Anti-Inflammatory Pyranochalcone Derivative Attenuates LPS-Induced Acute Kidney Injury via Inhibiting TLR4/NF-κB Pathway
Abstract
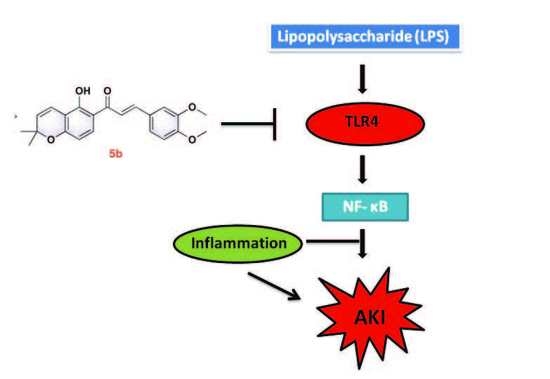
:1. Introduction
2. Results
2.1. Effect of 5b on LPS-Induced AKI Mice
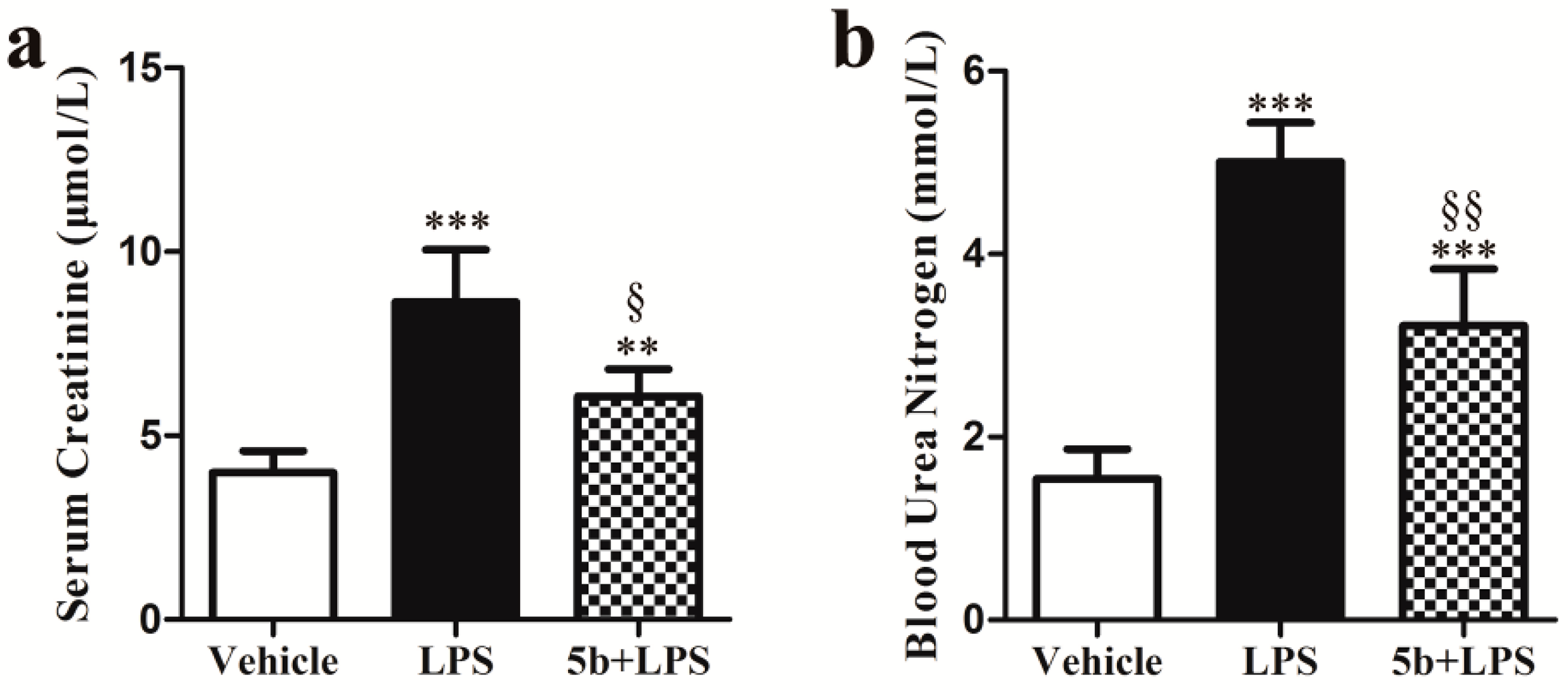
2.2. Effect of 5b on Renal Function in Mice
2.3. Effect of 5b on Proinflammatory Cytokine Gene Expressions in Renal Tissue
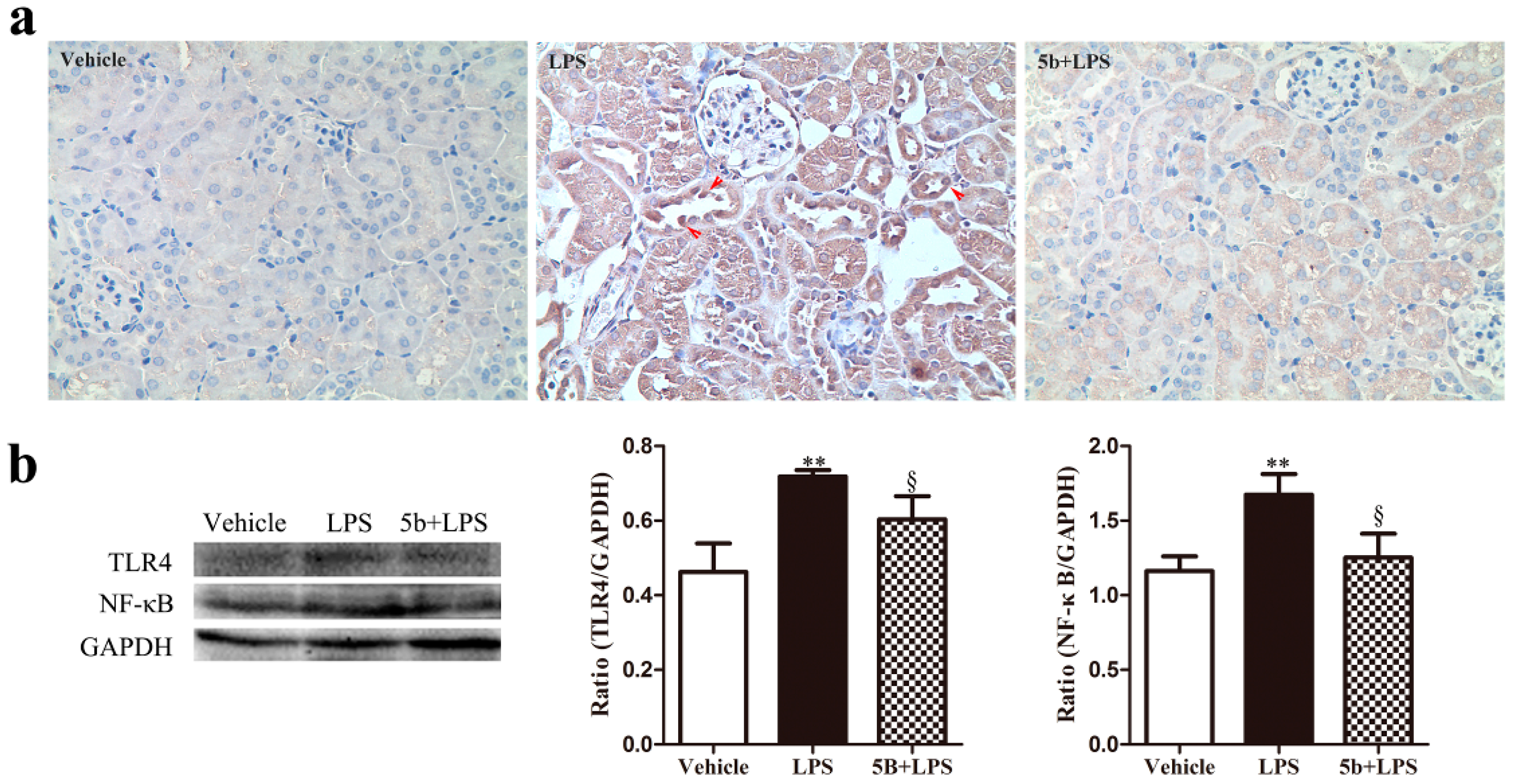
2.4. Effect of 5b on TLR4/NF-κB Pathway in LPS-Induced AKI Mice
2.5. Effect of 5b on Proinflammatory Cytokines in LPS-Induced HK-2 Cells
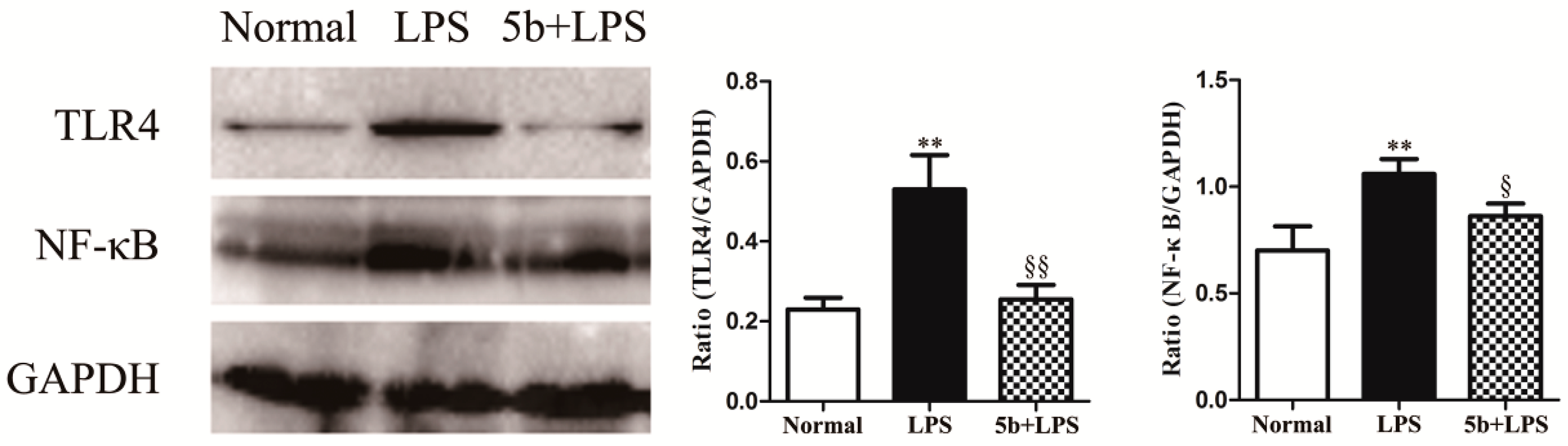
2.6. Effect of 5b on TLR4/NF-κB Pathway in LPS-Induced HK-2 Cells
3. Discussion
4. Materials and Methods
4.1. Animal and Treatment
4.2. Measurement of Renal Function
4.3. Histological Examination
4.4. Immunohistochemistry
4.5. Quantitative Real-Time PCR Analysis
4.6. Cell Culture
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Odutayo, A.; Wong, C.X.; Farkouh, M.; Altman, D.G.; Hopewell, S.; Emdin, C.A.; Hunn, B.H. AKI and Long-Term Risk for Cardiovascular Events and Mortality. J. Am. Soc. Nephrol. 2017, 28, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Acedillo, R.R.; Wald, R.; McArthur, E.; Nash, D.M.; Silver, S.A.; James, M.T.; Schull, M.J.; Siew, E.D.; Matheny, M.E.; House, A.A.; et al. Characteristics and Outcomes of Patients Discharged Home from an Emergency Department with AKI. Clin J. Am. Soc. Nephrol. 2017, 12, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Gomez, H.; Kellum, J.A. Sepsis-induced acute kidney injury. Curr. Opin. Crit. Care 2016, 22, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Zarjou, A.; Agarwal, A. Sepsis and acute kidney injury. J. Am. Soc. Nephrol. 