Antioxidant Activities and Chemical Constituents of Flavonoids from the Flower of Paeonia ostii
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sample Extraction and Preparation
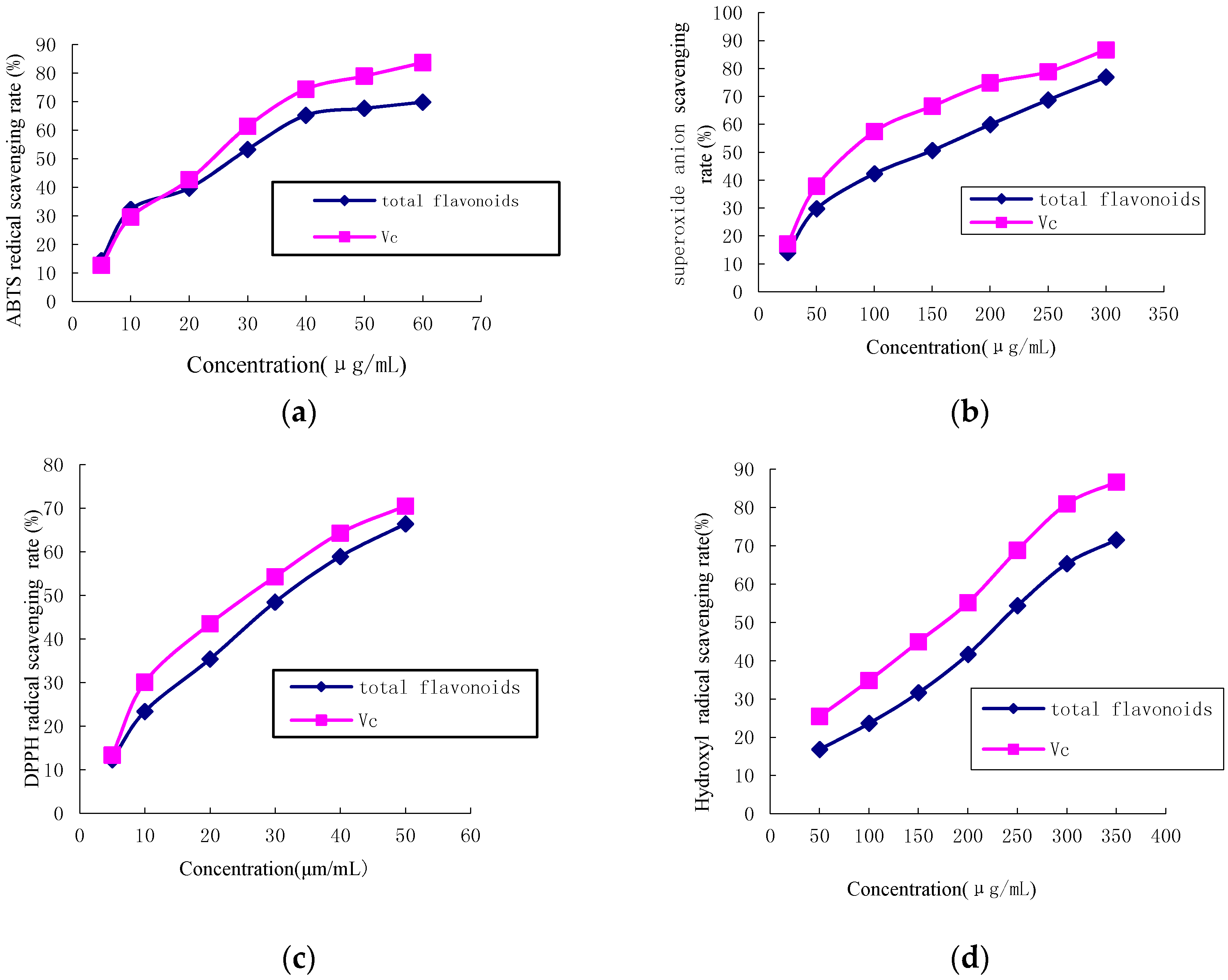
2.2. Antioxidant Activity
2.2.1. ABTS Radical Scavenging Activity
2.2.2. Superoxide Anion Free Radical Scavenging Activity
2.2.3. DPPH Radical Scavenging Activity
2.2.4. Hydroxyl Radical Scavenging Activity
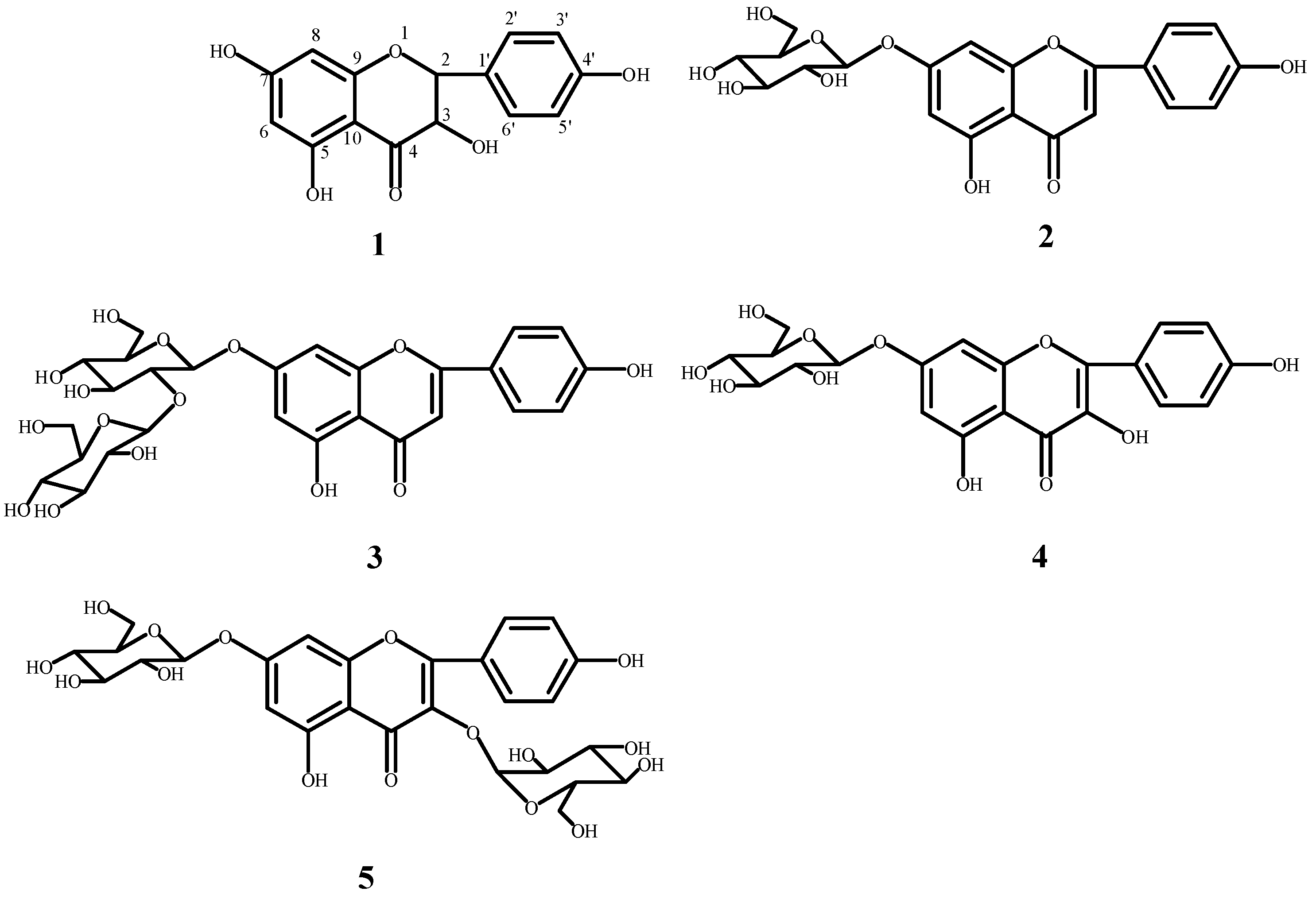
2.3. Structural Elucidation of the Isolated Compounds
2.4. Antioxidant Capacities of Isolated Compounds
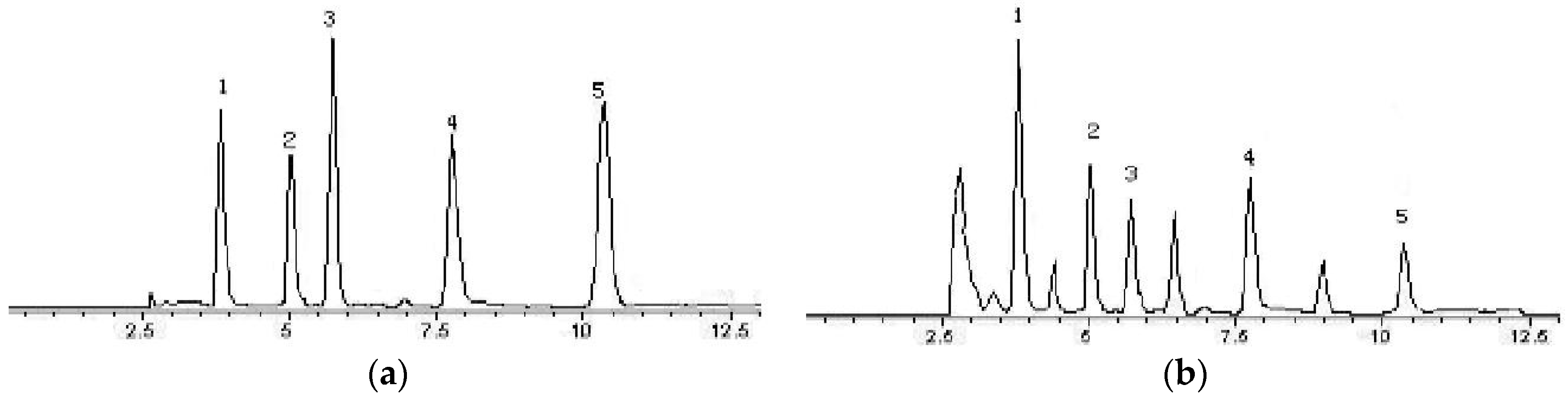
2.5. HPLC Analysis
3. Materials and Methods
3.1. Plant Material
3.2. Reagent and Chemicals
3.3. Apparatus
3.4. Sample Extraction and Preparation
3.5. Determination of Total Flavonoid Content
3.6. Antioxidant Activity
3.6.1. ABTS Radical Scavenging Activity
3.6.2. Superoxide Anion Free Radical Scavenging Activity
3.6.3. DPPH Radical Scavenging Activity Assay
3.6.4. Hydroxyl Radical Scavenging Activity
3.7. Isolation and Purification of Bioactive Compounds
3.8. Identification of the Isolated Compounds
3.9. Flavonoids Compounds Analysis by HPLC
3.10. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhong, J.Q.; Li, B.; Jia, Q.; Li, Y.M.; Zhu, W.L.; Chen, K.X. Advances in the structure-activity relationship study of natural flavonoids and its derivatives. Acta Pharm. Sin. 2011, 46, 622–630. [Google Scholar]
- Yang, B.; Kotani, A.; Arai, K.; Kusu, F. Estimation of the antioxidant activities of flavonoids from their oxidation potentials. Anal. Sci. 2001, 17, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; Katan, M.B. Dietary flavonoids: Intake, health effects and bioavailability. Food Chem. Toxicol. 1999, 37, 937–942. [Google Scholar] [CrossRef]
