan, P.; Hynes, M.J. The kinetics and mechanisms of the reactions of iron(III) with quercetin and morin. J. Inorg. Biochem. 2008, 102, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Pękal, A.; Biesaga, M.; Pyrzynska, K. Interaction of quercetin with copper ions: Complexation, oxidation and reactivity towards radicals. Biometals 2011, 24, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Samsonowicz, M.; Regulska, E. Spectroscopic study of molecular structure, antioxidant activity and biological effects of metal hydroxyflavonol complexes. Spectrochim. Acta Part A 2017, 173, 757–771. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Selvaraj, S.; Krishnaswamy, S.; Devashya, V.; Sethuraman, S.; Krishnan, U.M. Flavonoid-metal ion complexes: A novel class of therapeutic agents. Med. Res. Rev. 2014, 34, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Dowling, S.; Regan, F.; Hughes, H. The characterisation of structural and antioxidant properties of isoflavone metal chelates. J. Inorg. Biochem. 2010, 104, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Q.; Zhang, B.; Zhu, Q.T.; Wu, Z. Synthesis and Crystal Structure of Tri (3-Hydroxyflavone) Aluminum(III). Chem. J. Chin. Univ. 1998, 3, 410–413. [Google Scholar]
- Shao, M.; Gang, J.; Kim, S.; Yoon, M. A New Kaempferol-based Ru(II) Coordination Complex, Ru(kaem)Cl (DMSO) 3: Structure and Absorption–Emission Spectroscopy Study. Bull. Korean Chem. Soc. 2016, 37, 1625–1631. [Google Scholar] [CrossRef]
- El Amrani, F.B.A.; Perelló, L.; Real, J.A.; González-Alvarez, M.; Alzuet, G.; Borrás, J.; García-Granda, S.; Montejo-Bernardo, J. Oxidative DNA cleavage induced by an iron(III) flavonoid complex: Synthesis, crystal structure and characterization of chlorobis (flavonolato)(methanol) iron(III) complex. J. Inorg. Biochem. 2006, 100, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Sun, S.; Liang, Y.; Song, J. Antioxidant property of quercetin–Cr(III) complex: The role of Cr(III) ion. J. Mol. Struct. 2009, 918, 194–197. [Google Scholar] [CrossRef]
- Bukhari, S.B.; Memon, S.; Tahir, M.M.; Bhanger, M.I. Synthesis, characterization and investigation of antioxidant activity of cobalt–quercetin complex. J. Mol. Struct. 2008, 892, 39–46. [Google Scholar] [CrossRef]
- Panhwar, Q.K.; Memon, S. Synthesis of Cr(III)-morin complex: Characterization and antioxidant study. