Effect of Selected Plant Phenolics on Fe2+-EDTA-H2O2 System Mediated Deoxyribose Oxidation: Molecular Structure-Derived Relationships of Anti- and Pro-Oxidant Actions
Abstract
:1. Introduction
2. Results
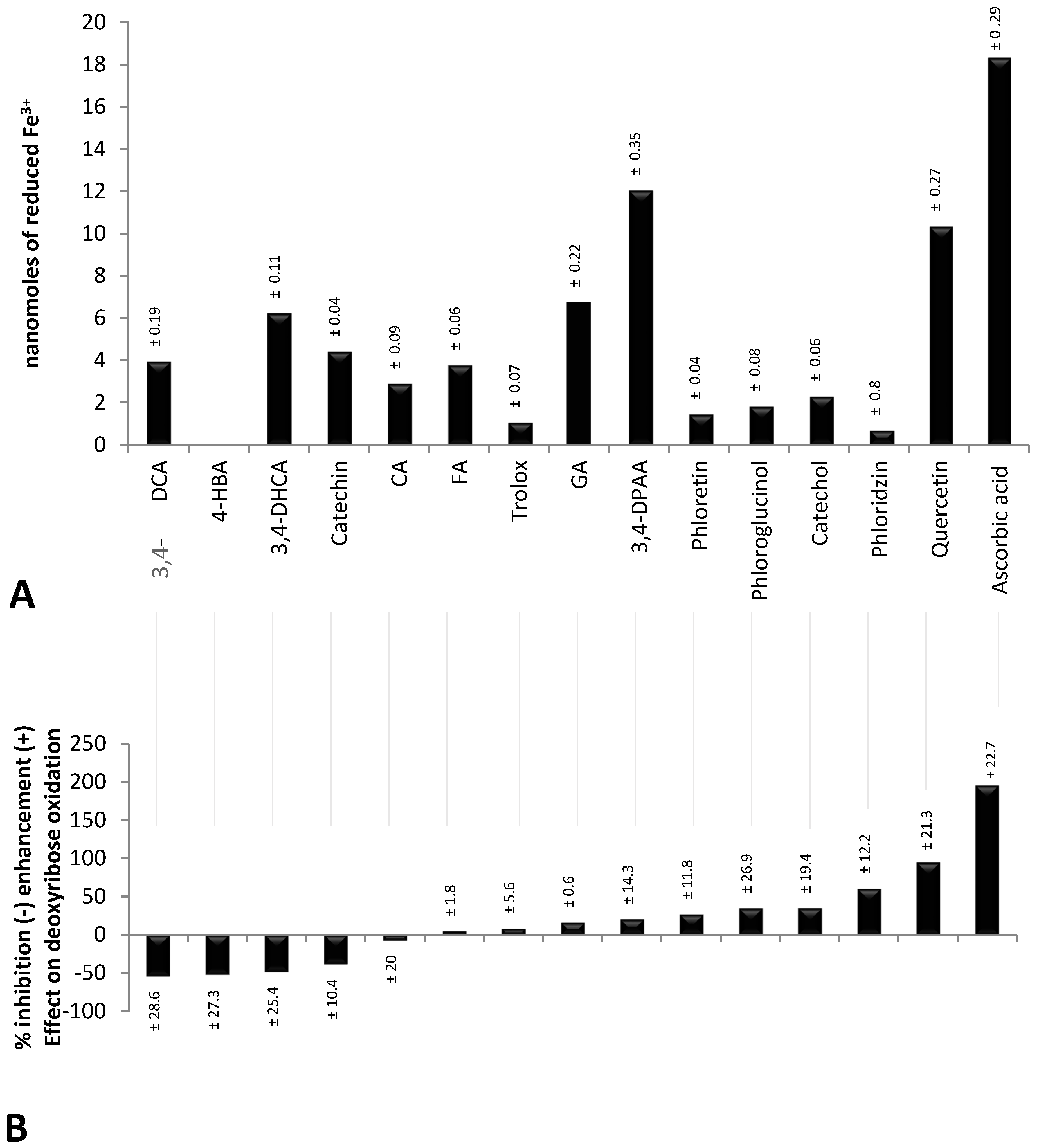
2.1. Validity of Experimental Conditions
2.2. Inhibitory Effect of Polyphenols on the Oxidative Degradation of Deoxyribose by the Fenton System
2.3. Enhancing Effect of Polyphenols on the Oxidative Degradation of Deoxyribose by the Fenton System
2.4. Factors Determining the Pro-Oxidant or Antioxidant Effect of Polyphenols on Deoxyribose Oxidation
3. Discussion
3.1. Plausible Mechanisms of the Anti- or Pro-Oxidant Activity of Polyphenols
3.2. Determinants of the Polyphenols Effect on Deoxyribose Oxidation by the Fenton System
3.3. Applicability to In Vivo Conditions
4. Materials and Methods
4.1. Reagents
4.2. Determining the Effect of Plant Phenolics on the Process of Oxidative Degradation of Deoxyribose in the Fenton System
4.3. Statistical Analysis
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
Appendix A—Chemical Compounds Studied in This Article
References
- Shen, Y.; Zhang, H.; Cheng, L.; Wang, L.; Qian, H.; Qi, X. In vitro and in vivo antioxidant activity of polyphenols extracted from black highland barley. Food Chem. 2016, 194, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; García, C.R.; Pinzón, J.R.; Chaur, M.N.; Brumaghim, J.L. Antioxidant and prooxidant effects of polyphenol compounds on copper-mediated DNA damage. J. Inorg. Biochem. 2011, 105, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Joubert, E.; Winterton, P.; Britz, T.J.; Gelderblom, W.C. Antioxidant and pro-oxidant activities of aqueous extracts and crude polyphenolic fractions of rooibos (Aspalathus linearis). J. Agric. Food Chem. 2005, 53, 10260–10267. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, Y.; Chang, W. Kinetic deoxyribose degradation assay and its application in assessing the antioxidant activities of phenolic compounds in a Fenton-type reaction system. Anal. Chim. Acta 2003, 478, 129–137. [Google Scholar] [CrossRef]
- Kurin, E.; Mučaji, P.; Nagy, M. In vitro antioxidant activities of three red wine polyphenols and their mixtures: An interaction study. Molecules 2012, 17, 14336–14348. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Singh, B.R.; Singh, R.L.; Prakash, D.; Dhakarey, R.; Upadhyay, G.; Singh, H.B. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem. Toxicol. 2009, 47, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Neergheen, V.S.; Soobrattee, M.A.; Bahorun, T.; Aruoma, O.I. Characterization of the phenolic constituents in Mauritian endemic plants as determinants of their antioxidant activities in vitro. J. Plant Physiol. 2006, 168, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Mozdzan, M.; Szemraj, J.; Rysz, J.; Nowak, D. Antioxidant properties of carnosine re-evaluated with oxidizing systems involving Fe and copper ions. Basic Clin. Pharmacol. Toxicol. 2005, 96, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Son, Y.O.; Pratheeshkumar, P.; Shi, X. Oxidative stress and metal carcinogenesis. Free Radic. Biol. Med. 2012, 53, 742–757. [Google Scholar] [CrossRef] [PubMed]