- Ameha, S.; Kaleab, A.; Fathy, K.E.F. Structure-radical scavenging activity relationships of flavonoids. Phytochemistry 2006, 67, 2058–2070. [Google Scholar]
- Bandyopadhyay, U.; Das, D.; Banerjee, R.K. Reactive oxygen species: Oxidative damage and pathogenesis. Curr. Sci. 1999, 77, 659–666. [Google Scholar]
- Finkel, T. Radical medicine: Treating ageing to cure disease. Nat. Rev. Mol. Cell Biol. 2005, 6, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Cotelle, N. Role of flavonoids in oxidative stress. Curr. Top. Med. Chem. 2001, 1, 569–590. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Kapoor, H. Antioxidants in fruits and vegetables-the millennium’s health. Int. J. Food Sci. Technol. 2001, 36, 703–725. [Google Scholar] [CrossRef]
- Miller, A.L. Antioxidant flavonoids: Structure, function and clinical usage. Altern. Med. Rev. 1996, 1, 103–111. [Google Scholar]
- Pietta, P. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J. Chinese Tree and Herbaceous Peonies; China Forestry Publishing House: Beijing, China, 1999; pp. 1–200. [Google Scholar]
- Zhang, C.; Wang, W.N.; Wang, Y.J.; Gao, S.L.; Du, D.N.; Fu, J.X.; Dong, L. Anthocyanin biosynthesis and accumulation in developing flowers of tree peony (Paeonia suffruticosa) ‘Luoyang Hong’. Postharvest Biol. Technol. 2014, 97, 11–22. [Google Scholar] [CrossRef]
- Fu, L. Study on the Components and Antioxidant Capacity of the Peony Flower Pigment of P. ostii. Master’s Thesis, North West Agriculture and Forestry University, Xianyang, China, 2011. [Google Scholar]
- Wu, S.H.; Wu, D.G.; Chen, Y.W. Chemical Constituents and Bioactivities of Plants from the Genus Paeonia. Chem. Biodivers. 2010, 7, 90–104. [Google Scholar] [CrossRef] [PubMed]