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.F.; De Giovani, W.F. Antioxidant properties of complexes of flavonoids with metal ions. Redox Rep. 2004, 9, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Bratu, M.M.; Birghila, S.; Miresan, H.; Negreanu-Pirjol, T.; Prajitura, C.; Calinescu, M. Biological activities of Zn(II) and Cu(II) complexes with quercetin and rutin: Antioxidant properties and UV-protection capacity. Rev. Chim. (Bucharest) 2014, 65, 544–549. [Google Scholar]
- Dehghan, G.; Khoshkam, Z. Tin(II)–quercetin complex: Synthesis, spectral characterisation and antioxidant activity. Food Chem. 2012, 131, 422–426. [Google Scholar] [CrossRef]
- Panhwar, Q.K.; Memon, S. Synthesis, spectral characterization and antioxidant activity of Tin(II)-morin complex. Pak. J. Anal. Environ. Chem. 2012, 13, 159–168. [Google Scholar]
- Panhwar, Q.K.; Memon, S. Synthesis, characterization and antioxidant study of Tin(II)–rutin complex: Exploration of tin packaging hazards. Inorg. Chim. Acta 2013, 407, 252–260. [Google Scholar] [CrossRef]
- Ravichandran, R.; Rajendran, M.; Devapiriam, D. Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food. Chem. 2014, 146, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.H.; Shi, X.Y.; Li, X.; Li, F.F.; Zhang, Q.Q.; Jiang, S.X.; Cui, J.Z.; Gao, H.L. Spectroscopic and electrochemical studies on the evaluation of the radical scavenging activities of luteolin by chelating iron. RSC Adv. 2014, 4, 25227–25233. [Google Scholar] [CrossRef]
