The Application of Ultra-High-Performance Liquid Chromatography Coupled with a LTQ-Orbitrap Mass Technique to Reveal the Dynamic Accumulation of Secondary Metabolites in Licorice under ABA Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Reagents
2.2. Sample Processing
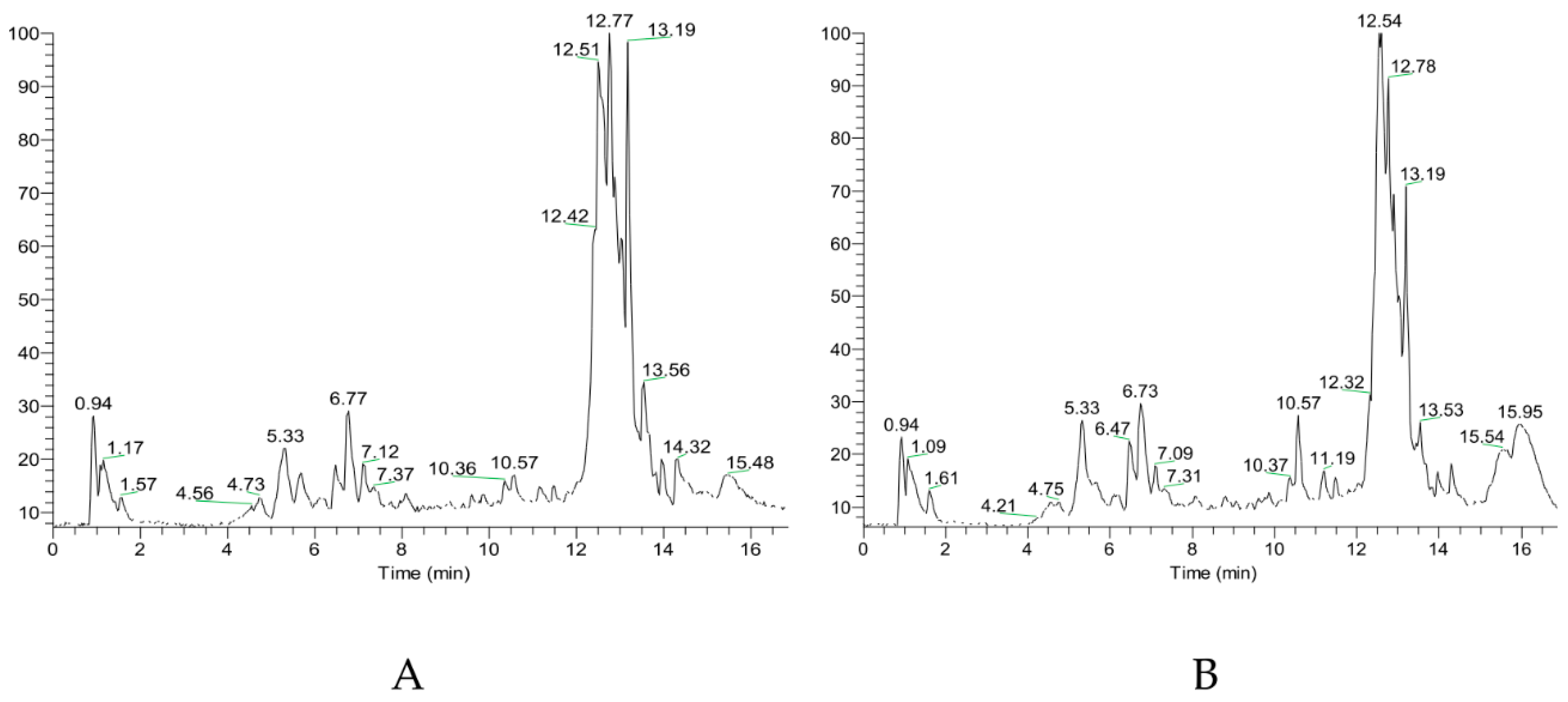
2.3. Chromatographic Conditions
2.4. MS Conditions
2.5. Statistical Analysis
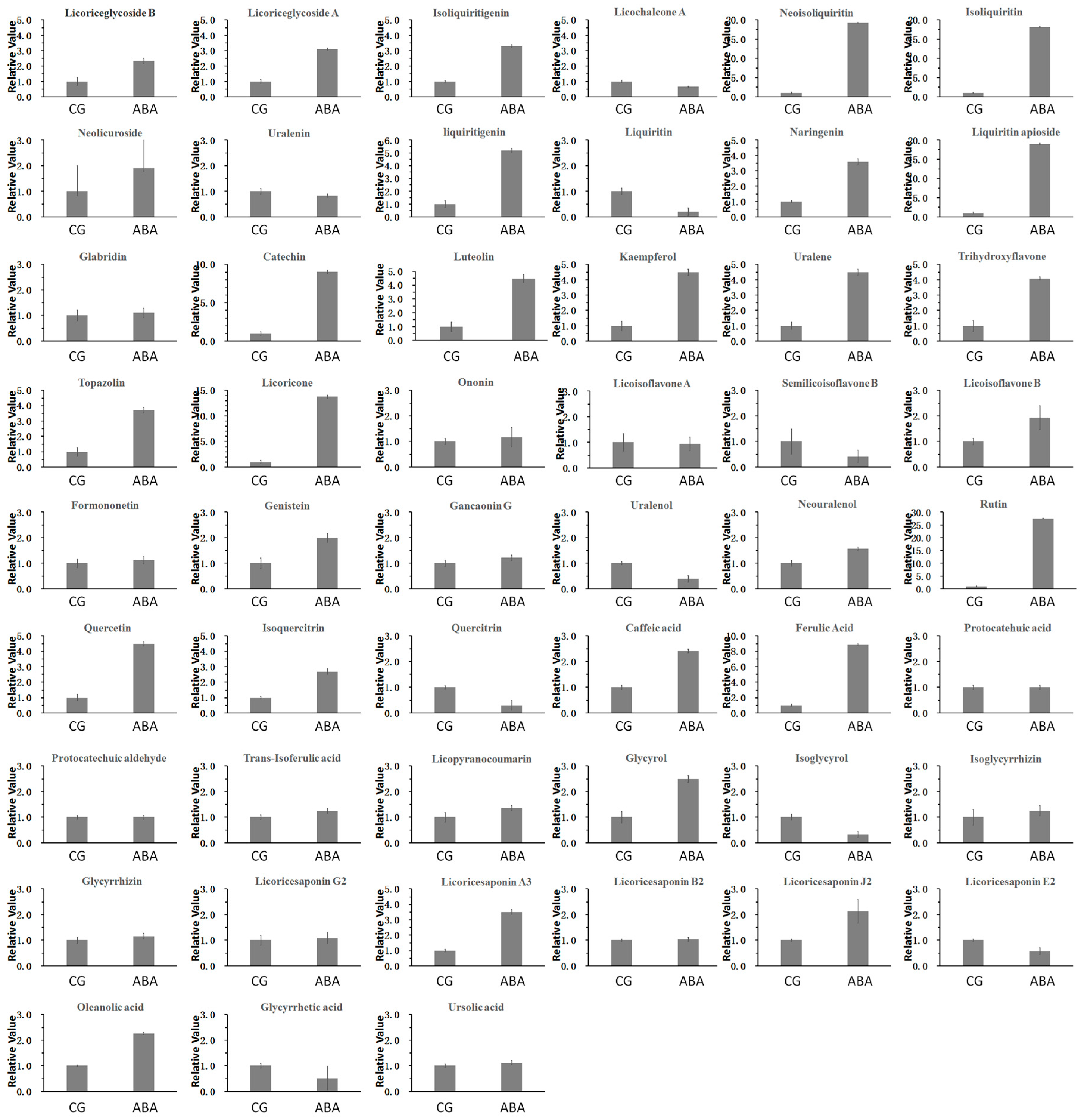
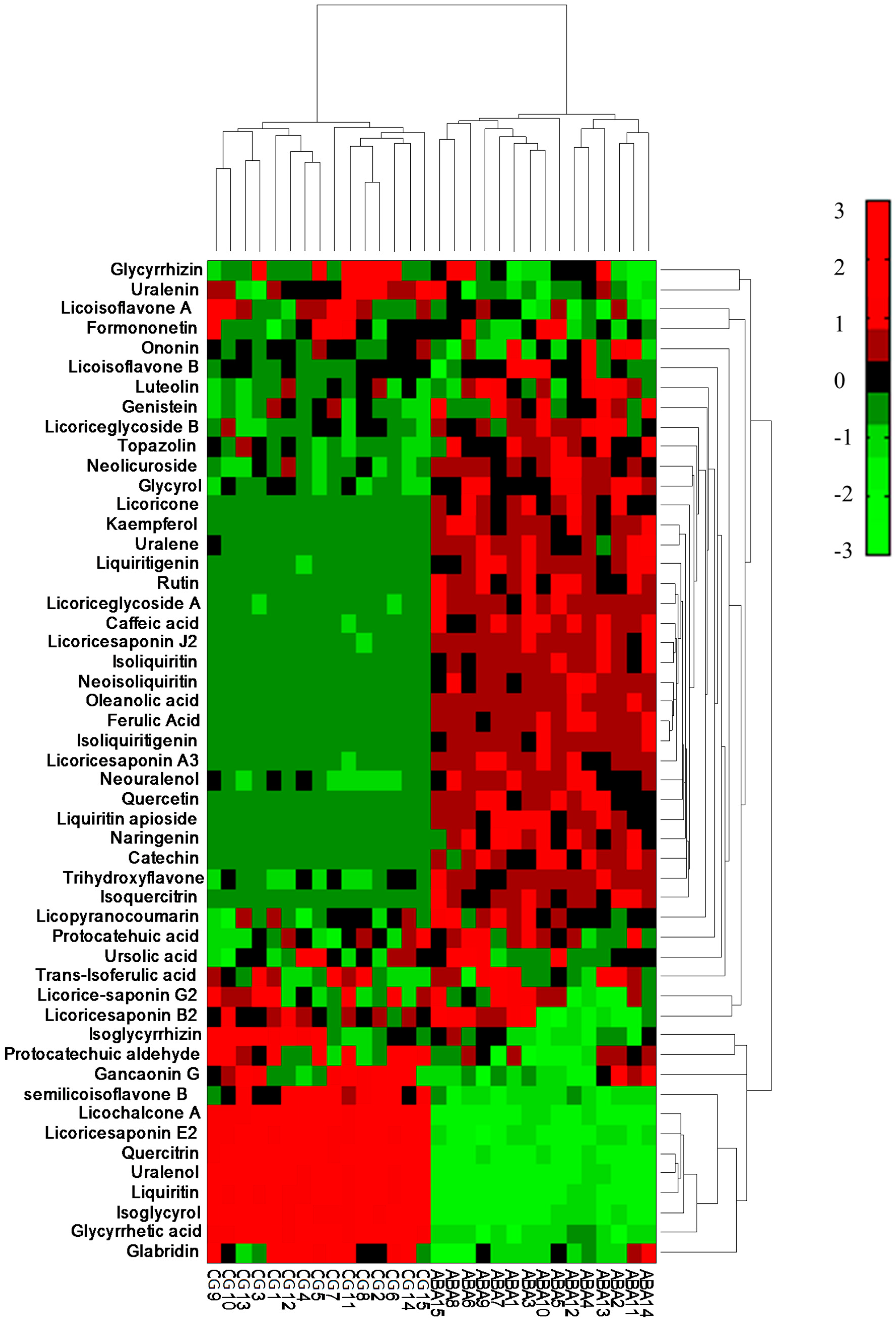
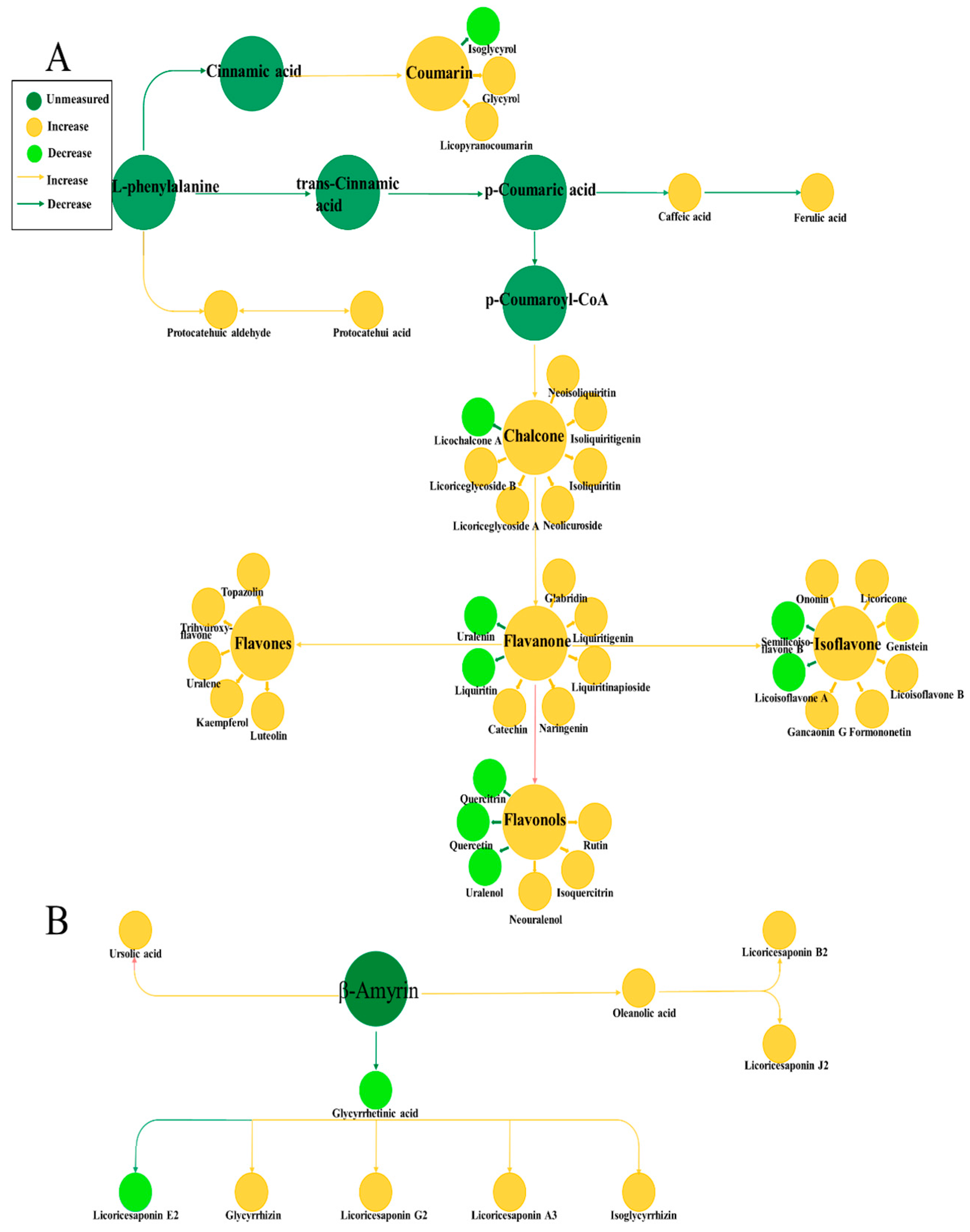
3. Result and Discussion
3.1. Flavonoids and Triterpenes in the Targeted Metabolomics Study
3.2. Comparison of Content of Licorice Samples in Two Groups
3.3. Principal Component Analysis
3.4. Partial Least Squares Discriminant Analysis
3.5. Analysis of Metabolic Pathways of Two Compounds
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, X.; Zhang, H.; Chen, L.; Shan, L.; Fan, G.; Gao, X. Liquorice, a unique “guide drug” of traditional Chinese medicine: A review of its role in drug interactions. Ethnopharmacol. J. 2013, 3, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Davituliani, V.V.; Korotkov, V.P.; Nikolaev, A.M. The physicochemical properties of a liquid extract of licorice root. Pharm. Chem. J. 1969, 3, 233–234. [Google Scholar] [CrossRef]
- Zhang, J.-Z.; Gao, W.-Y.; Gao, Y.; Liu, D.-L.; Huang, L.-Q. Analysis of influences of spaceflight on chemical constituents in licorice by HPLC–ESI-MS/MS. Acta Physiol. Plant. 2011, 33, 2511–2520. [Google Scholar] [CrossRef]
- Yang, R.; Wang, L.-Q.; Liu, Y. Antitumor Activities of Widely-used Chinese Herb—Licorice. Chin. Herb. Med. 2014, 6, 274–281. [Google Scholar] [CrossRef]