- He, C.N.; Peng, Y.; Zhang, Y.C.; Xu, L.J.; Gu, J.; Xiao, P.G. Phytochemical and Biological Studies of Paeoniaceae. Chem. Biodivers. 2010, 7, 805–838. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.W.; Deng, M.C.; Lv, Z.C.; Peng, Y.H. Evaluation of antioxidant activities of extracts from 19 Chinese edible flowers. SpringerPlus 2014, 3, 315. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Hashimoto, F.; Shiraishi, A.; Aoki, N. Phenetics in Tree Peony Species from China by Flower Pigment Cluster Analysis. J. Plant Res. 2001, 114, 213–221. [Google Scholar] [CrossRef]
- Wang, L.S.; Hashimoto, F.; Shiraishi, A.; Aoki, N.; Li, J.J.; Sakata, Y. Chemical taxonomy of the Xibei tree peony from China by floral pigmentation. J. Plant Res. 2004, 117, 47–55. [Google Scholar] [PubMed]
- Léonce, D. Sur la séparation du paeonoside et du malvoside au moyen de la chromatographie sur papier et sur couche mince de cellulose. J. Chromatogr. A 1968, 34, 425–427. [Google Scholar]
- Wang, X.; Cheng, C.G.; Sun, Q.L.; Li, F.W.; Liu, J.H.; Zheng, C.C. Isolation and purification of four flavonoid constituents from the flowers of Paeonia suffruticosa by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1075, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.L.; Lai, C.W.; Cheng, J.T. Antihyperglycemic effects of paeoniflorin and 8-debenzoylpaeoniflorin, glucosides from the root of Paeonia lactiflora. Planta Medica 1997, 63, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Comparative biochemistry of flavonoids—I: Distribution of chalcone and aurone pigments in plants. Phytochemistry 1966, 5, 111–115. [Google Scholar] [CrossRef]
- Hosoki, T.; Hamada, M.; Kando, T.; Moriwaki, R.; Inaba, K. Comparative study of anthocyanin in tree peony flowers. J. Jpn. Soc. Hortic. Sci. 1991, 60, 395–403. [Google Scholar] [CrossRef]
- Deng, R.X.; Liu, Z.; Qin, L.L.; Wang, L.; Liu, X.Q.; Liu, P. Optimization of Supercritical CO2 Extraction and Analysis of Chemical Composition of peony seed oil. Food Sci. 2010, 31, 142–145. [Google Scholar]
