Synthesis of Some New Pyridazine Derivatives for Anti-HAV Evaluation
Abstract
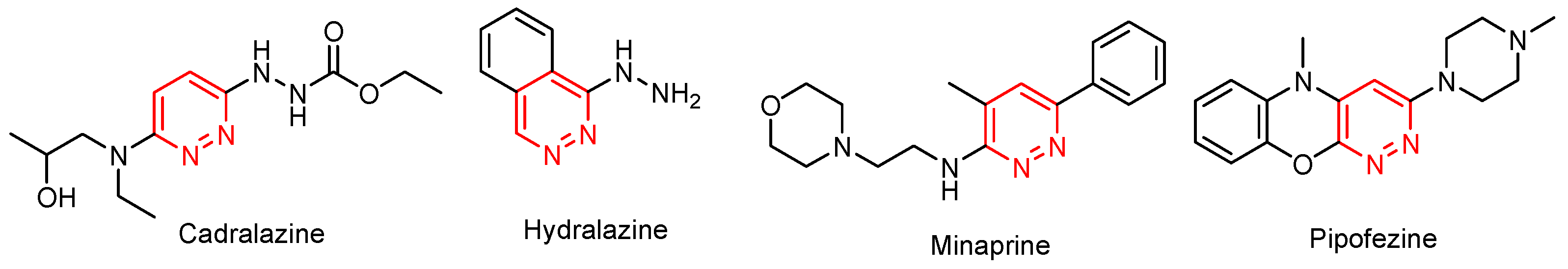
:1. Introduction
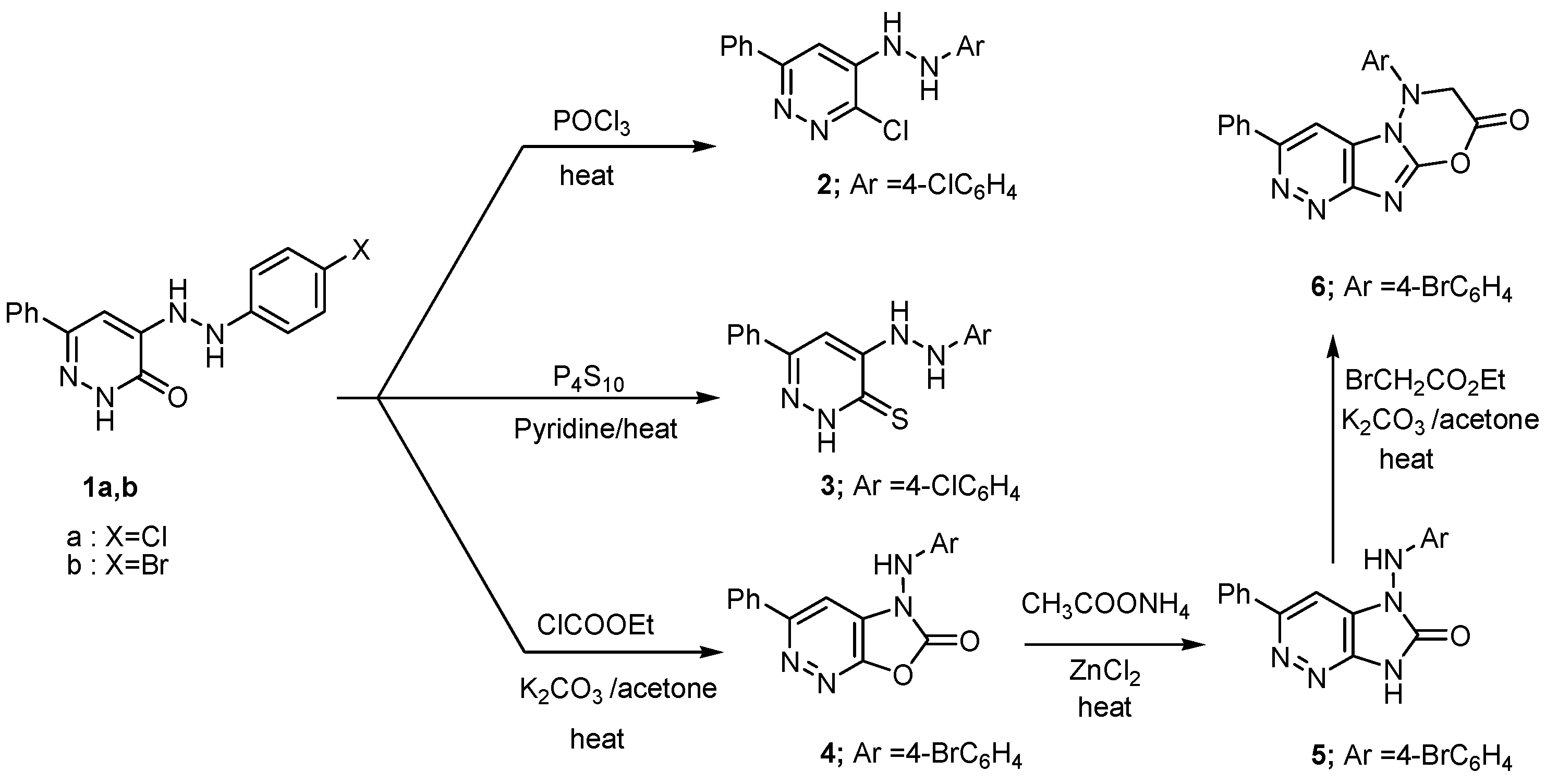
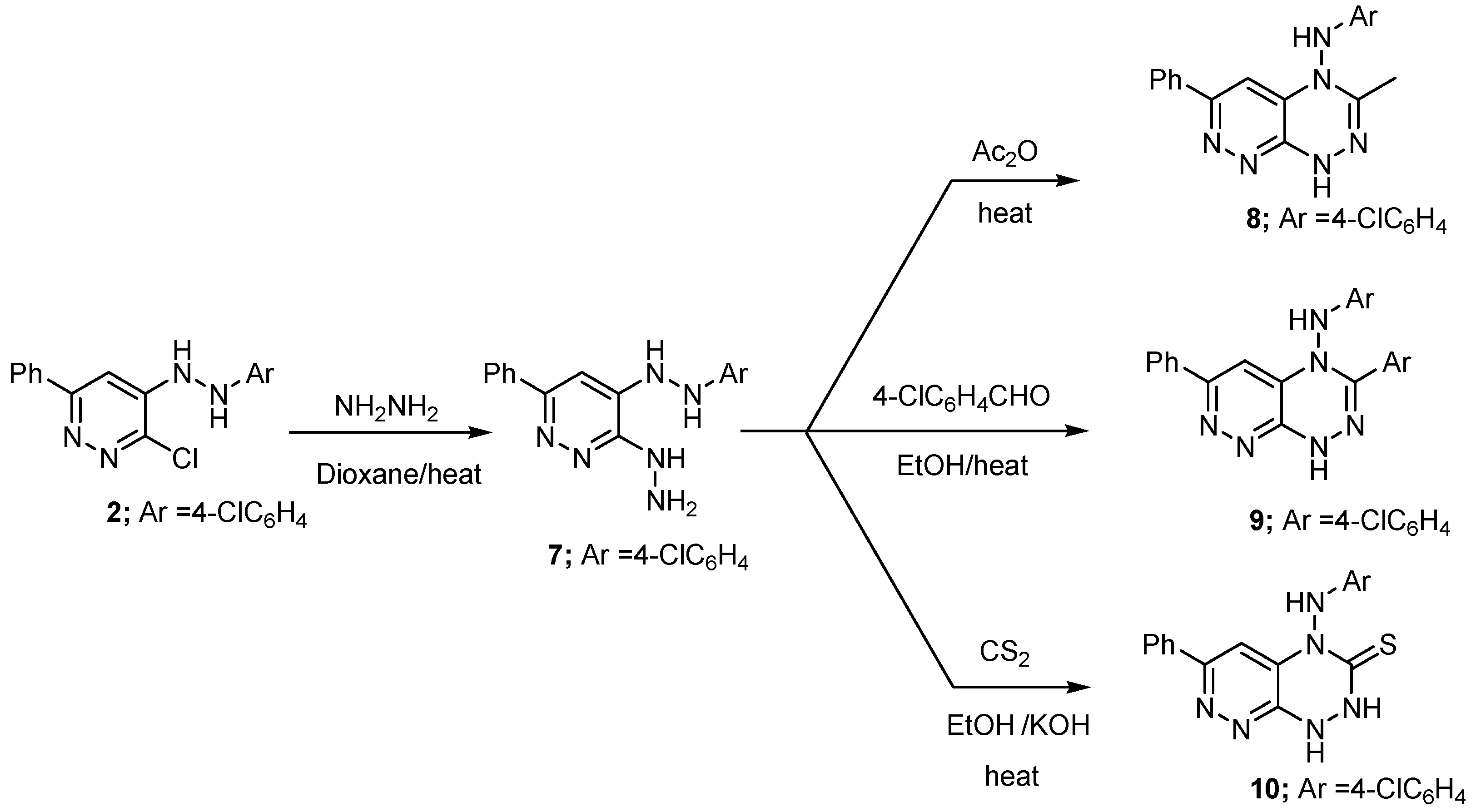
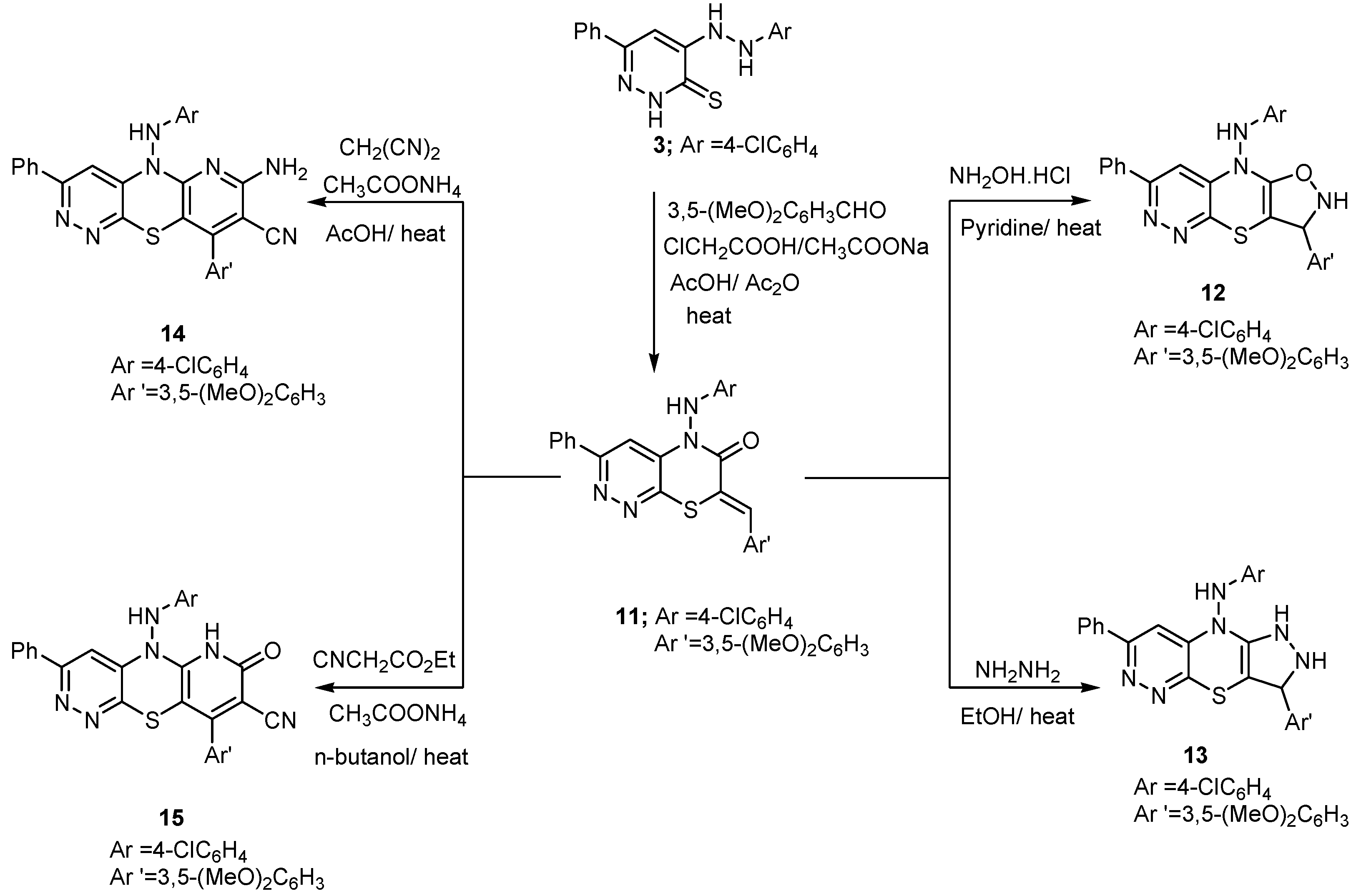
2. Results and Discussion
2.1. Chemistry
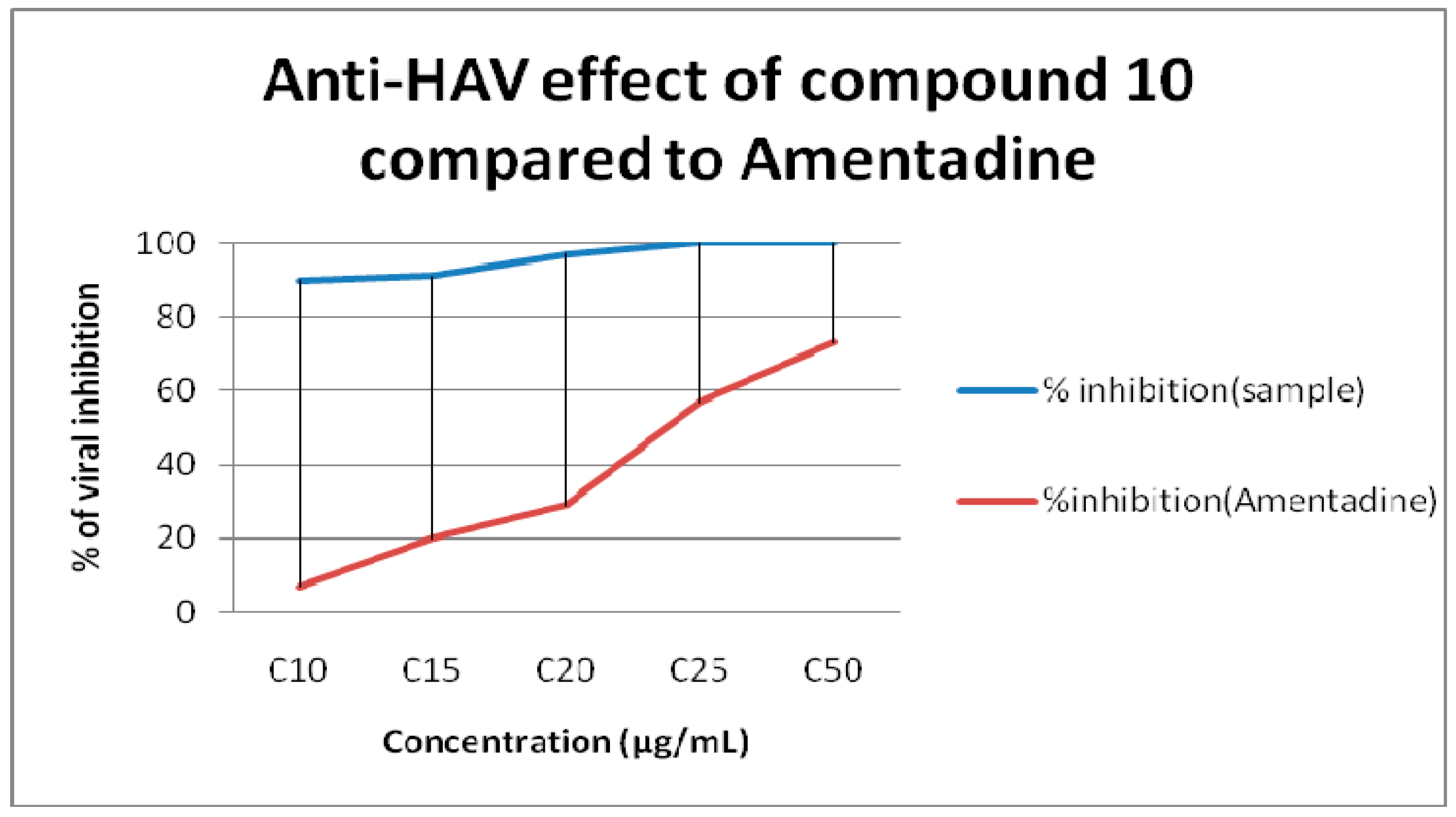
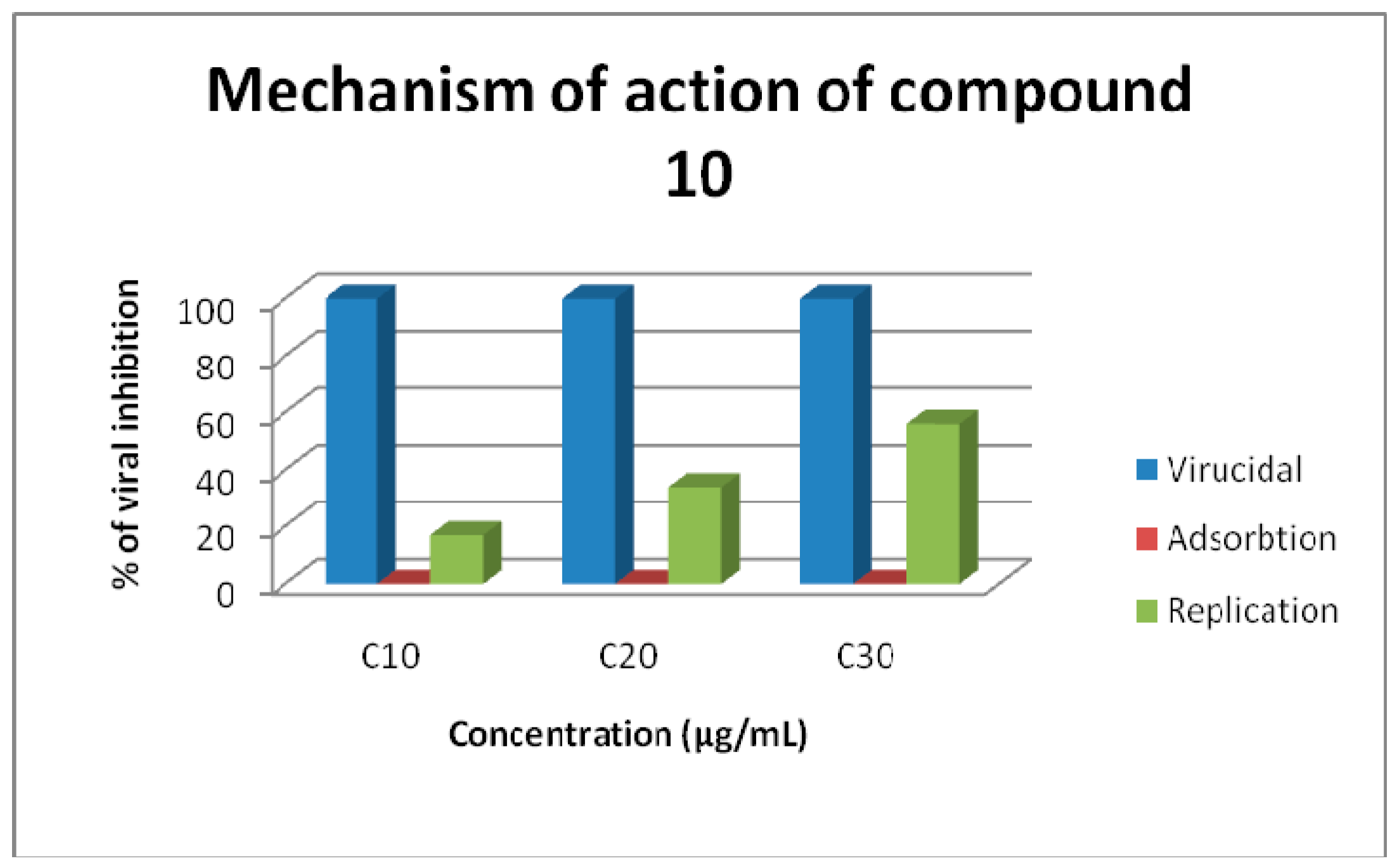
2.2. Antiviral Screening
3. Materials and Methods
3.1. General Inforation
3.2. Chemistry
3.2.1. 3-Chloro-4-(2-(4-chlorophenyl)hydrazinyl)-6-phenylpyridazine (2)
3.2.2. 4-(2-(4-Chlorophenyl)hydrazinyl)-6-phenylpyridazine-3(2H)-thione (3)
3.2.3. 5-(4-Bromophenylamino)-3-phenyloxazolo[5,4-c]pyridazin-6(5H)-one (4)
3.2.4. 5-(4-Bromophenylamino)-3-phenyl-5H-imidazo[4,5-c]pyridazin-6(7H)-one (5)
3.2.5. 6-(4-Bromophenyl)-3-phenyl-6,7-dihydro-8H-pyridazino[3′,4′:4,5]imidazo[2,1-b][1,3,4]oxadiazin-8-one (6)
3.2.6. 4-(2-(4-Chlorophenyl)hydrazinyl)-3-hydrazinyl-6-phenylpyridazine (7)
3.2.7. N-(4-Chlorophenyl)-3-methyl-6-phenylpyridazino[4,3-e][1,2,4]triazin-4(1H)-amine (8)