- Bukhari, S.B.; Memon, S.; Mahroof-Tahir, M.; Bhanger, M.I. Synthesis, characterization and antioxidant activity copper–quercetin complex. Spectrochim. Acta Part A 2009, 71, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hu, F.; Chang, Y.Q.; Zhou, Y.; Wang, P.; Zhang, J.P. Triplet excitation dynamics of two keto-carotenoids in n-hexane and in methanol as studied by ns flash photolysis spectroscopy. Chem. Phys. Lett. 2015, 633, 114–119. [Google Scholar] [CrossRef]
- Jouyban, A.; Soltanpour, S.; Chan, H.K. A simple relationship between dielectric constant of mixed solvents with solvent composition and temperature. Int. J. Pharm. 2004, 269, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Castro, G.T.; Blanco, S.E. Structural and spectroscopic study of 5,7-dihydroxy-flavone and its complex with aluminum. Spectrochim. Acta Part A 2004, 60, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.M.; Andrades, N.E.D.; Paulino, N.; Sawaya, A.C.H.F.; Eberlin, M.N.; Marcucci, M.C.; Favero, G.M.; Novak, E.M.; Bydlowski, S.P. Synthesis and Characterization of a Metal Complex Containing Naringin and Cu, and its Antioxidant, Antimicrobial, Antiinflammatory and Tumor Cell Cytotoxicity. Molecules 2007, 12, 1352–1366. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Mallick, S.; Chakraborty, T.; Ghosh, N.; Singh, A.K.; Manna, S.; Majumdar, S. Synthesis, characterisation and antioxidant activity of luteolin–vanadium(II) complex. Food Chem. 2015, 173, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.F.; Sussuchi, E.M.; De Giovani, W.F. Synthesis, electrochemical, spectral, and antioxidant properties of complexes of flavonoids with metal ions. Synth. React. Inorg. Met. 2003, 33, 1125–1144. [Google Scholar] [CrossRef]
- De Souza, R.F.; De Giovani, W.F. Synthesis, spectral and electrochemical properties of Al(III) and Zn(II) complexes with flavonoids. Spectrochim. Acta Part A 2005, 61, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Persson, I.; Persson, P.; Sandström, M.; Ullström, A.S. Structure of Jahn–Teller distorted solvated copper(II) ions in solution, and in solids with apparently regular octahedral coordination geometry. J. Chem. Soc. Dalton Trans. 2002, 7, 1256–1265. [Google Scholar] [CrossRef]
- Morrow, J.R.; Buttrey, L.A.; Berback, K.A. Transesterificationof a Phosphate Diester by Divalent and Trivalent Metal Ions. Inorg. Chem. 1992, 31, 16–20. [Google Scholar] [CrossRef]
- Lewandowski, A. Ionic solvent—I. Free energeis of transfer of copper(II) from water to alcohols and to their mixtures with water. Electorchim. Acta 1984, 29, 547–550. [Google Scholar] [CrossRef]
- Han, R.M.; Chen, C.H.; Tian, Y.X.; Zhang, J.P.; Skibsted, L.H. Fast regeneration of carotenoids from radical cations by isoflavonoid dianions: Importance of the carotenoid keto group for electron transfer. J. Phys. Chem. A 2010, 114, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Fang, H.; Han, Y.; Lai, W.; Fu, X.; Cao, R. Reactivity and mechanism studies of hydrogen evolution catalyzed by copper corroles. ACS Catal. 2015, 5, 5145–5153. [Google Scholar] [CrossRef]
- Chang, H.T.; Cheng, H.; Han, R.M.; Wang, P.; Zhang, J.P.; Skibsted, L.H. Regeneration of β-Carotene from Radical Cation by Eugenol, Isoeugenol, and Clove Oil in the Marcus Theory Inverted Region for Electron Transfer. J. Agric. Food Chem. 2017, 65, 908–912. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
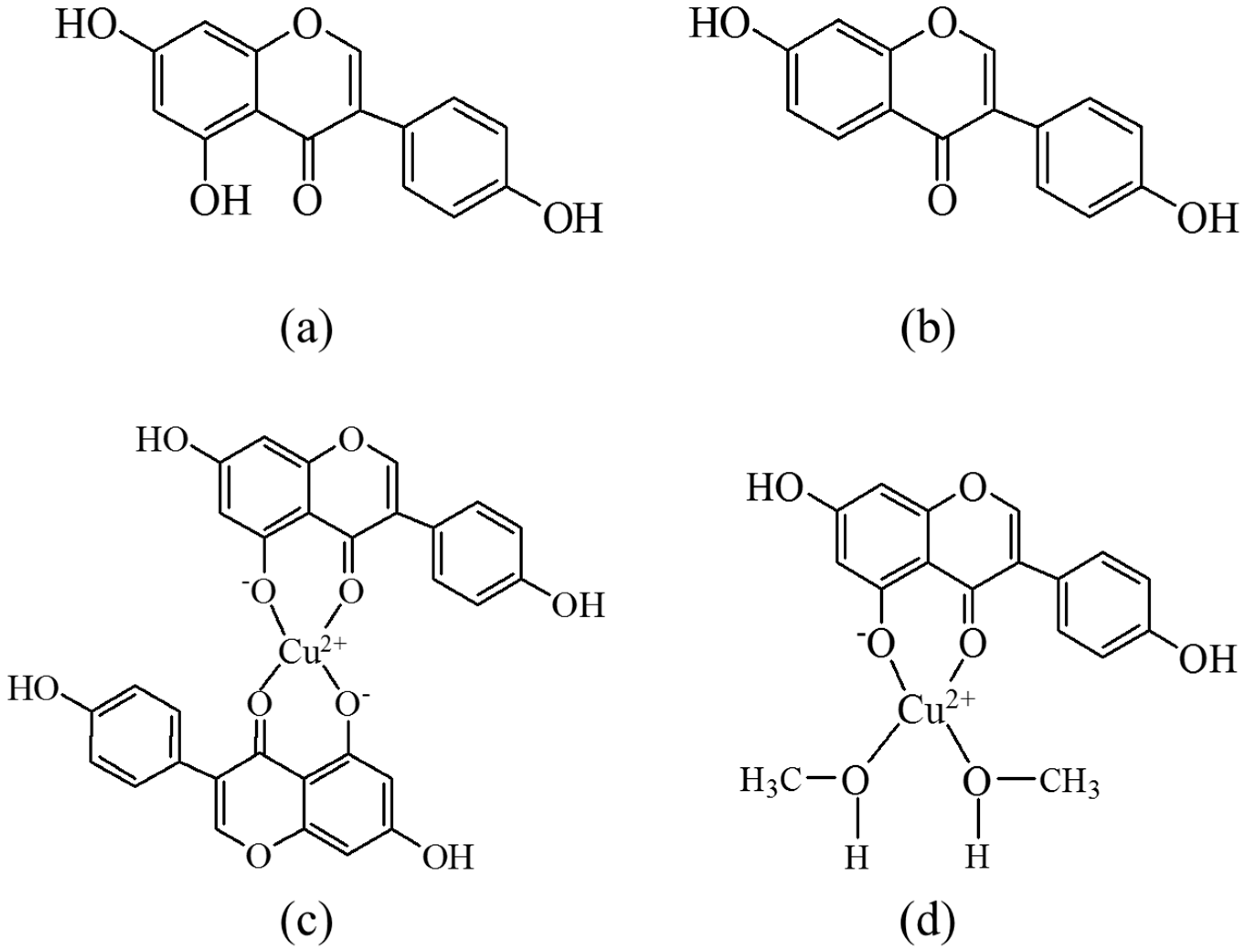
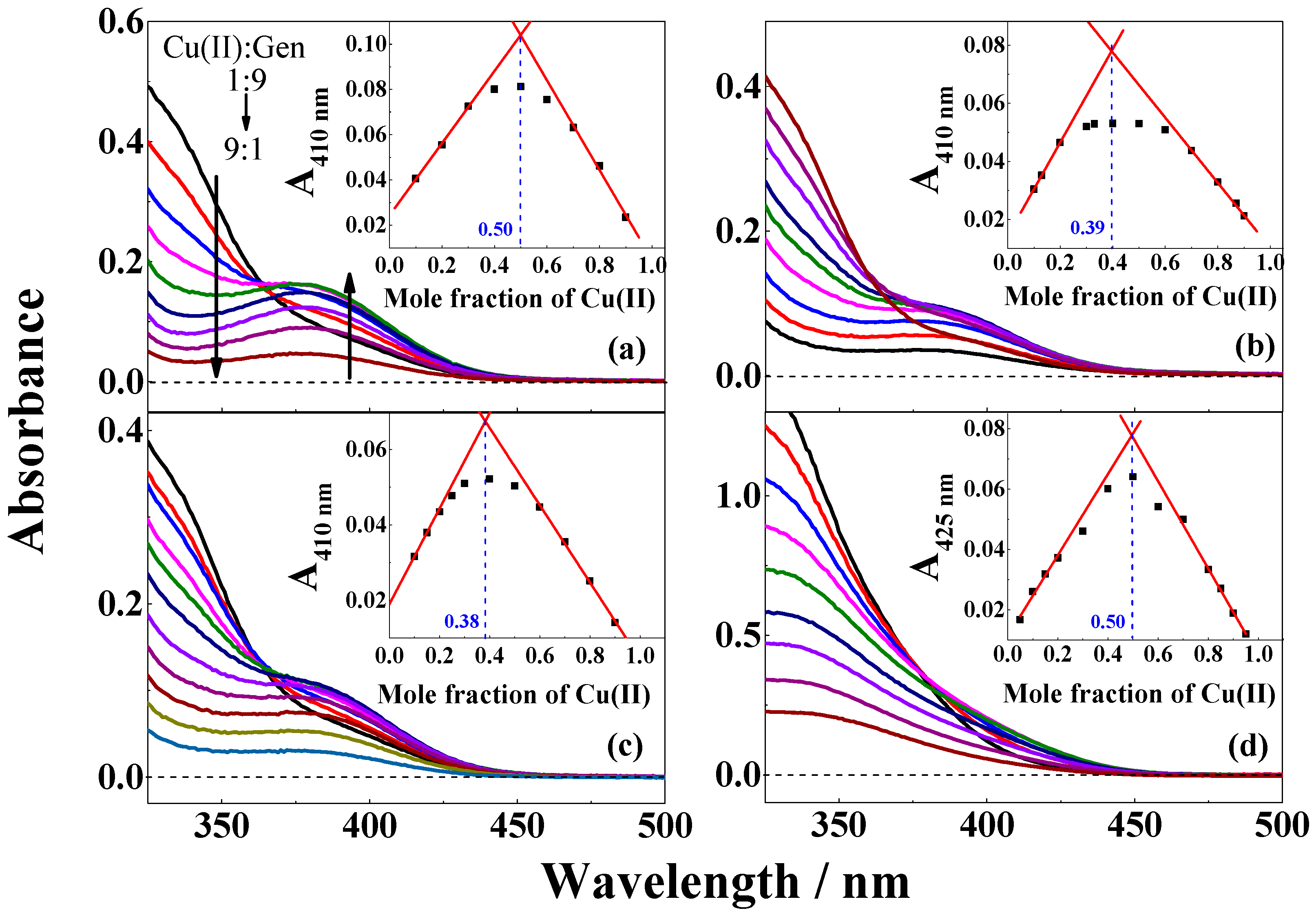