- De Graft-Johnson, J.; Kolodziejczyk, K.; Krol, M.; Nowak, P.; Krol, B.; Nowak, D. Ferric-reducing ability power of selected plant polyphenols and their metabolites: Implications for clinical studies on the antioxidant effects of fruits and vegetable consumption. Basic Clin. Pharmacol. Toxicol. 2007, 100, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, Y.; Wu, F.; Ni, Y.; Kokot, S. A colorimetric method of analysis for trace amounts of hydrogen peroxide with the use of the nano-properties of molybdenum disulfide. Analyst 2015, 140, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, W.I.; El-Sabban, F. Dimethyl sulfoxide (DMSO) and glycerol, hydroxyl radical scavengers, impair platelet aggregation within and eliminate the accompanying vasodilation of injured mouse pial arterioles. Stroke 1982, 13, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic metals, ascorbate and free radicals: Combinations to avoid. Radiat. Res. 1996, 145, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, S.; Oikawa, S.; Murata, M. Evaluation for safety of antioxidant chemopreventive agents. Antioxid. Redox Signal. 2005, 7, 1728–1739. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.; Aruoma, O.I. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem. 1987, 165, 215–219. [Google Scholar] [CrossRef]
- Gutteridge, J.M. Ferrous-salt-promoted damage to deoxyribose and benzoate. The increased effectiveness of hydroxyl-radical scavengers in the presence of EDTA. Biochem. J. 1987, 243, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.; Frei, B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999, 13, 1007–1024. [Google Scholar] [PubMed]
- Wang, S.Y.; Jio, H. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. J. Agric. Food Chem. 2000, 48, 5677–5684. [Google Scholar] [CrossRef] [PubMed]
- Minakata, K.; Fukushima, K.; Nakamura, M.; Iwahashi, H. Effect of some naturally occurring iron ion chelators on the formation of radicals in the reaction mixtures of rat liver microsomes with ADP, Fe and NADPH. J. Clin. Biochem. Nutr. 2011, 49, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Laughton, M.J.; Halliwell, B.; Evans, P.J.; Hoult, J.R.S. Antioxidant and pro-oxidant actions of the plant phenolics quercetin, gossypol and myricetin. Effects on lipid peroxidation, hydroxyl radical generation and bleomycin dependent damage to DNA. Biochem. Pharmacol. 1989, 38, 2859–2865. [Google Scholar] [CrossRef]