2011, 22, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Karl, I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003, 348, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.; Volpini, R.A.; Shimizu, M.H.; Sanches, T.R.; Camara, N.O.; Semedo, P.; Rodrigues, C.E.; Seguro, A.C.; Andrade, L. Erythropoietin prevents sepsis-related acute kidney injury in rats by inhibiting NF-kappaB and upregulating endothelial nitric oxide synthase. Am. J. Physiol. Renal. Physiol. 2012, 302, 1045–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Sun, H.; Song, Y.; Ma, Z.; Zhang, G.; Gu, X.; Zhao, L. Pterostilbene attenuates inflammation in rat heart subjected to ischemia-reperfusion: Role of TLR4/NF-kappaB signaling pathway. Int. J. Clin. Exp. Med. 2015, 8, 1737–1746. [Google Scholar] [PubMed]
- Ye, H.Y.; Jin, J.; Jin, L.W.; Chen, Y.; Zhou, Z.H.; Li, Z.Y. Chlorogenic Acid Attenuates Lipopolysaccharide-Induced Acute Kidney Injury by Inhibiting TLR4/NF-kappaB Signal Pathway. Inflammation 2017, 40, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, X.; Peng, K.; Chen, C.; Fu, L.; Wang, Z.; Feng, J.; Liu, Z.; Zhang, H.; Liang, G.; et al. Discovery and evaluation of novel anti-inflammatory derivatives of natural bioactive curcumin. Drug Des. Dev. Ther. 2014, 8, 161–171. [Google Scholar]
- Zhang, Y.; Zhao, C.; He, W.; Wang, Z.; Fang, Q.; Xiao, B.; Liu, Z.; Liang, G.; Yang, S. Discovery and evaluation of asymmetrical monocarbonyl analogs of curcumin as anti-inflammatory agents. Drug Des. Dev. Ther. 2014, 8, 373–382. [Google Scholar]
- Jamil, S.; Sirat, H.M.; Jantan, I.; Aimi, N.; Kitajima, M. A new prenylated dihydrochalcone from the leaves of Artocarpus lowii. J. Nat. Med. 2008, 62, 321–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mbaveng, A.T.; Ngameni, B.; Kuete, V.; Simo, I.K.; Ambassa, P.; Roy, R.; Bezabih, M.; Etoa, F.X.; Ngadjui, B.T.; Abegaz, B.M.; et al. Antimicrobial activity of the crude extracts and five flavonoids from the twigs of Dorstenia barteri (Moraceae). J. Ethnopharmacol. 