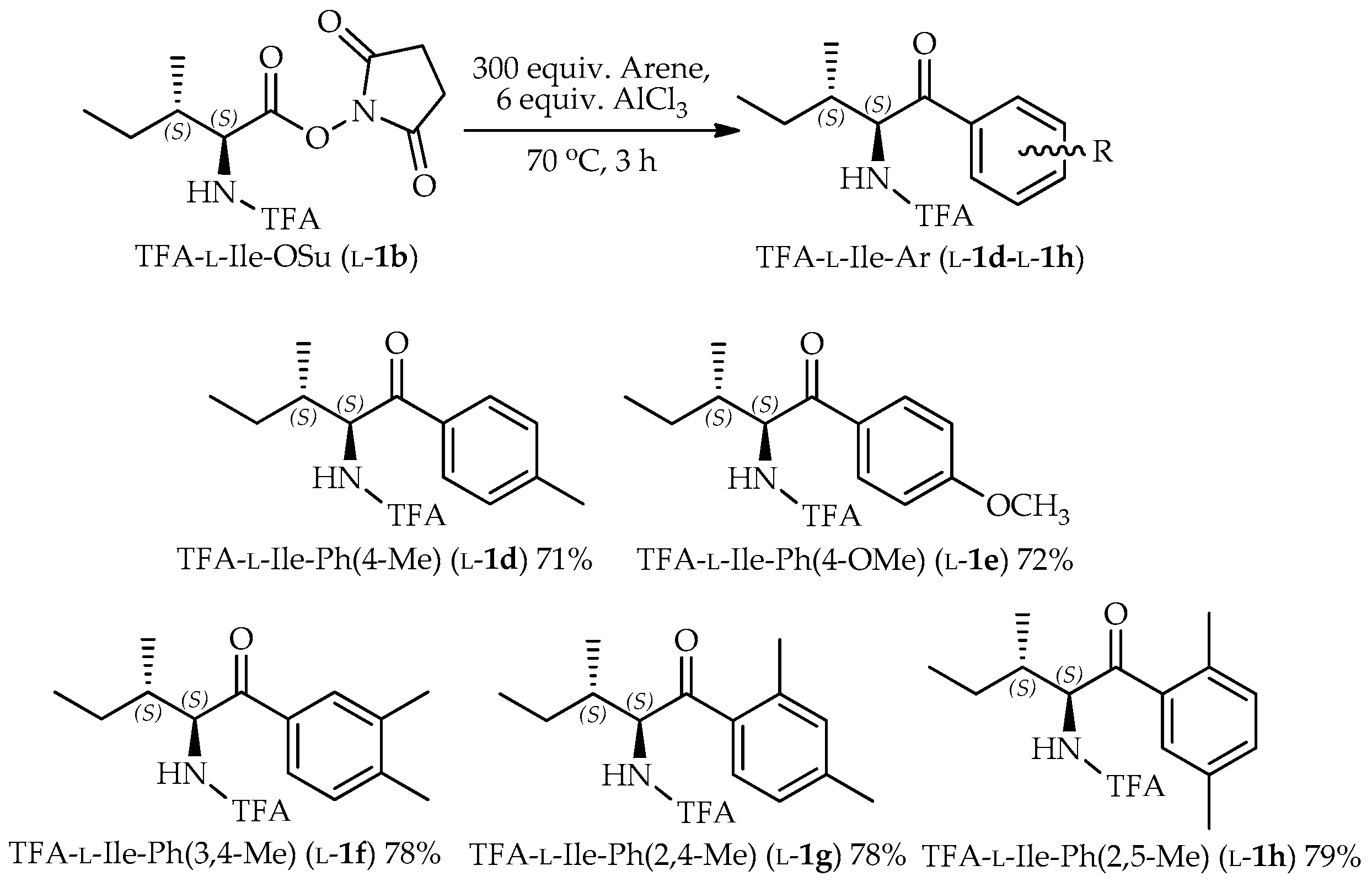
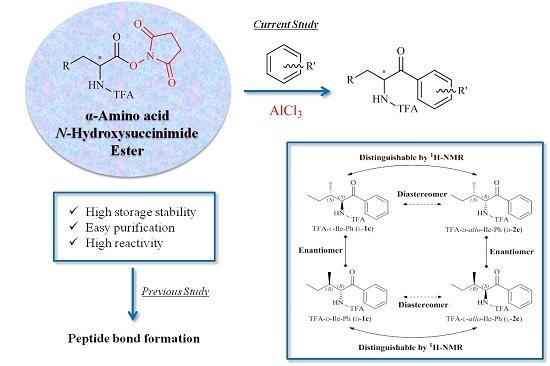
3.3. General Procedure for the Preparation of TFA-α-Amino Acid N-Hydroxysuccinimide Ester
NHS (N-hydroxysuccinimide, 1.1 equiv.) was added to a solution of TFA-α-amino acid (l-/d-1a–l-/d-2a, 3a, l-/d-4a–l-/d-8a, 1.0 mmol) in pre-cooled CH2Cl2 (10 mL). The suspension of WSCD-HCl (water soluble carbodiimide hydrochloride, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide monohydrochloride, 1.0 equiv.) in CH2Cl2 (10 mL) was added drop-wise at 0 °C and the reaction was stirred for 2–4 h. The solvent was removed by rotary evaporation. The residue that remained was dissolved in ethyl acetate; washed with water, sat. NaHCO3, sat. NaCl, dried over MgSO4, and then evaporated. The product was solidified by washing with hexane to be used for further reaction.
(2S,3S)-2,5-Dioxopyrrolidin-1-yl 3-methyl-2-(2,2,2-trifluoroacetamido)pentanoate (
TFA-l-Ile-OSu,
l-1b) [
24]: Colorless amorphous mass. [α]
D = −4.0 (
c 1.0, CHCl
3). IR (neat)
ν: 3265, 2965, 1785, 1740 cm
−1.