- Puppo, A. Effect of flavonoids on hydroxyl radical formation by Fenton-type reactions; influence of the iron chelator. Phytochemistry 1992, 31, 85–88. [Google Scholar] [CrossRef]
- Moran, J.F.; Klucas, R.V.; Grayer, R.J.; Abian, J.; Becana, M. Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: Prooxidant and antioxidant properties. Free Radic. Biol. Med. 1997, 22, 861–870. [Google Scholar] [CrossRef]
- Azam, S.; Hadi, N.; Khan, N.U.; Hadi, S.M. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: Implications for anticancer properties. Toxicol In Vitro 2004, 18, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Sutton, H.C.; Vile, G.F.; Winterbourn, C.C. Radical driven Fenton reactions--evidence from paraquat radical studies for production of tetravalent iron in the presence and absence of ethylenediaminetetraacetic acid. Arch. Biochem. Biophys. 1987, 256, 462–471. [Google Scholar] [CrossRef]
- Hussain, R.S.; Cillard, J.; Cillard, P. Hydroxyl radical scavenging activity of flavonoids. Phytochemistry 1987, 26, 2489–2491. [Google Scholar] [CrossRef]
- Heijnen, C.G.; Haenen, G.R.; van Acker, F.A.; van der Vijgh, W.J.; Bast, A. Flavonoids as peroxynitrite scavengers: The role of the hydroxyl groups. Toxicol In Vitro 2001, 15, 3–6. [Google Scholar] [CrossRef]
- Jung, H.A.; Jung, M.J.; Kim, J.Y.; Chung, H.Y.; Choi, J.S. Inhibitory activity of flavonoids from Prunus davidiana and other flavonoids on total ROS and hydroxyl radical generation. Arch. Pharm. Res. 2003, 26, 809–815. [Google Scholar] [CrossRef] [PubMed]
- D’Avila Farias, M.; Oliveira, P.S.; Dutra, F.S.; Fernandes, T.J.; de Pereira, C.M.; de Periera, C.M.; de Oliveira, S.Q.; Stefanello, F.M.; Lencina, C.L.; Barschak, A.G. Eugenol Derivatives as potential anti-oxidants: Is phenolic hydroxyl necessary to obtain an effect? J. Pharm. Pharmacol. 2014, 66, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Moniczewski, A.; Librowski, T.; Lochyński, S.; Strub, D. Evaluation of the irritating influence of carane derivatives and their antioxidant properties in a deoxyribose degradation test. Pharmacol. Rep. 2011, 63, 120–129. [Google Scholar] [CrossRef]
- Benzie, I.F.; Choi, S.W. Antioxidants in food: Content, measurement, significance, action, cautions, caveats, and research needs. Adv. Food Nutr. Res. 2014, 71, 1–53. [Google Scholar] [PubMed]
- Nguyen, M.T.; Kryachko, E.S.; Vanquickenborne, L.G. General and Theoretical Aspects of Phenols. In The Chemistry of Phenols; Rappoport, Z., Ed.; John Wiley & Sons: Chichester, UK, 2003; pp. 3–179. [Google Scholar]