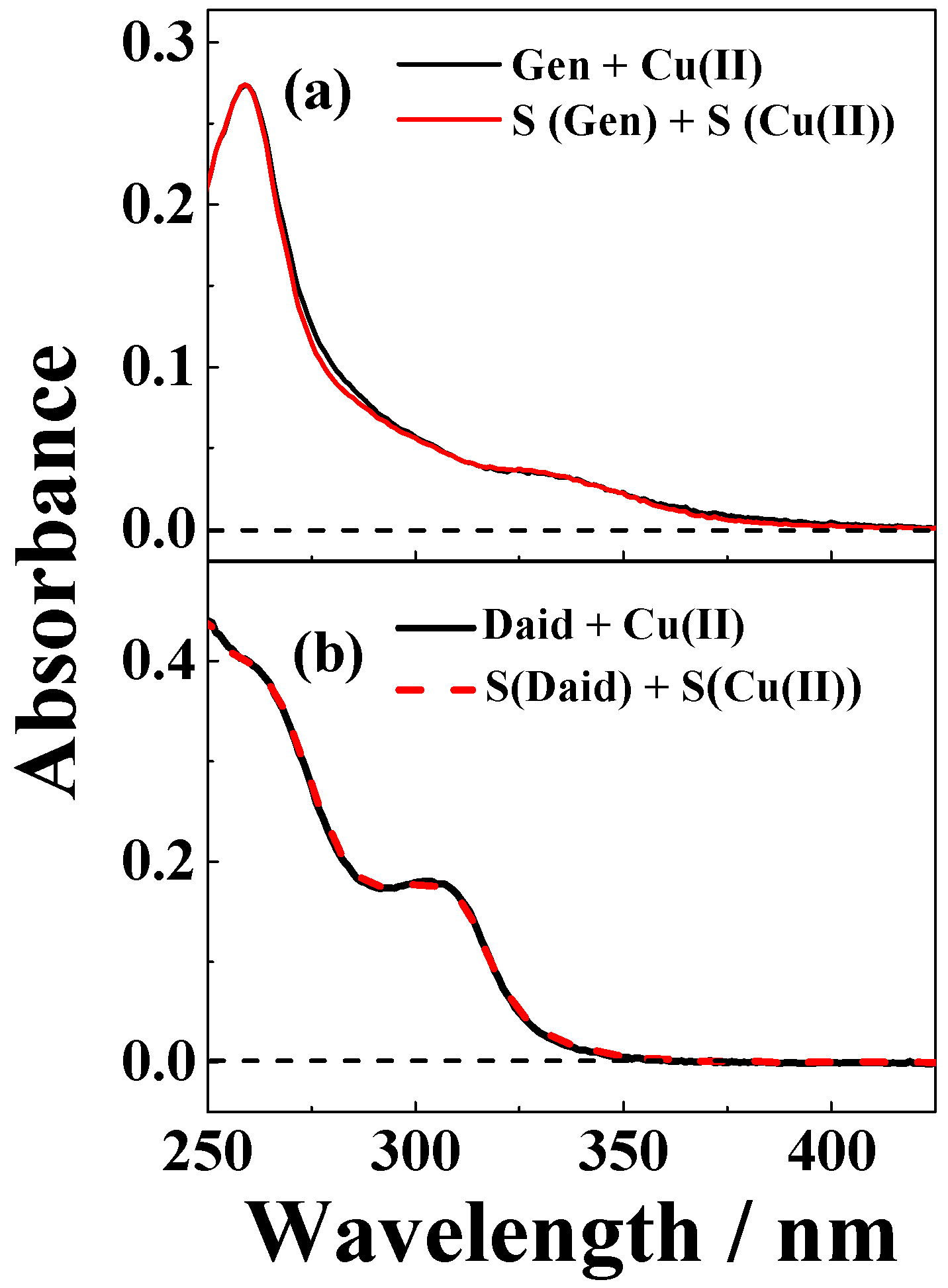
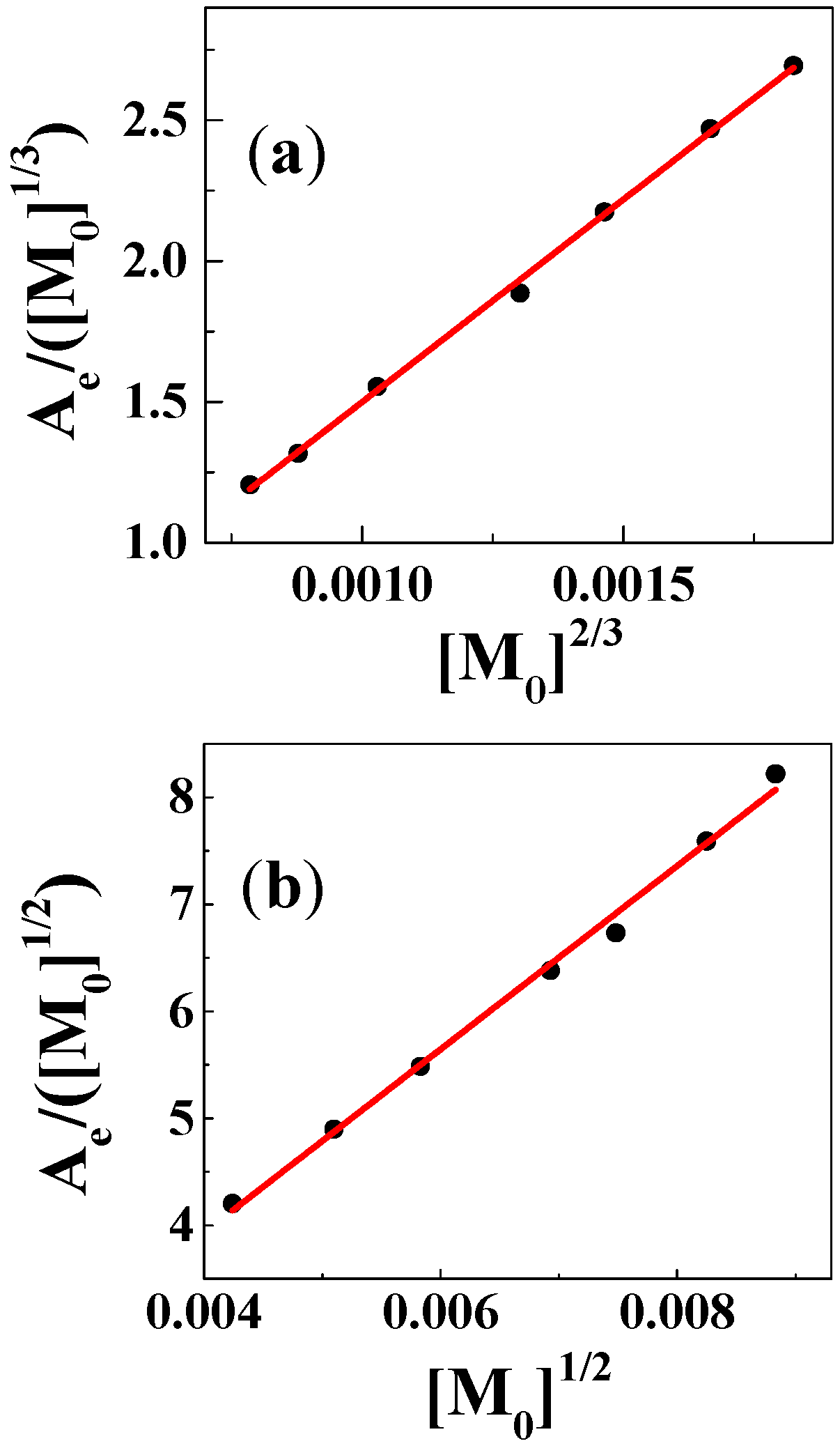
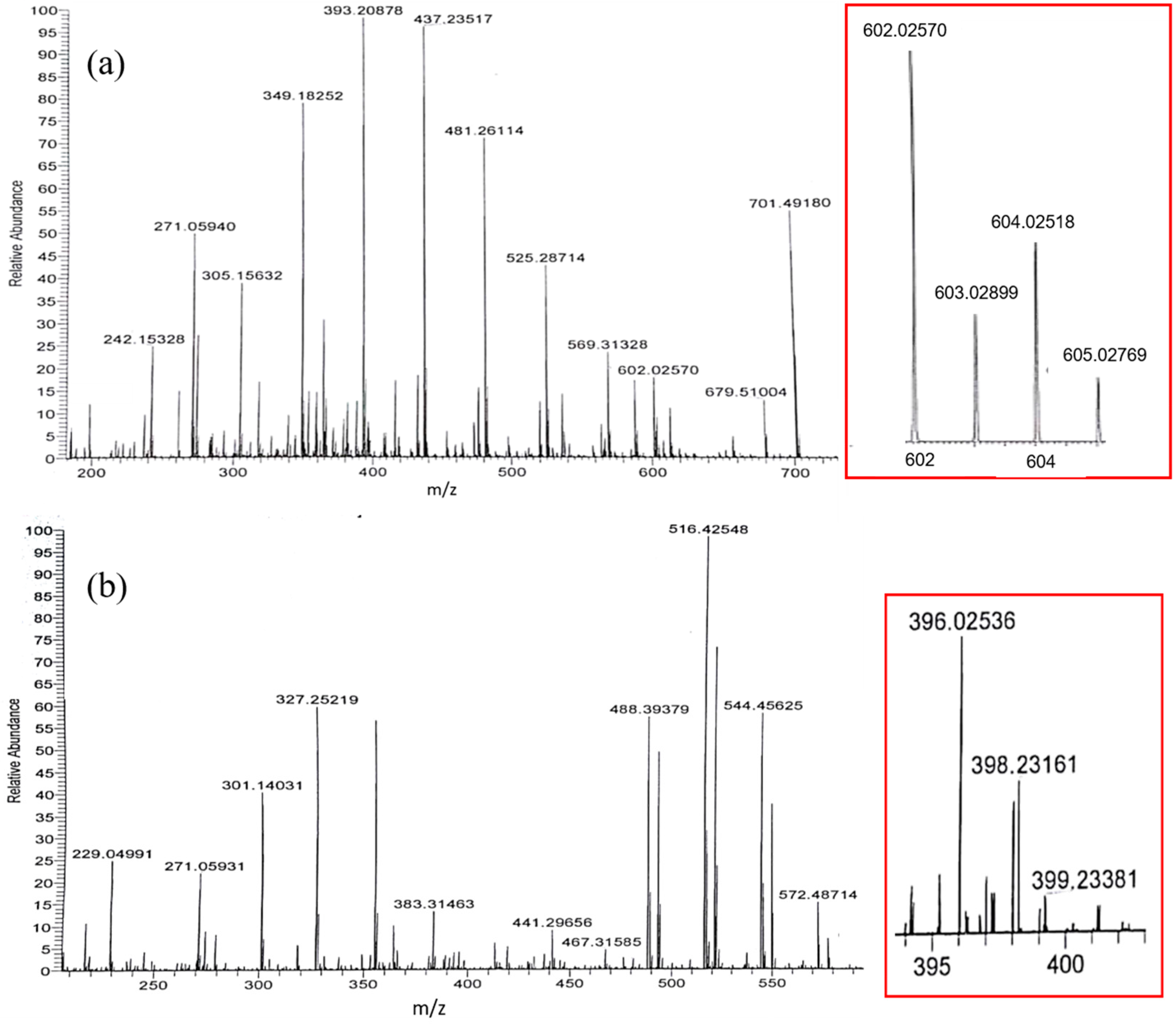
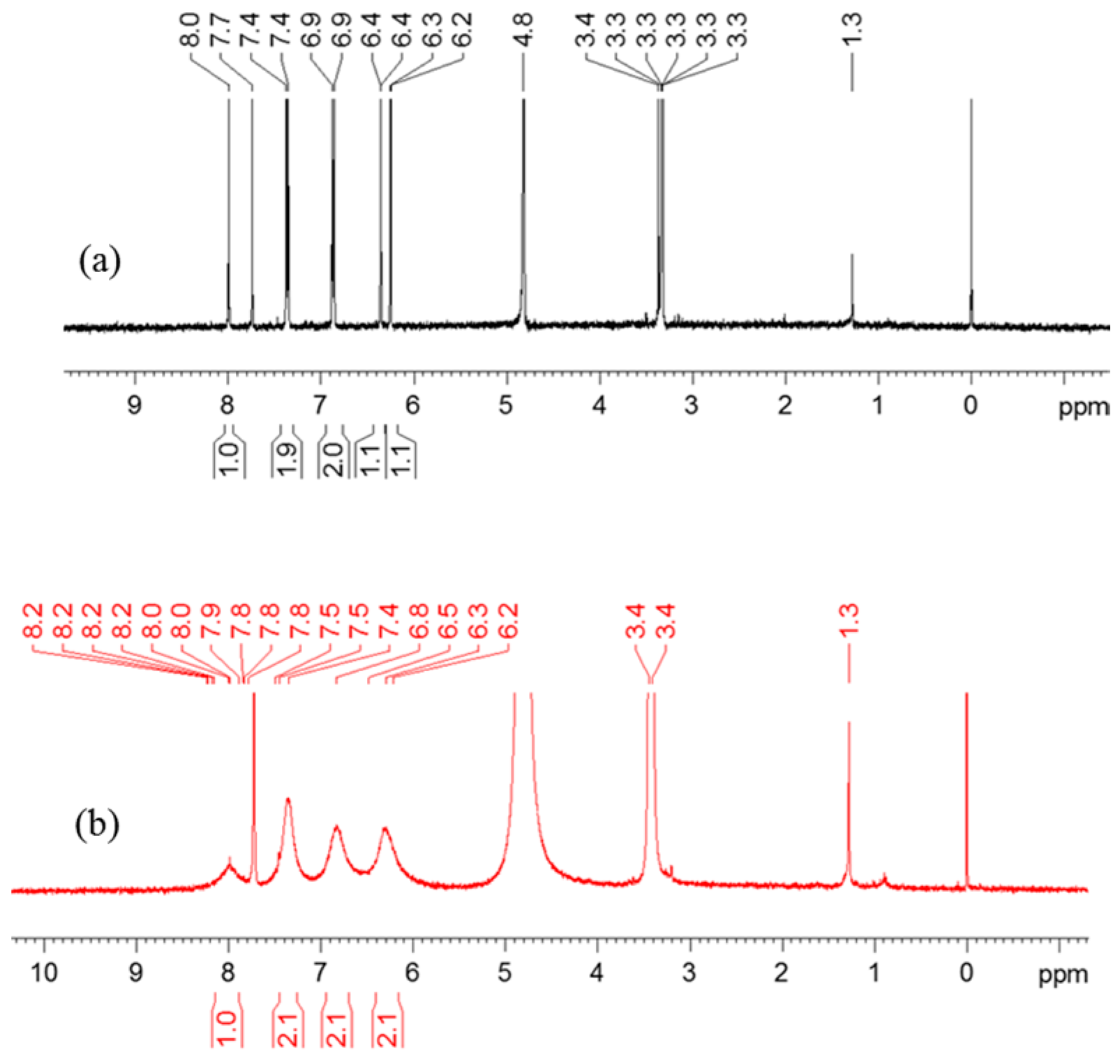
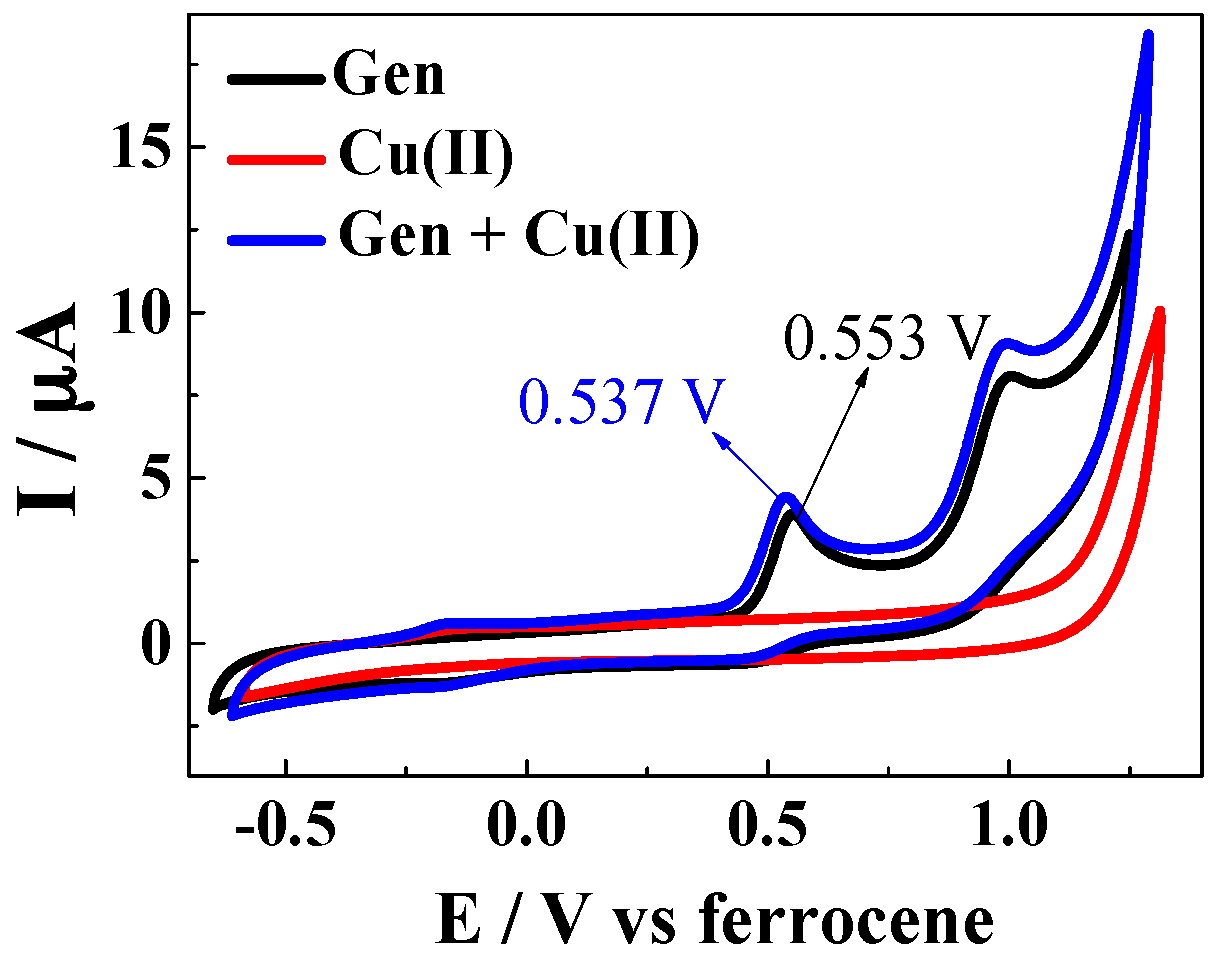
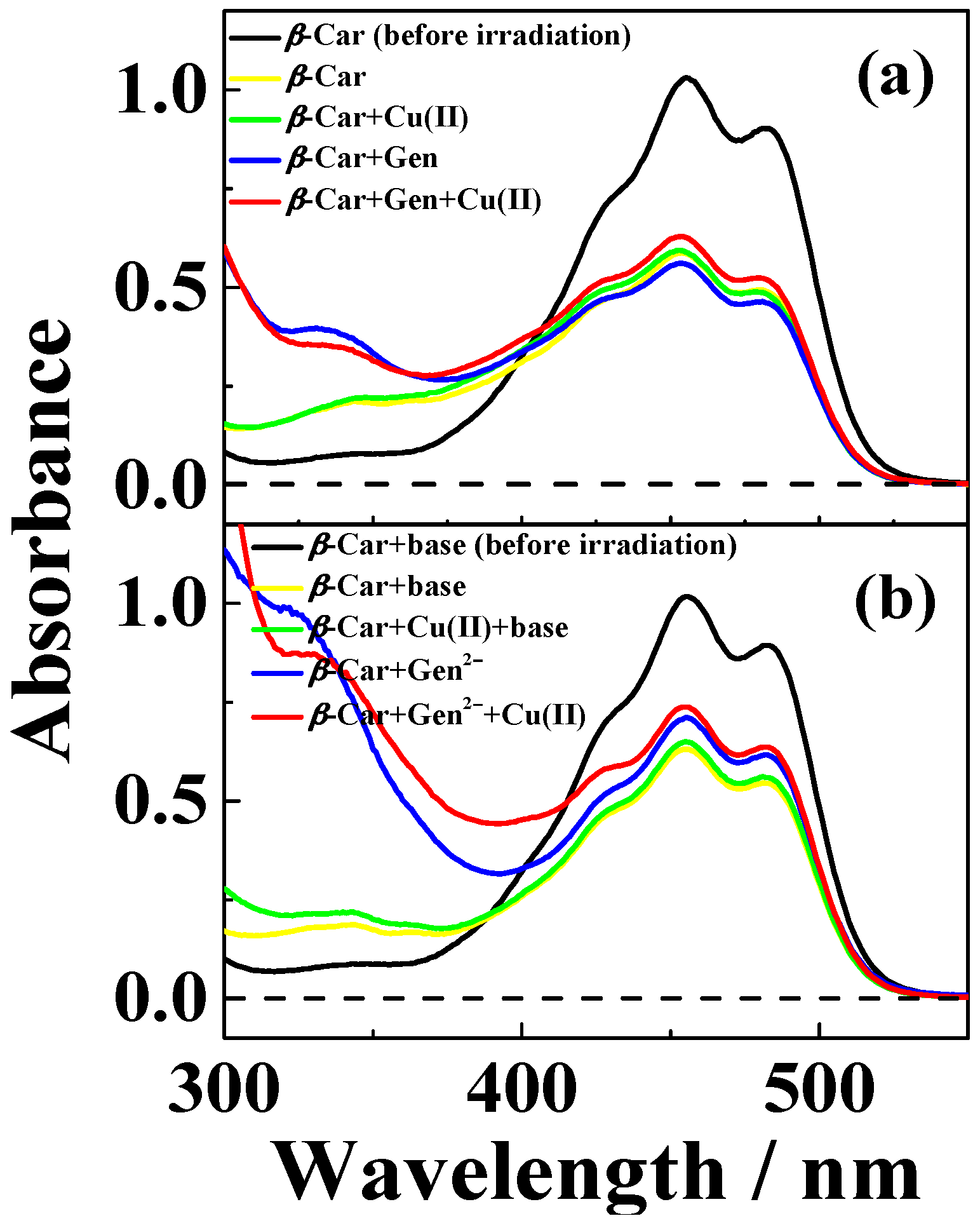
| Solvent | H2O | CH3OH | C2H5OH | CH3OH:CHCl3 = 7/3 | THF |
|---|---|---|---|---|---|
| Cu(II):Gen | ---/--- | a 1:1/b 1:1 | a 1:2/b 1:2 | a 1:2/b 1:1 | --- |
| KC | ---/--- | a 6.0 × 105/b 8.8 × 104 | a 1.1 × 1011/b 4.1 × 108 | a 1.1 × 1011/b 9.8 × 104 | --- |
| cP(ε) | 0.963 | 0.914 | 0.887 | 0.887 | 0.684 |
| Methanol:Chloroform = 7/3 | Methanol | ||||
|---|---|---|---|---|---|
| m/z Experimental | m/z Calculated | Cu(II)Gen2 | m/z Experimental | m/z Calculated | Cu(II)Gen |
| 271.0594 | 271.0607 | Gen + H+ | 271.0593 | 271.0607 | Gen + H+ |
| 349.1825 | 349.0090 | (Gen‒H) + Na+ + K+ + H2O | 327.2522 | 327.0271 | Gen + K+ + H2O |
| 393.2088 | 393.1397 | Gen + 5H2O + CH3OH + H+ | 396.0254 | 396.0370 | (Gen‒H) + 63Cu2+ + 2CH3OH |
| 437.2352 | 437.1241 | Gen + 4CH3OH + K+ | 398.2316 | 398.0252 | (Gen‒H) + 65Cu2+ + 2CH3OH |
| 481.2611 | 481.2285 | Gen + H2O + 6CH3OH + H+ | 488.3938 | 488.9968 | Gen‒2H + 4H2O + CH3OH + 3K+ |
| 525.2871 | 525.2159 | Gen + 4H2O + 5CH3OH + Na+ | 516.4255 | 516.0967 | Gen‒H + 4H2O + 3CH3OH + 2K+ |