- Bode, A.M.; Dong, Z. Chemopreventive Effects of Licorice and Its Components. Curr. Pharmacol. Rep. 2015, 1, 60–71. [Google Scholar] [CrossRef]
- Okuda-Tanino, A.; Sugawara, D.; Tashiro, T.; Iwashita, M.; Obara, Y.; Moriya, T.; Nakahata, N. Licochalcones extracted from Glycyrrhiza inflata inhibit platelet aggregation accompanied by inhibition of COX-1 activity. PLoS ONE 2017, 12, e0173628. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Tuvshintogtokh, I.; Mandakh, B.; Munkhjargal, B.; Uto, T.; Morinaga, O.; Shoyama, Y. Screening of Glycyrrhiza uralensis Fisch. ex DC. containing high concentrations of glycyrrhizin by Eastern blotting and enzyme-linked immunosorbent assay using anti-glycyrrhizin monoclonal antibody for selective breeding of licorice. J. Nat. Med. 2014, 68, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Sheng, D.; Duan, Q.; Liang, X.; Liang, Z.; Liu, Y. PEG and ABA trigger the burst of reactive oxygen species to increase Tanshinone production in Salvia miltiorrhiza Hairy roots. J. Plant Growth Regul. 2012, 31, 579–587. [Google Scholar] [CrossRef]
- Yu, X.; Qiao, X.; Zang, J.; Li, Y.; Liu, Y.; Liu, Y.; Sheng, C. An ultrahigh-performance liquid chromatography method with electrospray ionization tandem mass spectrometry for simultaneous quantification of five phytohormones in medicinal plant Glycyrrhiza uralensis under abscisic acid stress. J. Nat. Med. 2015, 3, 278–286. [Google Scholar]
- Dar, N.A.; Amin, I.; Wani, W.; Wani, S.A.; Shikari, A.B.; Wani, S.H.; Masoodi, K.Z. Abscisic acid: A key regulator of abiotic stress tolerance in plants. Plant Gene 2017. [Google Scholar] [CrossRef]