3.2.8. N,3-Bis(4-chlorophenyl)-6-phenylpyridazino[4,3-e][1,2,4]triazin-4(1H)-amine (9)
3.2.9. 4-(4-Chlorophenylamino)-6-phenyl-1,2-dihydropyridazino[4,3-e][1,2,4]triazine-3(4H)-thione (10)
3.2.10. 5-(4-Chlorophenylamino)-7-(3,5-dimethoxybenzylidene)-3-phenyl-5H-pyridazino[3,4-b][1,4]thiazin-6(7H)-one (11)
3.2.11. N-(4-Chlorophenyl)-3-(3,5-dimethoxyphenyl)-7-phenyl-2,3-dihydro-9H-isoxazolo[4,5-b]pyridazino[4,3-e][1,4]thiazin-9-amine (12)
3.2.12. N-(4-Chlorophenyl)-3-(3,5-dimethoxyphenyl)-7-phenyl-2,3-dihydropyrazolo[4,3-b]pyridazino[4,3-e][1,4]thiazin-9(1H)-amine (13)
3.2.13. 7-Amino-5-((4-chlorophenyl)amino)-9-(3,5-dimethoxyphenyl)-3-phenyl-5H-pyridazino[3,4-b]pyrido[2,3-e][1,4]thiazine-8-carbonitrile (14)
3.2.14. 5-((4-Chlorophenyl)amino)-9-(3,5-dimethoxyphenyl)-7-oxo-3-phenyl-6,7-dihydro-5H-pyridazino[3,4-b]pyrido[2,3-e][1,4]thiazine-8-carbonitrile (15)
3.3. Anti-Viral Bioassay
3.3.1. Cytotoxicity Assay
3.3.2. Plaque Infectivity Count Assay
3.3.3. Mechanism of Virus Inhabitation