- Liu, P.; Xu, Y.F.; Liu, Y.Q.; Hou, X.W.; Dong, J.Q.; Deng, R.X. Optimization of Ultrasonic-assisted Aqueous Extraction of peony seed oil. Sci. Technol. Cereals Oils Foods 2015, 23, 29–33. [Google Scholar]
- Liu, P.; Li, X.F.; Niu, Y.Q.; Deng, R.X.; Dong, J.Q.; Yin, W.P. Optimization extraction technology of oligostilbenes from seed cake of Peony for oil and its bioactivities. J. Chin. Cereal Oil Assoc. 2016, 31, 79–85. [Google Scholar]
- Tian, G.L.; Zhao, G.L. Food of Peony Flowers’s Exploitation and Development Prospects. Food Res. Dev. 2010, 31, 187–190. [Google Scholar]
- Li, A.F.; Sun, A.L.; Liu, R.M.; Zhang, Y.Q.; Cui, J.C. An efficient preparative procedure for main flavonoids from the peel of Trichosanthes kirilowii Maxim. Using polyamide resin followed by semi-preparative high performance liquid chromatography. J. Chromatogr. B 2014, 965, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hayat, K.; Zhang, X.; Tong, J.; Xia, S. Separation and purification of flavonoid from Ginkgo extract by polyamide resin. Sep. Sci. Technol. 2010, 45, 2413–2419. [Google Scholar] [CrossRef]
- He, J.; Feng, Y.; Ouyang, H.Z.; Yu, B.; Chang, Y.X.; Pan, G.X.; Dong, G.Y.; Wang, T.; Gao, X.M. A sensitive LC-MS/MS method for simultaneous determination of six flavonoids in rat plasma: Application to a pharmacokinetic study of total flavonoids from mulberry leaves. J. Pharm. Biomed. Anal. 2013, 84, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Fan, J.L.; Zhu, W.X.; Kang, H.B.; Ma, H.L.; Tao, G.J. Flavonoid constituents and antioxidant capacity in flowers of different Zhongyuan tree penoy cultivars. J. Funct. Foods 2012, 4, 147–157. [Google Scholar] [CrossRef]