- Li, W.D.; Hou, J.L.; Wang, W.Q.; Tang, X.M.; Liu, C.L.; Xing, D. Effect of water deficit on biomass production and accumulation of secondary metabolites in roots of Glycyrrhiza uralensis. Russ. J. Plant Physiol. 2011, 58, 538–542. [Google Scholar]
- Cheng, Q.; Shou, L.; Chen, C.; Shi, S.; Zhou, M. Application of ultra-high-performance liquid chromatography coupled with LTQ-Orbitrap mass spectrometry for identification, confirmation and quantitation of illegal adulterated weight-loss drugs in plant dietary supplements. J. Chromatogr. B 2017, 1064, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.M.A.; Elbashir, A.A.; Schmitz, O.J. Determination of acrylamide in Sudanese food by high performance liquid chromatography coupled with LTQ Orbitrap mass spectrometry. J. Food Chem. 2015, 176, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Cai, W.; Gao, W.; Liu, C. A novel glucuronosyltransferase has an unprecedented ability to catalyse continuous two-step glucuronosylation of glycyrrhetinic acid to yield glycyrrhizin. New Phytol. 2016, 212, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.-Q.; Xu, J.-D.; Zhu, H.; Kong, M.; Zhang, G.-H.; Li, S.-L. UPLC/ESI-QTOF-MS-based metabolomics survey on the toxicity of triptolide and detoxication of licorice. Chin. J. Nat. Med. 2017, 15, 474–480. [Google Scholar] [CrossRef]
- Xie, G.X.; Ni, Y.; Su, M.M.; Zhang, Y.Y.; Zhao, A.H.; Gao, X.F.; Jia, W. Application of ultra-performance LC-TOF MS metabolite profiling techniques to the analysis of medicinal Panax herbs. Metabolomics 2008, 4, 248–260. [Google Scholar] [CrossRef]