- Tsukimori, K.; Yoshitomi, T.; Morokuma, S.; Fukushima, K.; Wake, N. Serum uric acid levels correlate with plasma hydrogen peroxide and protein carbonyl levels in preeclampsia. Am. J. Hypertens. 2008, 21, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Ramavataram, D.V. Non transferrin bound iron: Nature, manifestations and analytical approaches for estimation. Indian J. Clin. Biochem. 2012, 27, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Prymont-Przyminska, A.; Zwolinska, A.; Sarniak, A.; Wlodarczyk, A.; Krol, M.; Nowak, M.; de Graft-Johnson, J.; Padula, G.; Bialasiewicz, P.; Markowski, J.; et al. Consumption of strawberries on a daily basis increases the non-urate 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging activity of fasting plasma in healthy subjects. J. Clin. Biochem. Nutr. 2014, 55, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.B.; Anstrom, K.J.; Clapp-Channing, N.E.; Knight, J.D.; Boineau, R.; Goertz, C.; Rozema, T.C.; Liu, D.M.; Nahin, R.L.; Rosenberg, Y.; et al. TACT Investigators. Quality-of-life outcomes with a disodium EDTA chelation regimen for coronary disease: Results from the trial to assess chelation therapy randomized trial. Circ. Cardiovasc. Qual. Outcomes 2014, 7, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.X.; Harich, K.C.; Cranton, E.M. A laboratory method to measure plasma EDTA levels. In A Textbook on EDTA Chelation Therapy; Cranton, E.M., Ed.; Hampton Roads Publishing Inc.: Newburyport, MA, USA, 2001; pp. 468–476. [Google Scholar]
- Hininger, I.; Waters, R.; Osman, M.; Garrel, C.; Fernholz, K.; Roussel, A.M.; Anderson, R.A. Acute prooxidant effects of vitamin C in EDTA chelation therapy and long-term antioxidant benefits of therapy. Free Radic. Biol. Med. 2005, 38, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.
| Polyphenol † | Chemical Structure | Inhibition of Deoxyribose Oxidation (%) |
|---|---|---|
| 3,4-Dihydroxycinnamic acid |  | 54.4 ± 28.6 (62.8) * |
| –OH: 3 | ||
| Catechol: 1 | ||
| –COOH: 1 | ||
| Aliphatic Substitute at the Catechol Ring: 1 | ||
| 4-Hydroxybenzoic acid |  | 52.4 ± 27.3 (56.3) * |
| –OH: 2 | ||
| Catechol: 0 | ||
| –COOH: 1 | ||
| Aliphatic Substitute at the Catechol Ring: 0 | ||
| 3,4-Dihydroxyhydrocinnamic acid |  | 48.5 ± 25.4 (52.5) * |
| –OH: 3 | ||
| Catechol: 1 | ||
| –COOH: 1 | ||
| Aliphatic Substitute at the Catechol Ring: 1 | ||
| Catechin |  | 38.5 ± 10.4 (44.4) * |
| –OH: 5 | ||
| Catechol: 1 | ||
| –COOH: 0 | ||
| Aliphatic Substitute at the Catechol Ring: 0 | ||
| Chlorogenic acid |  | 7.5 ± 20 (0.01) |
| –OH: 6 | ||
| Catechol: 1 | ||
| –COOH: 1 | ||
| Aliphatic Substitute at the Catechol Ring: 1 |
| Polyphenol † | Chemical Structure | Enhancement of Deoxyribose Oxidation (%) |
|---|---|---|
| Ferulic acid |  | 4.6 ± 1.8 (4.3) |
| –OH: 2 | ||
| Catechol: 0 | ||
| –COOH: 1 | ||
| Aliphatic Substitute at the Catechol Ring: 0 | ||
| Gallic acid |  | 16.1 ± 0.6 (15.2) * |
| –OH: 4 | ||
| Catechol: 2 | ||
| –COOH: 1 | ||
| Aliphatic Substitute at the Catechol Ring: 0 | ||
| 3,4-Dihydroxyphenylacetic acid |  | 20.3 ± 14.3 (13.7) * |
| –OH: 3 | ||
| Catechol: 1 | ||
| –COOH: 1 | ||
| Aliphatic Substitute at the Catechol Ring: 1 | ||
| Phloretin |  | 26.9 ± 11.8 (33.3) * |