1H-NMR (270 MHz, CDCl
3) δ: 7.41 (d,
J = 8.6 Hz, 1H, N
H), 4.97 (dd,
J = 8.6, 5.3 Hz, 1H, C
HNH), 2.89 (s, 4H, 2 × CH
2), 2.23–2.08 (m, 1H, C
HCH
3), 1.75–1.60 (1H, m, C
H2CH
3), 1.45–1.29 (1H, m, CH
2CH
3), 1.11 (d,
J = 6.9 Hz, 3H, CHC
H3), 1.02 (t,
J = 7.4 Hz, 3H, CH
2C
H3) ppm.
13C-NMR (67.5 MHz, CDCl
3) δ: 168.5 (2 × CO), 166.3, 156.9 (q,
2JCF = 38.4 Hz), 115.5 (q,
1JCF = 287.9 Hz), 55.3, 38.0, 25.5 (2 × CH
2), 24.7, 14.8, 11.3 ppm. HRMS-ESI (
m/
z) [M + Na]
+ calcd for C
12H
15F
3N
2O
5Na 347.0831, found 347.0822.
(2R,3R)-2,5-Dioxopyrrolidin-1-yl 3-methyl-2-(2,2,2-trifluoroacetamido)pentanoate (TFA-d-Ile-OSu, d-1b): Colorless amorphous mass. [α]D = +4.0 (c 1.0, CHCl3). IR (neat) ν: 3285, 2968, 1789, 1731 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 6.97 (d, J = 8.2 Hz, 1H, NH), 4.97 (dd, J = 8.6, 4.9 Hz, 1H, CHNH), 2.86 (s, 4H, 2 × CH2) 2.20–2.05 (m, 1H, CHCH3), 1.71–1.56 (m, 1H, CH2CH3), 1.41–1.21 (m, 1H, CH2CH3), 1.07 (d, J = 6.9 Hz, 3H, CHCH3), 1.00 (t, J = 7.4 Hz, 3H, CH2CH3) ppm. 13C NMR (67.5 MHz, CDCl3) δ: 168.7 (2 × CO), 166.2, 156.9 (q, 2JCF = 38.2 Hz), 115.5 (q, 1JCF = 287.5 Hz), 55.3, 37.7, 25.4 (2 × CH2), 24.6, 14.7, 11.1 ppm. HRMS-ESI (m/z) [M + Na]+ calcd for C12H15F3N2O5Na 347.0831, found 347.0833.
(2S,3R)-2,5-Dioxopyrrolidin-1-yl 3-methyl-2-(2,2,2-trifluoroacetamido)pentanoate (TFA-l-allo-Ile-OSu, l-2b): Colorless amorphous mass. [α]D = −4.0 (c 1.0, CHCl3). IR (neat) ν: 3316, 2929, 1788, 1752 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.06 (d, J = 9.2 Hz, 1H, NH), 5.10 (dd, J = 9.2, 4.0 Hz, 1H, CHNH), 2.86 (s, 4H, 2 × CH2), 2.31–2.15 (m, 1H, CHCH3), 1.54–1.41 (m, 1H, CH2CH3), 1.37–1.23 (m, 1H, CH2CH3), 1.05 (d, J = 6.9 Hz, 3H, CHCH3), 1.00 (t, J = 7.4 Hz, 3H, CH2CH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 168.4 (2 × CO), 166.8, 157.0 (q, 2JCF = 38.2 Hz), 115.5 (q, 1JCF = 287.7 Hz), 54.1, 38.2, 25.9, 25.5 (2 × CH2), 14.1, 11.6 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C12H16F3N2O5 325.1011, found 325.1013.
(2R,3S)-2,5-Dioxopyrrolidin-1-yl 3-methyl-2-(2,2,2-trifluoroacetamido)pentanoate (TFA-d-allo-Ile-OSu, d-2b): Colorless amorphous mass. [α]D = +4.0 (c 1.0, CHCl3). IR (neat) ν: 3327, 2971, 1752 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.07 (d, J = 9.2 Hz, 1H, NH), 5.10 (dd, J = 9.1, 4.1 Hz, 1H, CHNH), 2.86 (s, 4H, 2 × CH2), 2.28–2.17 (m, 1H, CHCH3), 1.57–1.39 (m, 1H, CH2CH3), 1.37–1.22 (m, 1H, CH2CH3), 1.05 (d, J = 6.9 Hz, 3H, CHCH3), 1.00 (t, J = 7.4 Hz, 3H, CH2CH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 168.7 (2 × CO), 166.7, 157.1 (q, 2JCF = 38.2 Hz), 115.5 (q, 1JCF = 287.3 Hz), 54.1, 37.9, 25.8, 25.4 (2 × CH2), 14.0, 11.4 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C12H16F3N2O5 325.1011, found 325.1014.
2,5-Dioxocyclopentyl 2-(2,2,2-trifluoroacetamido)acetate (
TFA-
Gly-
OSu,
3b) [
25]: Colorless amorphous mass. IR (neat)
ν: 3313, 2998, 1690 cm
−1.
1H-NMR (270 MHz, CD
3OD) δ: 4.44 (s, 2H, C
H2NH), 2.84 (s, 4H, 2 × CH
2) ppm.
13C NMR (67.5 MHz, ACETONE-
d6) δ: 170.1 (2 × CO), 165.7 158.2 (q,
2JCF = 37.4 Hz), 116.8 (q,
1JCF = 287.0 Hz), 39.5, 26.2 (2 × CH
2) ppm. HRMS-ESI (
m/
z) [M + H]
+ calcd for C
8H
7F
3N
2O
5Na 291.0205, found 291.0208.
(S)-2,5-Dioxopyrrolidin-1-yl 2-(2,2,2-trifluoroacetamido)propanoate (TFA-l-Ala-OSu, l-4b): Colorless amorphous mass. [α]D = −46 (c 1.0, CHCl3). IR (neat) ν: 3332, 2999, 1793, 1734 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.39 (d, J = 7.3 Hz, 1H, NH), 5.08–4.97 (m, 1H, CHCH3), 2.86 (s, 4H, 2 × CH2), 1.68 (d, J = 7.3 Hz, 3H, CHCH3) ppm. 13C NMR (67.5 MHz, CDCl3) δ: 168.8 (2 × CO), 167.4, 156.8 (q, 2JCF = 38.4 Hz), 115.6 (q, 1JCF = 287.2 Hz), 46.7, 25.5 (2 × CH2), 17.6 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C9H10F3N2O5 283.0542, found 283.0555.