Extract Affects Viral Particle Itself (Virucidal)
Extract Binds to Cell Receptor Preventing Viral Adsorption (Early Replication Step)
Extract Affects One of the Enzymes inside the Cell Needed by the Virus to Complete Its Replication Cycle (Late Replication Step)
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Akhtar, W.; Shaquiquzzaman, M.; Akhter, M.; Verma, G.; Khan, M.F.; Alam, M.M. The therapeutic journey of pyridazinone. Eur. J. Med. Chem. 2016, 123, 256–281. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Singh, A. Exploring potential, synthetic methods and general chemistry of pyridazine and pyridazinone: A brief introduction. Int. J. ChemTech Res. 2010, 2, 1112–1128. [Google Scholar]
- Costas, T.; Besada, P.; Piras, A.; Acevedo, L.; Yañez, M.; Orallo, F.; Laguna, R.; Terán, C. New pyridazinone derivatives with vasorelaxant and platelet antiaggregatory activities. Bioorg. Med. Chem. Lett. 2010, 20, 6624–6627. [Google Scholar] [CrossRef] [PubMed]
- Mantu, D.; Luca, M.C.; Moldoveanu, C.; Zbancioc, G.; Mangalagiu, I.I. Synthesis and antituberculosis activity of some new pyridazine derivatives. Part II. Eur. J. Med. Chem. 2010, 45, 5164–5168. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.; Whitmore, A. The tautomeric properties of 6-(2-pyrrolyl)pyridazin-3-one and 6-(2-pyrrolyl)pyridazin-3-thione. Arkivoc 2007, 11, 114–119. [Google Scholar]
- Cignarella, G.; Barlocco, D.; Pinna, G.A.; Loriga, M.; Curzu, M.M.; Tofanetti, O.; Germini, M.; Cazzulani, P.; Cavalletti, E. Synthesis and biological evaluation of substituted benzo[H] cinnolinones and 3H-benzo[6,7]cyclohepta[1,2-c]pyridazinones: Higher homologs of the antihypertensive and antithrombotic 5H-indeno[1,2-c]pyridazinones. J. Med. Chem. 1989, 32, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Salvadeo, A.; Villa, G.; Segagni, S.; Piazza, V.; Picardi, L.; Romano, M.; Parini, J. Cadralazine, a new vasodilator, in addition to a beta-blocker for long-term treatment of hypertension. Arzneim. Forsch. 1985, 35, 623–625. [Google Scholar]