2008, 116, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Ngameni, B.; Touaibia, M.; Patnam, R.; Belkaid, A.; Sonna, P.; Ngadjui, B.T.; Annabi, B.; Roy, R. Inhibition of MMP-2 secretion from brain tumor cells suggests chemopreventive properties of a furanocoumarin glycoside and of chalcones isolated from the twigs of Dorstenia turbinata. Phytochemistry 2006, 67, 2573–2579. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.; Casida, J.E. New bioactive flavonoids and stilbenes in cube resin insecticide. J. Nat. Prod. 1999, 62, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Wang, G.; Li, X.; Cao, D.; Yang, Z.; Ma, L.; Ye, H.; Liang, X.; Ran, Y.; Chen, J.; et al. Rational design, synthesis, and pharmacological properties of pyranochalcone derivatives as potent anti-inflammatory agents. Eur. J. Med. Chem. 2012, 54, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Lafrance, J.P.; Miller, D.R. Acute kidney injury associates with increased long-term mortality. J. Am. Soc. Nephrol. 2010, 21, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 2005, 294, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Zhang, L.; Yang, Y.Y.; Tang, Y.; Zhou, J.J.; Feng, Y.Y.; Cui, T.L.; Liu, F.; Fu, P. Resolvin D1 Protects Lipopolysaccharide-induced Acute Kidney Injury by Down-regulating Nuclear Factor-kappa B Signal and Inhibiting Apoptosis. Chin. Med. J. 2016, 129, 1100–1107. [Google Scholar] [PubMed]
- Yu, C.; Qi, D.; Sun, J.F.; Li, P.; Fan, H.Y. Rhein prevents endotoxin-induced acute kidney injury by inhibiting NF-kappaB activities. Sci. Rep. 2015, 5, 11822. [Google Scholar] [CrossRef] [PubMed]