(R)-2,5-Dioxopyrrolidin-1-yl 2-(2,2,2-trifluoroacetamido)propanoate (TFA-d-Ala-OSu, d-4b): Colorless amorphous mass. [α]D = +46 (c 1.0, CHCl3). IR (neat) ν: 3350, 2999, 1798, 1726 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.73 (d, J = 7.6 Hz, 1H, NH), 5.05–4.94 (m, 1H, CHCH3), 2.85 (s, 4H, 2 × CH2), 1.66 (d, J = 7.3 Hz, 3H, CHCH3) ppm.13C NMR (67.5 MHz, CDCl3) δ: 169.0 (2 × CO), 167.1, 156.8 (q, 2JCF = 38.2 Hz), 115.4 (q, 1JCF = 287.3 Hz), 46.7, 25.4 (2 × CH2), 17.1 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C9H10F3N2O5 283.0542, found 283.0553.
(S)-2,5-Dioxopyrrolidin-1-yl 3-methyl-2-(2,2,2-trifluoroacetamido)butanoate (
TFA-
l-
Val-
OSu,
l-5b) [
24]: Colorless amorphous mass. [α]
D = −22 (
c 1.0, CHCl
3). IR (neat)
ν: 3310, 2979, 1796, 1725 cm
−1.
1H-NMR (270 MHz, CD
3Cl
3) δ: 7.49 (d,
J = 8.9 Hz, 1H, N
H), 4.88 (dd,
J = 8.7, 5.4 Hz, 1H, C
HNH), 2.84 (s, 4H, 2 × CH
2), 2.47–2.34 (m, 1H, C
HCH
3), 1.08 (d,
J = 6.9 Hz, 6H, 2 × CH
3) ppm.
13C-NMR (67.5 MHz, CDCl
3) δ: 168.9 (2 × CO), 166.1, 157.1 (q,
2JCF = 38.2 Hz), 115.5 (q,
1JCF = 287.5 H), 55.9, 31.2, 25.4 (2 × CH
2), 18.3, 17.2 ppm. HRMS-ESI (
m/
z) [M + H]
+ calcd for C
11H
14F
3N
2O
5 311.0855, found 311.0858.
(R)-2,5-Dioxopyrrolidin-1-yl 3-methyl-2-(2,2,2-trifluoroacetamido)butanoate (TFA-d-Val-OSu, d-5b): Colorless amorphous mass. [α]D = +22 (c 1.0, CHCl3). IR (neat) ν: 3298, 2975, 1725 cm−1. 1H-NMR (270 MHz, CD3Cl3) δ: 6.93 (d, J = 8.6 Hz, 1H, NH), 4.95 (dd, J = 8.9, 4.9 Hz, 1H, CHNH), 2.87 (s, 4H, 2 × CH2), 2.53–2.35 (m, 1H, CHCH3), 1.09 (d, J = 6.9 Hz, 6H, 2 × CH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 168.4 (2 × CO), 166.4, 157.0 (q, 2JCF = 38.0 Hz), 115.5 (q, 1JCF = 287.7 Hz), 55.9, 31.7, 25.5 (2 × CH2), 18.4, 17.2 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C11H14F3N2O5 311.0855, found 311.0857.
(S)-2,5-Dioxopyrrolidin-1-yl 4-methyl-2-(2,2,2-trifluoroacetamido)pentanoate (
TFA-
l-
Leu-
OSu,
l-6b) [
24]: Colorless amorphous mass. [α]
D = −42 (
c 1.0, CHCl
3). IR (neat)
ν: 3295, 2967, 1734, 1712 cm
−1.
1H-NMR (270 MHz, CD
3Cl
3) δ: 6.86 (d,
J = 8.6 Hz, 1H, N
H), 5.04 (td,
J = 8.9, 4.9 Hz, 1H, C
HNH), 2.86 (s, 4H 2 × CH
2), 2.00–1.72 (m, 3H, C
H2C
H), 1.02 (d,
J = 2.3 Hz, 3H, CH
3), 1.00 (d,
J = 2.3 Hz, 3H, CH
3) ppm.
13C-NMR (67.5 MHz, CDCl
3) δ 168.6 (2 × CO), 167.2, 156.9 (q,
2JCF = 38.2 Hz), 115.5 (q,
1JCF = 287.7 Hz), 49.3, 41.0, 25.5 (2 × CH
2), 24.7, 22.6, 21.5 ppm. HRMS-ESI (
m/
z) [M + Na]
+ calcd for C
12H
15F
3N
2O
5Na 347.0831, found 347.0832.
(R)-2,5-Dioxopyrrolidin-1-yl 4-methyl-2-(2,2,2-trifluoroacetamido)pentanoate (TFA-d-Leu-OSu, d-6b): Colorless amorphous mass. [α]D = +42 (c 1.0, CHCl3). IR (neat) ν: 3305, 2965, 1733, 1719 cm−1. 1H-NMR (270 MHz, CD3Cl3) δ: 6.97 (br s, 1H, NH), 5.05 (td, J = 8.7, 4.9 Hz, 1H), 2.86 (s, 4H, 2 × CH2), 2.00–1.74 (m, 3H, CH2CH), 1.03–0.98 (m, 6H, 2 × CH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 168.6 (2 × CO), 167.2, 156.9 (q, 2JCF = 38.2 Hz), 115.5 (q, 1JCF = 287.7 Hz), 49.3, 41.0, 25.5 (2 × CH2), 24.7, 22.6, 21.5 ppm. HRMS-ESI (m/z) [M + Na]+ calcd for C12H15F3N2O5Na 347.0831, found 347.0830.