- Wang, X.; Shi, X.G.; Zheng, C.C.; Liu, J.H. Effect of extract from Peony flowers on removal of reactive oxygen species and preventing DNA damage caused by hydroxyl radical. Food Ferment. Ind. 2004, 30, 54–58. [Google Scholar]
- Zhu, S.Y. Optimised extraction and antioxidant of polyphenols from Peony flowers. Biotechnology 2014, 24, 78–82. [Google Scholar]
- Sanchez-Moreno, C. Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Int. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Soares, J.R.; Dinis, T.C.P.; Cunha, A.P.; Almeida, L.M. Antioxidant activity of some extracts of Thymus zygis. Free Radic. Res. 1997, 26, 459–478. [Google Scholar]
- Wu, P.P.; Ma, G.Z.; Li, N.H.; Deng, Q.; Yin, Y.Y.; Huang, R.Q. Investigation of in vitro and in vivo antioxidant activities of flavonoids rich extract from the berries of Rhodomyrtus tomentosa (Ait.) Hassk. Food Chem. 2015, 173, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.H.; Shylesh, B.S.; Padikkala, J. Antioxidant and hepato protective effect of Alanthus icicifocus. Fitoterapia 2001, 72, 272–277. [Google Scholar] [CrossRef]
- Li, Y.S.; Li, D.M.; Jiang, L.Y.; Liu, G.M. Study on Chemical Constituents of Pinus yunnanensis. Chin. J. Exp. Tradit. Med. Formulae 2012, 18, 119–120. [Google Scholar]
- Tan, X.Q.; Guo, L.J.; Chen, H.S.; Wu, L.S.; Kong, F.F. Study on the Flavonoids constituents of Trchelospermum jasminoides. J. Chin. Med. Mater. 2010, 33, 58–60. [Google Scholar]
- Li, Y.Q.; Lei, X.X.; Feng, Y.L.; Xu, Q.M.; Xu, L.Z.; Yang, S.L. Study on the Chemical Constituents of Terpinia arguta. Chin. Pharm. J. 2012, 47, 261–264. [Google Scholar]
- Qu, Y.K.; Dou, D.Q.; Pei, Y.P.; Yoshikawa, M.; Matsuda, H.; Chen, Y.J. Chemical Constituents of Opuntia dillenii. J. China Pharm. Univ. 2005, 36, 213–215. [Google Scholar]
- Yu, R.M.; Li, X.; Zhang, H.J.; Wu, L.J.; Zhu, T.R.; Li, W.; Liu, L.Y.; Zhang, G. Study on the Chemical Constituents of Oxytropis glabra DC. J. Integr. Plant 1992, 34, 369–377. [Google Scholar]