- Dirkes, S. Sepsis and inflammation: Impact on acute kidney injury. Nephrol. Nurs. J. 2013, 40, 125–132. [Google Scholar] [PubMed]
- Reilly, J.P.; Anderson, B.J.; Hudock, K.M.; Dunn, T.G.; Kazi, A.; Tommasini, A.; Charles, D.; Shashaty, M.G.; Mikkelsen, M.E.; Christie, J.D.; et al. Neutropenic sepsis is associated with distinct clinical and biological characteristics: A cohort study of severe sepsis. Crit. Care 2016, 20, 222. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Jia, J.; Ji, K.; Gong, X.; Wang, R.; Zhang, X.; Wang, H.; Zang, B. The neutrophil elastase inhibitor, sivelestat, attenuates sepsis-related kidney injury in rats. Int. J. Mol. Med. 2016, 38, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Quinto, B.M.; Iizuka, I.J.; Monte, J.C.; Santos, B.F.; Pereira, V.; Durao, M.S.; Dalboni, M.A.; Cendoroglo, M.; Santos, O.F.; Batista, M.C. TNF-alpha depuration is a predictor of mortality in critically ill patients under continuous veno-venous hemodiafiltration treatment. Cytokine 2015, 71, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Fan, H.J.; Li, H.; Ding, H.; Lv, Q.; Hou, S.K. Zingerone ameliorates lipopolysaccharide-induced acute kidney injury by inhibiting Toll-like receptor 4 signaling pathway. Eur. J. Pharmacol. 2016, 772, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zheng, Q.; Hu, X.; Shen, H.; Li, F. Betulin attenuates kidney injury in septic rats through inhibiting TLR4/NF-kappaB signaling pathway. Life Sci. 2016, 144, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Duann, P.; Lianos, E.A.; Ma, J.; Lin, P.H. Autophagy, Innate Immunity and Tissue Repair in Acute Kidney Injury. Int. J. Mol. Sci. 2016, 17, 662. [Google Scholar] [CrossRef] [PubMed]