- Semeraro, C.; Dorigotti, L.; Banfi, S.; Carpi, C. Pharmacological studies on cadralazine: A new antihypertensive vasodilator drug. J. Cardiovasc. Pharmacol. 1981, 3, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Fung, M.; Thornton, A.; Mybeck, K.; Wu, J.H.-H.; Hornbuckle, K.; Muniz, E. Evaluation of the characteristics of safety withdrawal of prescription drugs from worldwide pharmaceutical markets-1960 to 1999. Drug Inf. J. 2001, 35, 293–317. [Google Scholar] [CrossRef]
- Biziere, K.; Worms, P.; Kan, J.; Mandel, P.; Garattini, S.; Roncucci, R. Minaprine, a new drug with antidepressant properties. Drugs Exp. Clin. Res. 1984, 11, 831–840. [Google Scholar]
- Biziere, K.; Kan, J.; Souilhac, J.; Muyard, J.; Roncucci, R. Pharmacological evaluation of minaprine dihydrochloride, a new psychotropic drug. Arzneim. Forsch. 1981, 32, 824–831. [Google Scholar]
- Imad, S.; Nisar, S.; Maqsood, Z.T. A study of redox properties of hydralazine hydrochloride, an antihypertensive drug. J. Saudi Chem. Soc. 2010, 14, 241–245. [Google Scholar] [CrossRef]