| 569.3133 | 569.2212 | Gen + 2H2O + 7CH3OH + K+ | 544.4503 | 544.2731 | Gen + 8CH3OH + H2O + H+ |
| 602.0257 | 602.0374 | 2(Gen‒H) + 63Cu2+ + H+ | |||
| 604.0252 | 604.0256 | 2(Gen‒H) + 65Cu2+ + H+ | |||
| 701.4918 | 701.2446 | 2Gen + 5CH3OH + H+ | |||
| Chemical Shift/ppm | 2-H | 6-H | 8-H | 2’,6’-H | 3’,5’-H |
|---|---|---|---|---|---|
| Gen | 8.0 | 6.2 | 6.4 | 7.4 | 6.9 |
| Cu(II)Gen2 | 8.2 | 6.3 | 6.5 | 7.5 | 6.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Xu, Y.; Liu, H.-Y.; Han, R.-M.; Zhang, J.-P.; Skibsted, L.H. Genistein Binding to Copper(II)—Solvent Dependence and Effects on Radical Scavenging. Molecules 2017, 22, 1757. https://doi.org/10.3390/molecules22101757
Yang J, Xu Y, Liu H-Y, Han R-M, Zhang J-P, Skibsted LH. Genistein Binding to Copper(II)—Solvent Dependence and Effects on Radical Scavenging. Molecules. 2017; 22(10):1757. https://doi.org/10.3390/molecules22101757
Chicago/Turabian StyleYang, Jing, Yi Xu, Hao-Yu Liu, Rui-Min Han, Jian-Ping Zhang, and Leif H. Skibsted. 2017. "Genistein Binding to Copper(II)—Solvent Dependence and Effects on Radical Scavenging" Molecules 22, no. 10: 1757. https://doi.org/10.3390/molecules22101757