- Zuk, A.; Bonventre, J.V. Acute Kidney Injury. Annu. Rev. Med. 2016, 67, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Leemans, J.C.; Butter, L.M.; Teske, G.J.; Stroo, I.; Pulskens, W.P.; Florquin, S. The toll interleukin-1 receptor (IL-1R) 8/single Ig domain IL-1R-related molecule modulates the renal response to bacterial infection. Infect. Immun. 2012, 80, 3812–3820. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.W.; Wong, C.K.; Lam, C.W. Tumor necrosis factor-alpha up-regulates the expression of CCL2 and adhesion molecules of human proximal tubular epithelial cells through MAPK signaling pathways. Immunobiology 2008, 213, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. Inflammatory processes in renal fibrosis. Nat. Rev. Nephrol. 2014, 10, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Akcay, A.; Nguyen, Q.; Edelstein, C.L. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009, 2009, 137072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, D.; Bao, Y.; Shi, Y.; Cui, Y.; Guo, M. Nerolidol Protects Against LPS-induced Acute Kidney Injury via Inhibiting TLR4/NF-kappaB Signaling. Phytother. Res. 2017, 31, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.S.; Zhou, J.J.; Feng, Y.Y.; Shi, M.; Guo, F.; Gou, S.J.; Salerno, S.; Ma, L.; Fu, P. Pharmacological Inhibition of Macrophage Toll-like Receptor 4/Nuclear Factor-kappa B Alleviates Rhabdomyolysis-induced Acute Kidney Injury. Chin. Med. J. 2017, 130, 2163–2169. [Google Scholar] [PubMed]
- Zhao, L.; Xu, L.; Tao, X.; Han, X.; Yin, L.; Qi, Y.; Peng, J. Protective Effect of the Total Flavonoids from Rosa laevigata Michx Fruit on Renal Ischemia-Reperfusion Injury through Suppression of Oxidative Stress and Inflammation. Molecules 2016, 21, 952. [Google Scholar] [CrossRef] [PubMed]