- Cohn, J.N.; Johnson, G.; Ziesche, S.; Cobb, F.; Francis, G.; Tristani, F.; Smith, R.; Dunkman, W.B.; Loeb, H.; Wong, M. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N. Engl. J. Med. 1991, 325, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Asif, M. The pharmacological importance of some diazine containing drug molecules. Sop Trans. Org. Chem. 2014, 1, 1–16. [Google Scholar] [CrossRef]
- Li, D.; Zhan, P.; Liu, H.; Pannecouque, C.; Balzarini, J.; de Clercq, E.; Liu, X. Synthesis and biological evaluation of pyridazine derivatives as novel HIV-1 NNRTIs. Bioorg. Med. Chem. 2013, 21, 2128–2134. [Google Scholar] [CrossRef] [PubMed]
- Flefel, E.M.; Abdel-Mageid, R.E.; Tantawy, W.A.; Ali, M.A.; Amr, A.G. Heterocyclic compounds based on 3-(4-bromophenyl)azo-5-phenyl-2(3H)-furanone: Anti-avian influenza virus (H5N1) activity. Acta Pharm. 2012, 62, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, M.; Yao, X.; Li, Y.; Tan, J.; Wang, L.; Qiao, W.; Geng, Y.; Liu, Y.; Wang, Q. Design, synthesis and antiviral activity of novel pyridazines. Eur. J. Med. Chem. 2012, 54, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Fan, H.