- Wu, L.Q.; Zhu, W.X.; Yi, J.P.; Zhang, Y.X.; Zhong, L.J. Study on Determination of Classification and Extraction Technology of Red Pigment in Peony Flower. Trans. Chin. Soc. Agric. Mach. 2015, 36, 77–80. [Google Scholar]
- Lu, J.; Qin, P.Z.; Han, X.; Wang, Y.P.; Li, Z.H. Evaluation of antioxidant and antibacterial properties of extracts from Trollius chinensis Bunge. Eur. Food Res. Technol. 2015, 240, 301–310. [Google Scholar] [CrossRef]
- Tai, Z.G.; Chen, A.Y.; Qin, B.D.; Cai, L.; Xu, Y.Q. Chemical constituents and antioxidant activity of the Musa basjoo flower. Eur. Food Res. Technol. 2014, 239, 501–508. [Google Scholar] [CrossRef]
- Ammar, R.B.; Bhouri, W.; Sghaier, M.B.; Boubaker, J.; Skandrani, I.; Neffat, A.; Bouhlel, I.; Kilani, S.; Mariotte, A.M.; Leila, C.G.; et al. Antioxidant and free radical-scavenging properties of three flavonoids isolated from the leaves of Rhamnus alaternus L. (Rhamnaceae): A structure-activity relationship study. Food Chem. 2009, 116, 258–264. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, Y.T.; Guo, Y.R.; Liu, F.X.; Chen, F. Determination of phenolic contents and antioxidant activities of extracts of Jatropha curcas L. seed shell, a by-product, a new source of natural antioxidant. Ind. Crop. Prod. 2014, 58, 265–270. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Agatia, G.; Azzarellob, E.; Pollastrib, S.; Tattinic, M. Flavonoids as antioxidant in Plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, Ì.; Elias, R.; Gepdiremen, A.; Taoubi, K.; Köksal, E. Antioxidant secoiridoids from fringe tree (Chionanthus virginicus L.). Wood Sci. Technol. 2009, 43, 195–212. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–5 are available from the authors.