- Shen, J.; Pu, Z.-J.; Kai, J.; Kang, A.; Tang, Y.-P.; Shang, L.-L.; Duan, J.-A. Comparative metabolomics analysis for the compatibility and incompatibility of kansui and licorice with different ratios by UHPLC-QTOF/MS and multivariate data analysis. J. Chromatogr. B 2017, 1057, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.J.; Qiu, S.-X.; Wufuer, A.; Shum, L. Simultaneous determination of glycyrrhizin, a marker component in radix Glycyrrhizae, and its major metabolite glycyrrhetic acid in human plasma by LC–MS/MS. J. Chromatogr. B 2005, 814, 201–207. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wu, H.; Nie, J.; Yan, P.; Yang, T.-B.; Yang, Z.-Y.; Pei, R. Accurate recognition and Feature qualify for Flavonoid extracts from Liang-wai Gan Cao by Liquid Chromatography-High Resolution-Mass Spectrometry and Computational MS/MS Fragmentation. J. Pharm. Biomed. Anal. 2017, 146, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Chu, J.; Sun, X.; Wang, J.; Yan, C. Simple, rapid, and simultaneous assay of multiple carboxyl containing phytohormones in wounded tomatoes by UPLC-MS/MS using single SPE purification and isotope dilution. Anal. Sci. 2012, 28, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Giannarelli, S.; Muscatello, B.; Bogani, P.; Spiriti, M.M.; Buiatti, M.; Fuoco, R. Comparative determination of some phytohormones in wild-type and genetically modified plants by gas chromatography-mass spectrometry and high-performance liquid chromatography-tandem mass spectrometry. Anal. Biochem. 