- Fattori, V.; Borghi, S.M.; Guazelli, C.F.S.; Giroldo, A.C.; Crespigio, J.; Bussmann, A.J.C.; Coelho-Silva, L.; Ludwig, N.G.; Mazzuco, T.L.; Casagrande, R.; et al. Vinpocetine reduces diclofenac-induced acute kidney injury through inhibition of oxidative stress, apoptosis, cytokine production, and NF-kappaB activation in mice. Pharmacol. Res. 2017, 120, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Park, J.K.; Kirsch, T.; Yagita, H.; Akiba, H.; Boenisch, O.; Haller, H.; Najafian, N.; Habicht, A. The TIM-1:TIM-4 pathway enhances renal ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2011, 22, 484–495. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 5b are available from the authors. |




| Mouse Gene | Sequence (5′-3′) |
|---|---|
| F-IL-1β | TGGGCCTCAAAGGAAAGAAT |
| R-IL-1β | CAGGCTTGTGCTCTGCTTGT |
| F-IL-6 | ACAACCACGGCCTTCCCTACTT |
| R-IL-6 | CACGATTTCCCAGAGAACATGTG |
| F-TNF-α | ACCCTCACACTCAGATCATCTTC |
| R-TNF-α | TGGTGGTTTGCTACGACGT |
| F-GAPDH | GTATGACTCCACTCACGGCAAA |
| R-GAPDH | GGTCTCGCTCCTGGAAGATG |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, M.; Zeng, X.; Guo, F.; Huang, R.; Feng, Y.; Ma, L.; Zhou, L.; Fu, P. Anti-Inflammatory Pyranochalcone Derivative Attenuates LPS-Induced Acute Kidney Injury via Inhibiting TLR4/NF-κB Pathway. Molecules 2017, 22, 1683. https://doi.org/10.3390/molecules22101683
Shi M, Zeng X, Guo F, Huang R, Feng Y, Ma L, Zhou L, Fu P. Anti-Inflammatory Pyranochalcone Derivative Attenuates LPS-Induced Acute Kidney Injury via Inhibiting TLR4/NF-κB Pathway. Molecules. 2017; 22(10):1683. https://doi.org/10.3390/molecules22101683
Chicago/Turabian StyleShi, Min, Xiaoxi Zeng, Fan Guo, Rongshuang Huang, Yanhuan Feng, Liang Ma, Li Zhou, and Ping Fu. 2017. "Anti-Inflammatory Pyranochalcone Derivative Attenuates LPS-Induced Acute Kidney Injury via Inhibiting TLR4/NF-κB Pathway" Molecules 22, no. 10: 1683. https://doi.org/10.3390/molecules22101683