(S)-2,5-Dioxopyrrolidin-1-yl 2-(2,2,2-trifluoroacetamido)pentanoate (TFA-l-Nva-OSu, l-7b): Colorless amorphous mass. [α]D = −27 (c 1.0, CHCl3). IR (neat) ν: 3325, 2962, 1744, 1711 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 6.83 (d, J = 7.3 Hz, 1H, NH), 5.03 (td, J = 8.0, 5.5 Hz, 1H, CHNH), 2.87 (s, 4H, 2 × CH2), 2.17–2.02 (m, 1H, CHCH2), 1.99–1.85 (m, 1H, CHCH2), 1.59–1.40 (m, 2H, CH2CH3), 1.01 (t, J = 7.3 Hz, 3H, CH2CH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 168.6 (2 × CO), 166.9, 156.9 (q, 2JCF = 38.0 Hz), 115.5 (q, 1JCF = 287.7 Hz), 50.7, 34.0, 25.5 (2 × CH2), 18.2, 13.3 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C11H14F3N2O5 311.0855, found 311.0856.
(R)-2,5-Dioxopyrrolidin-1-yl 2-(2,2,2-trifluoroacetamido)pentanoate (TFA-d-Nva-OSu, d-7b): Colorless amorphous mass. [α]D = +27 (c 1.0, CHCl3). IR (neat) ν: 3322, 2962, 1744, 1711 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.11 (d, J = 8.2 Hz, 1H, NH), 5.02 (td, J = 8.1, 5.4 Hz, 1H, CHNH), 2.86 (s, 4H, 2 × CH2), 2.15–2.01 (m, 1H, CHCH2), 1.98–1.84 (m, 1H, CHCH2), 1.60–1.43 (m, 2H, CH2CH3), 1.00 (t, J = 7.3 Hz, 3H, CH2CH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 168.6 (2 × CO), 166.9, 156.9 (q, 2JCF = 38.2 Hz), 115.5 (q, 1JCF = 287.9 Hz), 50.7, 34.0, 25.5 (2 × CH2), 18.2, 13.3 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C11H14F3N2O5 311.0855, found 311.0861.
(S)-2,5-Dioxopyrrolidin-1-yl 2-(2,2,2-trifluoroacetamido)hexanoate (TFA-l-Nle-OSu, l-8b): Colorless amorphous mass. [α]D = −18 (c 1.0, CHCl3). IR (neat) ν: 3332, 2958, 1721, 1703 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.34 (d, J = 8.2 Hz, 1H, NH), 4.98 (td, J = 8.2, 5.3 Hz, 1H, CHNH), 2.85 (s, 4H, 2 × CH2), 2.16–2.03 (m, 1H, CHCH2), 1.99–1.85 (m, 1H, CHCH2), 1.52–1.32 (m, 4H, 2 × CH2), 0.93 (t, J = 7.1 Hz, 3H, CH2CH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 168.8 (2 × CO), 166.8, 156.9 (q, 2JCF = 38.2 Hz), 115.5 (q, 1JCF = 287.7 Hz), 50.8, 31.5, 26.9, 25.4 (2 × CH2), 21.9, 13.5 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C12H16F3N2O5 325.1011, found 325.1021.
(R)-2,5-Dioxopyrrolidin-1-yl 2-(2,2,2-trifluoroacetamido)hexanoate (TFA-d-Nle-OSu, d-8b): Colorless amorphous mass. [α]D = +18 (c 1.0, CHCl3). IR (neat) ν: 3324, 2958, 1724, 1708 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 6.89 (br s, 1H, NH), 5.01 (td, J = 7.8, 5.5 Hz, 1H, CHNH), 2.87 (s, 4H, 2 × CH2), 2.18–2.04 (m, 1H, CHCH2), 2.00–1.86 (m, 1H, CHCH2), 1.52–1.36 (m, 4H, 2 × CH2), 0.94 (t, J = 7.1 Hz, 3H, CH2CH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 168.6 (2 × CO), 166.9, 156.9 (q, 2JCF = 38.4 Hz), 115.5 (q, 1JCF = 287.3 Hz), 50.8, 31.7, 26.8, 25.5 (2 × CH2), 21.9, 13.6 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C12H16F3N2O5 325.1011, found 325.1016.
3.4. General Procedure for the Preparation of TFA-Protected α-Amino Aryl-Ketones
TFA-α-amino acid-OSu (l-/d-1b–l-/d-2b, 3b, l-/d-4b–l-/d-8b, 0.9–1.6 mmol) was suspended in arene. Into the suspension, pulverized AlCl3 (6 equiv.) was added and then stirred at a temperature of 70 °C. The reaction was monitored by the consumption of starting material on TLC. Then, the mixture was poured into an ethyl acetate-H2O two-phase system to quench the reaction. The organic layer was washed with H2O, sat. NaCl, dried over MgSO4, and then evaporated. The crude product was purified by silica column chromatography (ethyl acetate/hexane 1:3 l-/d-1c, l-/d-2c, 3c, and l-/d-4b–l-/d-8b; and diethyl ether/hexane 1:6 l-/d-1d–l-/d-1h).
2,2,2-Trifluoro-N-((2S,3S)-3-methyl-1-oxo-1-phenylpentan-2-yl)acetamide (TFA-l-Ile-Ph, l-1c): Colorless needles. [α]D = +70 (c 2.0, CHCl3). IR (neat) ν: 3317, 3073, 2972, 1722, 1694 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.97 (d, J = 7.4 Hz, 2H, Ar-H), 7.66 (t, J = 7.4 Hz, 1H, Ar-H), 7.53 (t, J = 7.4 Hz, 2H, Ar-H), 5.60 (dd, J = 8.6, 4.3 Hz, 1H, CHNH), 2.10–1.95 (m, 1H, CHCH3), 11.40–1.25 (m, 1H, CH2CH3), 1.12–0.95 (m, 4H, overlap CH2CH3 and CHCH3), 0.82 (t, J = 7.3 Hz, 3H, CH2CH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 197.5, 157.1 (q, 2JCF = 37.4 Hz), 134.7, 134.3, 129.0 (2 × CH), 128.7 (2 × CH), 115.9 (q, 1JCF = 288.1 Hz), 58.4, 38.7, 23.7, 16.2, 11.4 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C14H17F3NO2 288.1211, found 288.1212.