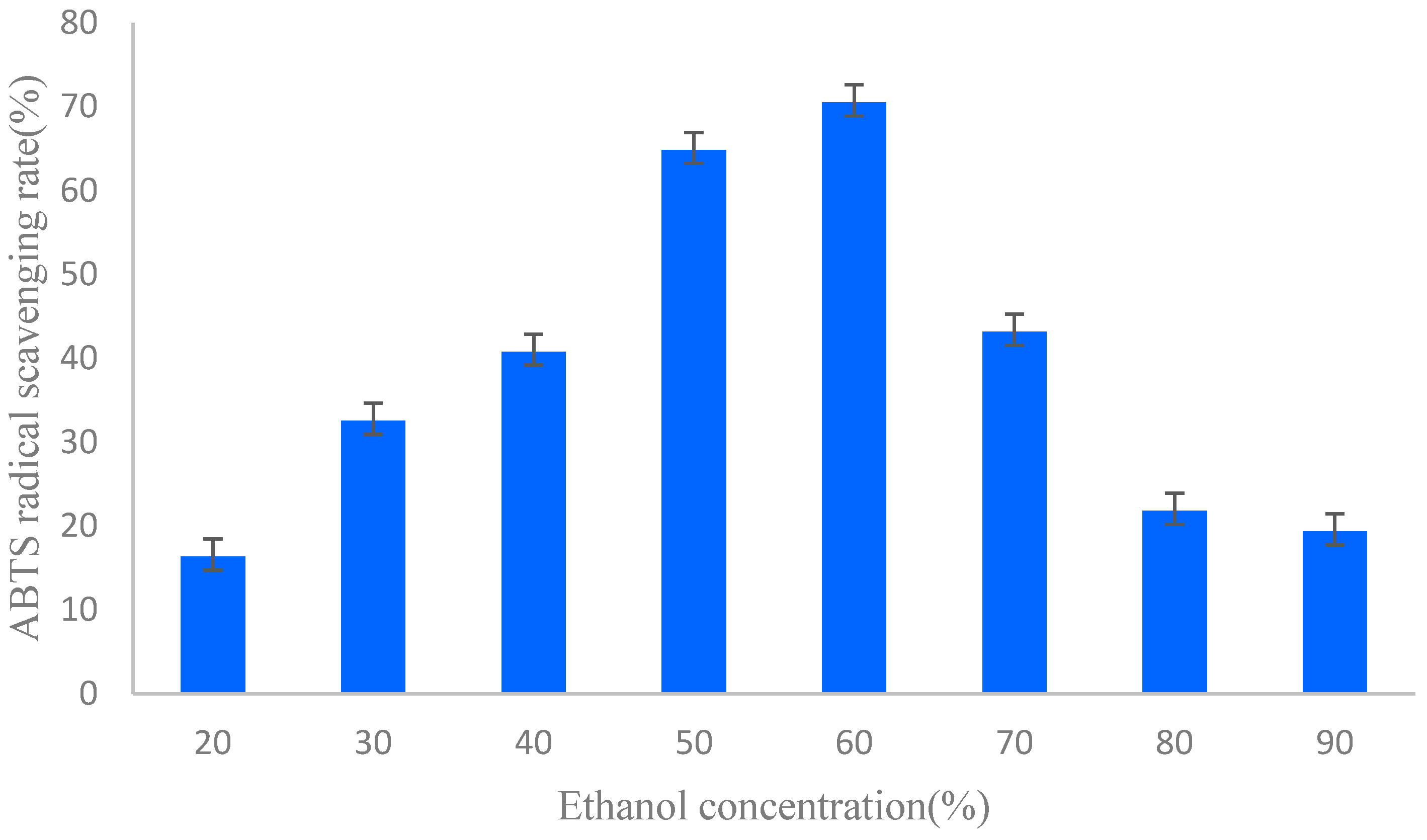
| Ethanol Concentration (%) | Weight (g) | Flavonoids Content (mg·g−1) |
|---|---|---|
| 20 | 0.83 | 43.4 ± 5.21 |
| 30 | 1.25 | 81.4 ± 3.26 |
| 40 | 2.21 | 108.5 ± 7.14 |
| 50 | 10.24 | 276.0 ± 6.58 |
| 60 | 7.62 | 257.8 ± 10.23 |
| 70 | 4.22 | 124.5 ± 8.56 |
| 80 | 2.67 | 61.4 ± 3.12 |
| 90 | 0.48 | 47.8 ± 2.89 |
| Compounds | IC50 (μg·mL−1) | |||
|---|---|---|---|---|
| ABTS | O•2− | DPPH• | •OH | |
| 1 | 25.4 ± 0.68 | 75.8 ± 0.11 | 24.6 ± 0.41 | 141.9 ± 0.17 |
| 2 | 36.5 ± 0.76 | 86.4 ± 0.21 | 34.2 ± 0.98 | 155.2 ± 0.25 |
| 3 | 41.1 ± 0.24 | 84.2 ± 0.09 | 40.1 ± 0.13 | 157.3 ± 0.17 |
| 4 | 35.7 ± 0.35 | 80.1 ± 0.14 | 35.3 ± 0.21 | 134.8 ± 0.12 |
| 5 | 23.5 ± 0.44 | 69.4 ± 0.17 | 20.9 ± 0.27 | 121.8 ± 0.14 |
| Vc | 28.7 ± 2.54 | 72.3 ± 2.56 | 26.4 ± 0.21 | 132.1 ± 3.15 |
| Samples | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Flavonoid-rich extracts | 32.18 ± 1.21 | 84.75 ± 1.24 | 183.42 ± 2.74 | 106.25 ± 3.15 | 66.17 ± 2.23 |
| Dried flower | 0.62 ± 0.08 | 1.66 ± 0.34 | 10.25 ± 0.35 | 6.44 ± 0.45 | 1.02 ± 0.08 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, X.; Wu, K.; Wang, M.; Liu, P.; Wang, X.; Deng, R. Antioxidant Activities and Chemical Constituents of Flavonoids from the Flower of Paeonia ostii. Molecules 2017, 22, 5. https://doi.org/10.3390/molecules22010005
Zhang H, Li X, Wu K, Wang M, Liu P, Wang X, Deng R. Antioxidant Activities and Chemical Constituents of Flavonoids from the Flower of Paeonia ostii. Molecules. 2017; 22(1):5. https://doi.org/10.3390/molecules22010005
Chicago/Turabian StyleZhang, Huifang, Xiaofang Li, Ke Wu, Mengke Wang, Pu Liu, Xinsheng Wang, and Ruixue Deng. 2017. "Antioxidant Activities and Chemical Constituents of Flavonoids from the Flower of Paeonia ostii" Molecules 22, no. 1: 5. https://doi.org/10.3390/molecules22010005