| –OH: 4 | ||
| Catechol: 0 | ||
| –COOH: 0 | ||
| Aliphatic Substitute at the Catechol Ring: 0 | ||
| Phloroglucinol |  | 34.8 ± 26.9 (30.8) * |
| –OH: 3 | ||
| Catechol: 0 | ||
| –COOH: 0 | ||
| Aliphatic Substitute at the Catechol Ring: 0 | ||
| Catechol |  | 34.9 ± 19.4 (38.1) * |
| –OH: 2 | ||
| Catechol: 1 | ||
| –COOH: 0 | ||
| Aliphatic Substitute at Catechol Ring: 0 | ||
| Phloridzin |  | 60.6 ± 12.2 (55.7) * |
| –OH: 7 | ||
| Catechol: 0 | ||
| –COOH: 0 | ||
| Aliphatic Substitute at the Catechol Ring: 0 | ||
| Quercetin |  | 95.0 ± 21.3 (97.5) * |
| –OH: 5 | ||
| Catechol: 1 | ||
| –COOH: 0 | ||
| Aliphatic Substitute at the Catechol Ring: 0 |
| Dependent Variable | Independent Variables | Entry into Model | Multiple r | Squared Multiple r | p | Zero Order r |
|---|---|---|---|---|---|---|
| Inhibition of deoxyribose oxidation (n = 5) | Catechol ring | Out | 0.177 | |||
| Aliphatic substitute | In | |||||
| –OH substitutions | In | 0.588 | 0.346 | 0.004 | 0.570 | |
| –COOH substitute | In | −0.053 | ||||
| Enhancement of deoxyribose oxidation (n = 8) | Aliphatic substitute | Out | −0.22 | |||
| –OH substitutions | In | 0.572 | 0.327 | 0.001 | 0.590 | |
| –COOH substitute | In | |||||
| Catechol ring | In | −0.166 |
| No. | Sample | Volumes of Working Solutions of Reagents and Tested Polyphenols (µL) | ||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | ||
| Deoxyribose | Polyphenol | DMSO | FeSO4 | EDTA | H2O | H2O2 | ||
| 1 | Blank | 460 | - | - | - | - | 40 | - |
| 2 | Positive | 460 | - | - | 10 | 10 | 10 | 10 |
| 3 | Polyphenol effect | 460 | 10 | - | 10 | 10 | - | 10 |
| 4 | DMSO control * | 460 | - | 10 | 10 | 10 | - | 10 |
| Additional Controls | ||||||||
| 5 | Incomplete system ** | 460 | - | - | 10 | 10 | 20 | - |
| 6 | Deoxyribose with polyphenol † | 460 | 10 | - | - | - | 30 | - |
| 7 | Polyphenol alone †† | - | 10 | - | - | - | 490 | - |
| 8 | Polyphenol with H2O2 ††† | - | 10 | - | - | - | 480 | 10 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Graft-Johnson, J.; Nowak, D. Effect of Selected Plant Phenolics on Fe2+-EDTA-H2O2 System Mediated Deoxyribose Oxidation: Molecular Structure-Derived Relationships of Anti- and Pro-Oxidant Actions. Molecules 2017, 22, 59. https://doi.org/10.3390/molecules22010059
De Graft-Johnson J, Nowak D. Effect of Selected Plant Phenolics on Fe2+-EDTA-H2O2 System Mediated Deoxyribose Oxidation: Molecular Structure-Derived Relationships of Anti- and Pro-Oxidant Actions. Molecules. 2017; 22(1):59. https://doi.org/10.3390/molecules22010059
Chicago/Turabian StyleDe Graft-Johnson, Jeffrey, and Dariusz Nowak. 2017. "Effect of Selected Plant Phenolics on Fe2+-EDTA-H2O2 System Mediated Deoxyribose Oxidation: Molecular Structure-Derived Relationships of Anti- and Pro-Oxidant Actions" Molecules 22, no. 1: 59. https://doi.org/10.3390/molecules22010059