2,2,2-Trifluoro-N-((2R,3R)-3-methyl-1-oxo-1-phenylpentan-2-yl)acetamide (TFA-d-Ile-Ph, d-1c): Colorless needles. [α]D = −70 (c 2.0, CHCl3). IR (neat) ν: 3337, 3069, 2969, 1721, 1699 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.98 (d, J = 7.6 Hz, 2H, Ar-H), 7.66 (t, J = 7.6 Hz, 1H, Ar-H), 7.53 (t, J = 7.6 Hz, 2H, Ar-H), 5.60 (dd, J = 8.6, 4.0 Hz, 1H, CHNH), 2.10–1.95 (m, 1H, CHCH3), 1.40–1.23 (m, 1H, CH2CH3), 1.12–0.95 (m, 4H, overlap CH2CH3 and CHCH3), 0.82 (t, J = 7.3 Hz, 3H, CH2CH3) ppm.13C-NMR (67.5 MHz, CDCl3) δ: 197.5, 157.1 (q, 2JCF = 37.2 Hz), 134.7, 134.3, 129.0 (2 × CH), 128.7 (2 × CH), 115.9 (q, 1JCF = 288.3 Hz), 58.4, 38.7, 23.7, 16.2, 11.4 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C14H17F3NO2 288.1211, found 288.1215.
2,2,2-Trifluoro-N-((2S,3S)-3-methyl-1-oxo-1-(p-tolyl)pentan-2-yl)acetamide (TFA-l-Ile-Ph(4-Me), l-1d): Colorless needles. [α]D = +83 (c 1.0, CHCl3). IR (neat) ν: 302, 3023, 2925, 1700 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.87 (d, J = 8.2 Hz, 2H, Ar-H), 7.32 (d, J = 8.6 Hz, 2H, Ar-H), 5.56 (dd, J = 8.6, 4.3 Hz, 1H, CHNH), 2.45 (s, 3H, CH3), 2.06–1.95 (m, 1H, CHCH3), 1.39–1.26 (m, 1H, CH2CH3), 1.11–0.94 (m, 4H, overlap CH2CH3 and CHCH3), 0.82 (t, J = 7.3 Hz, 3H, CH2CH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 197.0, 157.1 (q, 2JCF = 37.2 Hz), 145.5, 132.1 (2 × CH), 129.7 (2 × CH), 128.8, 115.9 (q, 1JCF = 287.9 Hz), 58.3, 38.9, 23.7, 21.8, 16.2, 11.4 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C15H19F3NO2 302.1368 found 302.1353.
2,2,2-Trifluoro-N-((2S,3S)-1-(4-methoxyphenyl)-3-methyl-1-oxopentan-2-yl)acetamide (TFA-l-Ile-Ph(4-OMe), l-1e): Colorless oil. [α]D = +66 (c 1.0, CHCl3). IR (neat) ν: 3320, 3079, 2934, 1726, 1675 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.96 (d, J = 8.9 Hz, 2H, Ar-H), 6.99 (d, J = 8.9 Hz, 2H, Ar-H), 5.53 (dd, J = 8.7, 4.5 Hz, 1H, CHNH), 3.90 (s, 3H, OCH3), 2.08–1.93 (m, 1H, CHCH3), 1.42-1.20 (m, 1H, CH2CH3), 1.10–0.95 (m, 4H, overlap CH2CH3 and CHCH3), 0.82 (t, J = 7.4 Hz, 3H, CH2CH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 195.6, 164.5, 157.0 (q, 2JCF = 37.2 Hz), 131.1 (2 × CH), 127.5, 115.9 (q, 1JCF = 288.6 Hz), 114.2 (2 × CH), 58.0, 55.6, 39.0, 23.7, 16.2, 11.4 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C15H18F3NO3Na 340.1136 found 340.1145.
N-((2S,3S)-1-(3,4-Dimethylphenyl)-3-methyl-1-oxopentan-2-yl)-2,2,2-trifluoroacetamide (TFA-l-Ile-Ph(3,4-Me), l-1f): Colorless needles. [α]D = +82 (c 1.0, CHCl3). IR (neat) ν: 3343, 3075, 2979, 1742, 1691 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.71 (d, J = 11.2 Hz, 2H, Ar-H), 7.29 (s, 1H, Ar-H), 5.56 (dd, J = 8.7, 4.1 Hz, 1H, CHNH), 2.35 (s, 6H, 2 × CH3), 2.06–1.95 (m, 1H, CHCH3), 1.40–1.26 (m, 1H, CH2CH3), 1.08–0.93 (m, 4H, overlap CH2CH3 and CHCH3), 0.81 (t, J = 7.4 Hz, 3H, CH2CH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 197.2, 157.1 (q, 2JCF = 37.2 Hz), 144.3, 137.6, 132.5, 130.2, 129.7, 126.5, 115.9 (q, 1JCF = 287.9 Hz), 58.3, 38.9, 23.7, 20.1, 19.8, 16.2, 11.4 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C16H21F3NO2 316.1524, found 316.1506.
N-((2S,3S)-1-(2,4-Dimethylphenyl)-3-methyl-1-oxopentan-2-yl)-2,2,2-trifluoroacetamide (TFA-l-Ile-Ph(2,4-Me), l-1g): Colorless needles. [α]D = +49 (c 0.25, CHCl3). IR (neat) ν: 3294, 3097, 2969, 1714, 1685 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.63 (d, J = 8.6 Hz, 1H, Ar-H), 7.14-7.02 (m, 2H, Ar-H), 5.49 (dd, J = 8.4, 4.1 Hz, 1H, CHNH), 2.49 (s, 3H, CH3), 2.38 (s, 3H, CH3), 1.99–1.87 (m, 1H, CHCH3), 1.32–1.19 (m, 1H, CH2CH3), 1.07–0.87 (m, 4H, overlap CH2CH3 and CHCH3), 0.81 (t, J = 7.3 Hz, 3H, CH2CH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 199.9, 157.1 (q, 2JCF = 37.4 Hz), 143.2, 139.5, 133.3, 132.2, 129.4, 126.6, 115.9 (q, 1JCF = 288.3 Hz), 59.8, 38.5, 24.1, 21.2, 21.0, 15.9, 11.2 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C16H21F3NO2 316.1524, found 316.1526.