2010, 398, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, S.; Chen, W.; Qu, L.; Gai, Y.; Jiang, X. Simultaneous determination of 24 or more acidic and alkaline phytohormones in femtomole quantities of plant tissues by high-performance liquid chromatography-electrospray ionization-ion trap mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Sakakibara, H. Highly sensitive high-throughput profiling of six phytohormones using MS-probe modification and liquid chromatography-tandem mass spectrometry. Methods Mol. Biol. 2012, 918, 151–164. [Google Scholar] [PubMed]
- Wu, L.; Liang, W.; Chen, W.; Li, S.; Cui, Y.; Qi, Q.; Zhang, L. Screening and Analysis of the Marker Components in Ganoderma lucidum by HPLC and HPLC-MSn with the Aid of Chemometrics. Molecules 2017, 22, 584. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Chen, W.; Wu, L.; Li, S.; Qi, Q.; Cui, Y.; Liang, L.; Ye, T.; Zhang, L. Quality evaluation and chemical markers screening of salvia miltiorrhiza Bge. (Danshen) based on HPLC fingerprints and HPLC-MSn coupled with chemometrics. Molecules 2017, 22, 478. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.T.; Kong, D.D.; Luo, J.Y.; Kong, W.J.; Guo, W.Y.; Yang, M.H. Quantitative and fingerprinting analysis of Atractylodes rhizome based on gas chromatography with flame ionization detection combined with chemometrics. J. Sep. Sci. 2016, 39, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, R.; Yuan, B.; Liu, Y.; Liu, C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm. Sin. B 2015, 5, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Hu, J.; Li, Z.Y.; Qin, X.M.; Zhang, L.Z.; Guo, X.Q. Species classification and quality assessment of Chaihu (Radix Bupleuri) based on