N-((2S,3S)-1-(2,5-Dimethylphenyl)-3-methyl-1-oxopentan-2-yl)-2,2,2-trifluoroacetamide (TFA-l-Ile-Ph(2,5-Me), l-1h): Colorless needles. [α]D = +68 (c 1.0, CHCl3). IR (neat) ν: 3302, 3024, 2934, 1714, 1686, 1567 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.5 (s, 1H, Ar-H), 7.3 (d, J = 7.6 Hz, 1H, Ar-H), 7.2 (d, J = 7.6 Hz, 1H, Ar-H), 5.5 (dd, J = 8.6, 4.0 Hz, 1H, CHNH), 2.4 (s, 3H, CH3), 2.4 (s, 3H, CH3), 2.0–1.8 (m, 1H, CHCH3), 1.4–1.2 (m, 1H, CH2CH3), 1.1–0.9 (m, 3H overlap CH2CH3 and CHCH3), 0.8 (t, J = 7.3 Hz, 3H, CH2CH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 200.7, 157.1 (q, 2JCF = 37.2 Hz), 135.9, 135.6, 135.0, 133.3, 132.3, 129.3, 115.9 (q, 1JCF = 287.9 Hz), 60.1, 38.4, 24.1, 20.7, 20.3, 15.9, 11.3 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C16H21F3NO2 316.1524, found 316.1530.
2,2,2-Trifluoro-N-((2S,3R)-3-methyl-1-oxo-1-phenylpentan-2-yl)acetamide (TFA-l-allo-Ile-Ph, l-2c): Colorless needles. [α]D = +79 (c 2.0, CHCl3). IR (neat) ν: 3335, 3068, 2971, 1741, 1693 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.98 (d, J = 7.6 Hz, 2H, Ar-H), 7.65 (t, J = 7.6 Hz, 1H, Ar-H), 7.53 (t, J = 7.6 Hz, 2H, Ar-H), 5.74 (dd, J = 8.9, 3.0 Hz, 1H, CHNH), 2.09–1.94 (m, 1H, CHCH3), 1.62–1.49 (m, 1H, CH2CH3), 1.38–1.21 (m, 1H, CH2CH3), 1.06 (t, J = 7.3 Hz, 3H, CH2CH3), 0.76 (d, J = 6.9 Hz, 3H, CHCH3) ppm. 13C NMR (67.5 MHz, CDCl3) δ: 197.1, 157.3 (q, 2JCF = 37.1 Hz), 134.4, 134.0, 129.1 (2 × CH), 128.7 (2 × CH), 115.9 (q, 1JCF = 287.7 Hz), 57.1, 38.7, 27.3, 13.4, 12.0 ppm. HRMS-ESI (m/z) [M + Na]+ calcd for C14H16F3NO2Na 310.1031, found 310.1039.
2,2,2-Trifluoro-N-((2R,3S)-3-methyl-1-oxo-1-phenylpentan-2-yl)acetamide (TFA-d-allo-Ile-Ph, d-2c): Colorless needles. [α]D = −79 (c 2.0, CHCl3). IR (neat) ν: 3331, 3067, 2969, 1738, 1692 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.98 (d, J = 7.4 Hz, 2H, Ar-H), 7.66 (t, J = 7.4 Hz, 1H, Ar-H), 7.53 (t, J = 7.4 Hz, 2H, Ar-H), 5.74 (dd, J = 8.7, 2.8 Hz, 1H, CHNH), 2.07–1.94 (m, 1H, CHCH3), 1.66–1.48 (m, 1H, CH2CH3), 1.38–1.21 (m, 1H, CH2CH3), 1.06 (t, J = 7.3 Hz, 3H, CH2CH3), 0.76 (d, J = 6.9 Hz, 3H, CHCH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 197.1, 157.3 (q, 2JCF = 37.4 Hz), 134.4, 134.1, 129.1 (2 × CH), 128.7 (2 × CH), 115.9 (q, 1JCF = 287.9 Hz), 57.1, 38.7, 27.3, 13.4, 12.0 ppm. HRMS-ESI (m/z) [M + Na]+ calcd for C14H16F3NO2Na 310.1031, found 310.1040.
2,2,2-Trifluoro-N-(2-oxo-2-phenylethyl)acetamide (
TFA-
Gly-
Ph,
3c) [
2]: Colorless oil. IR (neat)
ν: 3327, 3103, 2927, 1733, 1703 cm
−1.
1H-NMR (270 MHz, CDCl
3) δ: 7.99 (d,
J = 7.4 Hz, 2H, Ar-H), 7.68 (t,
J = 7.4 Hz, 1H, Ar-H), 7.54 (t,
J = 7.4 Hz, 2H, Ar-H), 4.83 (d,
J = 4.3 Hz, 2H, C
H2NH) ppm.
13C NMR (67.5 MHz, CDCl
3) δ: 192.1, 157.1 (q,
2JCF = 37.6 Hz), 134.6, 133.6, 129.1 (2 × CH), 127.9 (2 × CH), 115.7 (q,
1JCF = 287.3 Hz), 46.1 ppm. HRMS-ESI (
m/
z) [M + H]
+ calcd for C
10H
9F
3NO
2 232.0585, found 232.0595.
(S)-2,2,2-trifluoro-N-(1-oxo-1-phenylpropan-2-yl)acetamide (
TFA-
l-
Ala-
Ph,
l-
4c) [
7,
12,
26]: Colorless oil. = −7.0 (
c 1.0, CHCl
3). Lit. [
26] [α]
D = −8.6 (
c 0.17, CHCl
3). IR (neat)
ν: 3331, 3070, 2991, 1738, 1701 cm
−1.
1H-NMR (270 MHz, CDCl
3) δ: 7.99 (d,
J = 7.4 Hz, 2H, Ar-H), 7.67 (t,
J = 7.4 Hz, 1H, Ar-H), 7.54 (t,
J = 7.4 Hz, 2H, Ar-H), 5.60–5.50 (m, 1H, C
HCH
3), 1.53 (d,
J = 7.3 Hz, 3H, CHC
H3) ppm.
13C NMR (67.5 MHz, CDCl
3) δ: 197.0, 156.5 (q,
2JCF = 37.6 Hz), 134.5, 132.9, 129.1 (2 × CH), 128.8 (2 × CH), 115.7 (q,
1JCF = 287.3 Hz), 50.8, 19.2 ppm. HRMS-ESI (
m/
z) [M + H]
+ calcd for C
11H
11F
3NO
2 246.0742, found 246.0748.
(R)-2,2,2-Trifluoro-N-(1-oxo-1-phenylpropan-2-yl)acetamide (TFA-d-Ala-Ph, d-4c): Colorless oil. [α]D = +7.0 (c 1.0, CHCl3). IR (neat) ν: 3337, 3091, 2948, 1725, 1700 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.99 (d, J = 7.3 Hz, 2H, Ar-H), 7.67 (t, J = 7.4 Hz, 1H, Ar-H), 7.54 (t, J = 7.4 Hz, 2H, Ar-H), 5.60–5.49 (m, 1H, CHCH3), 1.53 (d, J = 6.9 Hz, 3H, CHCH3) ppm. 13C NMR (67.5 MHz, CDCl3) δ: 197.0, 156.5 (q, 2JCF = 37.6 Hz), 134.5, 132.9, 129.1 (2 × CH), 128.8 (2 × CH), 115.7 (q, 1JCF = 287.7 Hz), 50.8, 19.2 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C11H11F3NO2 246.0742, found 246.0750.
(S)-2,2,2-Trifluoro-N-(3-methyl-1-oxo-1-phenylbutan-2-yl)acetamide (
TFA-
l-
Val-
Ph,
l-
5c) [
7]: Colorless oil. [α]
D = +83 (
c 1.0, CHCl
3). IR (neat)
ν: 3347, 3071, 2972, 1728, 1679 cm
−1. (270 MHz, CDCl
3) δ: 7.99 (d,
J = 7.7 Hz, 2H, Ar-H), 7.66 (t,
J = 7.7 Hz, 1H, Ar-H), 7.53 (t,
J = 7.7 Hz, 2H, Ar-H), 7.32 (br s, 1H, N
H), 5.61 (dd,
J = 8.6, 4.0 Hz, 1H, C
HNH), 2.39–2.23 (m, 1H, C
HCH
3), 1.07 (d,
J = 6.9 Hz, 3H, CHC
H3), 0.80 (d,
J = 6.9 Hz, 3H, CHC
H3) ppm.
13C-NMR (67.5 MHz, CDCl
3) δ: 197.2, 157.3 (q,
2JCF = 37.6 Hz), 134.4, 129.1 (2 × CH), 128.7 (2 × CH), 115.9 (q,
1JCF = 287.7 Hz), 58.6, 32.2, 20.0, 16.4 ppm. HRMS-ESI (
m/
z) [M + H]
+ calcd for C
13H
15F
3NO
2 274.1055, found 274.1057.
(R)-2,2,2-Trifluoro-N-(3-methyl-1-oxo-1-phenylbutan-2-yl)acetamide (TFA-d-Val-Ph, d-5c): Colorless oil. [α]D = −83 (c 1.0, CHCl3). IR (neat) ν: 3344, 3070, 2972, 1720, 1672 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.99 (d, J = 7.3 Hz, 2H, Ar-H), 7.66 (t, J = 7.3 Hz, 1H, Ar-H), 7.53 (t, J = 7.3 Hz, 2H, Ar-H), 7.30 (br s, 1H, NH), 5.61 (dd, J = 8.7, 3.8 Hz, 1H, CHNH), 2.39–2.22 (m, 1H, CHCH3), 1.07 (d, J = 6.9 Hz, 3H, CHCH3), 0.79 (d, J = 6.9 Hz, 3H, CHCH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 197.2, 157.3 (q, 2JCF = 36.7 Hz), 134.4, 129.1 (2 × CH), 128.7 (2 × CH), 115.9 (q, 1JCF = 287.7 Hz), 58.6, 32.1, 19.9, 16.3 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C13H15F3NO2 274.1055, found 274.1057.
(S)-2,2,2-Trifluoro-N-(4-methyl-1-oxo-1-phenylpentan-2-yl)acetamide (TFA-l-Leu-Ph, l-6c): Colorless oil. [α]D = +26 (c 2.0, CHCl3). IR (neat) ν: 3334, 3092, 2963, 1726, 1683 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.98 (d, J = 7.3 Hz, 2H, Ar-H), 7.66 (t, J = 7.4 Hz, 1H, Ar-H), 7.54 (t, J = 7.6 Hz, 2H, Ar-H), 5.68 (td, J = 8.9, 2.6 Hz, 1H, CHNH), 1.79–1.49 (m, 3H, CH2CH), 1.10 (d, J = 5.9 Hz, 3H, CHCH3), 0.89 (d, J = 6.3 Hz, 3H, CHCH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 197.4, 156.9 (q, 2JCF = 37.4 Hz), 134.4, 133.6, 129.1 (2 × CH), 128.7 (2 × CH), 115.8 (q, 1JCF = 287.7 Hz), 52.9, 42.7, 25.1, 23.3, 21.7 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C14H17F3NO2 288.1211, found 288.1214.