high-performance liquid chromatographic fingerprint and combined chemometrics methods. Arch. Pharm. Res. 2011, 34, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Shibano, M.; Nukui, H.; Kita, S.; Kusano, G.; Shibata, T.; Watanabe, H.; Ohashi, H. Studies on natural medicines index compounds for HPLC Glycyrrhiza macedonica. Nat. Med. 1999, 53, 166–172. [Google Scholar]
- Lunn, J.E.; Delorge, I.; Figueroa, C.M.; van Dijck, P.; Stitt, M. Trehalose metabolism in plants. Plant J. Cell Mol. Biol. 2014, 79, 544–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nägele, T.; Doerfler, H.; Fragner, L.; Chaturvedi, P.; Nukarinen, E.; Bellaire, A.; Huber, W.; Weiszmann, J.; Engelmeier, D.; et al. System level analysis of cacao seed ripening reveals a sequential interplay of primary and secondary metabolism leading to polyphenol accumulation and preparation of stress resistance. Plant J. Cell Mol. Biol. 2016, 87, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhang, J. Effect of Abscisic acid on active oxygen species, antioxidative defence system, and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.K.; Gray, D.J.; Lu, J.; Gu, L. Effects of exogenous abscisic acid on antioxidant capacities, anthocyanins, and flavonol contents of muscadine grape (Vitis rotundifolia) skins. Food Chem. 2010, 126, 982–988. [Google Scholar] [CrossRef]
- Fatima, Z.; Bano, A. The effects of plant growth regulators and temperate shock on IAA-oxidase activity of roots and endogenous ABA level of leaves. Pak. J. Biol. 1999, 2, 1601–1603. [Google Scholar]