(R)-2,2,2-Trifluoro-N-(4-methyl-1-oxo-1-phenylpentan-2-yl)acetamide (TFA-d-Leu-Ph, d-6c): Colorless oil. [α]D = −26 (c 2.0, CHCl3). IR (neat) ν: 3335, 3094, 2931, 1731, 1685 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.98 (d, J = 7.3 Hz, 2H, Ar-H), 7.66 (t, J = 7.3 Hz, 1H, Ar-H), 7.54 (t, J = 7.3 Hz, 2H, Ar-H), 5.68 (td, J = 9.1, 2.5 Hz, 1H, CHNH), 1.79–1.53 (m, 3H, CH2CH), 1.10 (d, J = 5.9 Hz, 3H, CHCH3), 0.89 (d, J = 5.9 Hz, 3H, CHCH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 197.4, 156.9 (q, 2JCF =37.4 Hz), 134.4, 133.6, 129.1 129.1 (2 × CH), 128.7 129.1 (2 × CH), 115.8 (q, 1JCF = 287.7 Hz), 52.9, 42.6, 25.1, 23.2, 21.7 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C14H17F3NO2 288.1211, found 288.1220.
(S)-2,2,2-Trifluoro-N-(1-oxo-1-phenylpentan-2-yl)acetamide (TFA-l-Nva-Ph, l-7c): Colorless oil. [α]D = +46 (c 1.0, CHCl3). IR (neat) ν: 3341, 3074, 2979, 1733 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.98 (d, J = 7.3 Hz, 2H, Ar-H), 7.67 (t, J = 7.3 Hz, 1H, Ar-H), 7.54 (t, J = 7.3 Hz, 2H, Ar-H), 7.44 (br s, 1H, NH), 5.62 (td, J = 7.4, 4.5 Hz, 1H, CHNH), 2.08–1.95 (m, 1H, CHCH2), 1.76–1.62 (m, 1H, CHCH2), 1.48–1.17 (m, 2H, CH2CH3) 0.90 (t, J = 7.3 Hz, 3H, CH2CH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 197.0, 156.8 (q, 2JCF = 37.1 Hz), 134.5, 133.6, 129.1 (2 × CH), 128.7 (2 × CH), 115.8 (q, 1JCF = 286.8 Hz), 54.4, 35.2, 18.0, 13.7 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C13H15F3NO2 274.1055, found 274.1064.
(R)-2,2,2-Trifluoro-N-(1-oxo-1-phenylpentan-2-yl)acetamide (TFA-d-Nva-Ph, d-7c): Colorless oil. [α]D = −46 (c 1.0, CHCl3). IR (neat) ν: 3339, 3073, 2977, 1732 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.99 (d, J = 7.4 Hz, 2H, Ar-H), 7.67 (t, J = 7.4 Hz, 1H, Ar-H), 7.54 (t, J = 7.4 Hz, 2H, Ar-H), 7.45 (br s, 1H, NH), 5.62 (td, J = 7.3, 4.4 Hz, 1H, CHNH), 2.08–1.95 (m, 1H, CHCH2), 1.76–1.62 (m, 1H, CHCH2), 1.48–1.13 (m, 2H, CH2CH3), 0.90 (t, J = 7.3 Hz, 3H, CH2CH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 197.0, 156.8 (q, 2JCF = 37.4 Hz), 134.5, 133.6, 129.1 (2 × CH), 128.7 (2 × CH), 115.8 (q, 1JCF = 288.3 Hz), 54.4, 35.2, 18.1, 13.7 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C13H15F3NO2 274.1055, found 274.1058.
(S)-2,2,2-Trifluoro-N-(1-oxo-1-phenylhexan-2-yl)acetamide (TFA-l-Nle-Ph, l-8c): Colorless oil. [α]D = +60 (c 0.5, CHCl3). IR (neat) ν: 3326, 3068, 2960, 1728, 1687 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.98 (d, J = 7.4 Hz, 2H, Ar-H), 7.66 (t, J = 7.4 Hz, 1H, Ar-H), 7.53 (t, J = 7.4 Hz, 2H, Ar-H), 7.46 (br s, 1H, NH), 5.61 (td, J = 7.3, 4.6 Hz, 1H, CHNH), 2.12–1.98 (m, 1H, CHCH2), 1.77–1.63 (m, 1H, CHCH2), 1.41–1.15 (m, 4H, 2 × CH2), 0.83 (t, J = 6.9 Hz, 3H, CH2CH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 197.0, 156.8 (q, 2JCF = 37.4 Hz), 134.4, 133.6, 129.0 (2 × CH), 128.6 (2 × CH), 115.8 (q, 1JCF = 287.7 Hz), 54.5, 32.7, 26.7, 22.2, 13.6 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C14H17F3NO2 288.1211, found 288.1224.
(R)-2,2,2-Trifluoro-N-(1-oxo-1-phenylhexan-2-yl)acetamide (TFA-d-Nle-Ph, d-8c): Colorless oil. [α]D = −60 (c 0.5, CHCl3). IR (neat) ν: 3327, 3069, 2961, 1732, 1691 cm−1. 1H-NMR (270 MHz, CDCl3) δ: 7.99 (d, J = 7.3 Hz, 2H, Ar-H), 7.67 (t, J = 7.3 Hz, 1H, Ar-H), 7.54 (t, J = 7.3 Hz, 2H, Ar-H), 7.47 (br s, 1H, NH), 5.61 (td, J = 7.3, 4.6 Hz, 1H, CHNH), 2.12–1.98 (m, 1H, CHCH2), 1.78–1.63 (m, 1H, CHCH2), 1.39–1.14 (m, 4H, 2 × CH2), 0.83 (t, J = 6.9 Hz, 3H, CH2CH3) ppm. 13C-NMR (67.5 MHz, CDCl3) δ: 197.0, 156.8 (q, 2JCF = 37.4 Hz), 134.4, 133.6, 129.1 (2 × CH), 128.6 (2 × CH), 115.8 (q, 1JCF = 287.7 Hz), 54.5, 32.7, 26.7, 22.2, 13.6 ppm. HRMS-ESI (m/z) [M + H]+ calcd for C14H17F3NO2 288.1211, found 288.1216.