- Liu, C.-L.; Xu, P.; Xu, L.-P.; Wang, M.-Y.; Yu, P. Study on correlation between synthesis of liquiritin and abscisic acid in Glycyrrhiza uralensis Fisch. under drought stress. CJTCMP 2011, 26, 2052–2055. [Google Scholar]
Sample Availability: Samples of the compounds in this paper are available from the authors. |


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Xu, G.; Ren, G.; Sun, Y.; Huang, Y.; Liu, C. The Application of Ultra-High-Performance Liquid Chromatography Coupled with a LTQ-Orbitrap Mass Technique to Reveal the Dynamic Accumulation of Secondary Metabolites in Licorice under ABA Stress. Molecules 2017, 22, 1742. https://doi.org/10.3390/molecules22101742
Li D, Xu G, Ren G, Sun Y, Huang Y, Liu C. The Application of Ultra-High-Performance Liquid Chromatography Coupled with a LTQ-Orbitrap Mass Technique to Reveal the Dynamic Accumulation of Secondary Metabolites in Licorice under ABA Stress. Molecules. 2017; 22(10):1742. https://doi.org/10.3390/molecules22101742
Chicago/Turabian StyleLi, Da, Guojie Xu, Guangxi Ren, Yufeng Sun, Ying Huang, and Chunsheng Liu. 2017. "The Application of Ultra-High-Performance Liquid Chromatography Coupled with a LTQ-Orbitrap Mass Technique to Reveal the Dynamic Accumulation of Secondary Metabolites in Licorice under ABA Stress" Molecules 22, no. 10: 1742. https://doi.org/10.3390/molecules22101742
APA StyleLi, D., Xu, G., Ren, G., Sun, Y., Huang, Y., & Liu, C. (2017). The Application of Ultra-High-Performance Liquid Chromatography Coupled with a LTQ-Orbitrap Mass Technique to Reveal the Dynamic Accumulation of Secondary Metabolites in Licorice under ABA Stress. Molecules, 22(10), 1742. https://doi.org/10.3390/molecules22101742