-T.; Song, B.-A.; Jin, L.-H.; Bhadury, P.S.; Hu, D.-Y.; Yang, S. Synthesis and Antiviral Activities of α-Aminophosphonate Derivatives Containing a Pyridazine Moiety. Phosphorus Sulfur Silicon Relat. Elem. 2010, 186, 81–87. [Google Scholar] [CrossRef]
- Ferro, S.; Agnello, S.; Barreca, M.L.; de Luca, L.; Christ, F.; Gitto, R. Synthesis of new pyridazine derivatives as potential anti-HIV-1 agents. J. Heterocycl. Chem. 2009, 46, 1420–1424. [Google Scholar] [CrossRef]
- Galtier, C.; Mavel, S.; Snoeck, R.; Andrei, G.; Pannecouque, C.; Witvrouw, M.; Balzarini, J.; de Clercq, E.; Gueiffier, A. Synthesis and antiviral activities of 3-aralkylthiomethylimidazo[1,2-b]pyridazine derivatives. Antivir. Chem. Chemother. 2003, 14, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Özadalı, K.; Özkanlı, F.; Jain, S.; Rao, P.P.N.; Velázquez-Martínez, C.A. Synthesis and biological evaluation of isoxazolo[4,5-d]pyridazin-4-(5H)-one analogues as potent anti-inflammatory agents. Bioorg. Med. Chem. 2012, 20, 2912–2922. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, K.; Takita, S.; Eiraku, T.; Kojima, A.; Iwase, K.; Kishi, T.; Fukuchi, K.; Yasue, T.; Adams, D.R.; Allcock, R.W.; et al. Phosphodiesterase inhibitors. Part 3: Design, synthesis and structure–activity relationships of dual PDE3/4-inhibitory fused bicyclic heteroaromatic-dihydropyridazinones with anti-inflammatory and bronchodilatory activity. Bioorg. Med. Chem. 2012, 20, 1644–1658. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.M.; Khalil, N.A.; Ahmed, E.M.; Eissa, K.I. Synthesis and anti-inflammatory activity of novel pyridazine and pyridazinone derivatives as non-ulcerogenic agents. Arch. Pharm. Res. 2012, 35, 2077–2092. [Google Scholar] [CrossRef] [PubMed]
- Tewari, A.K.; Dubey, R.; Mishra, A. 2-Substituted-8-methyl-3,6-dihydroimidazo[4,5-c]pyrazolo[3,4-e]pyridazine as an anti-inflammatory agent. Med. Chem. Res. 2011, 20, 125–129. [Google Scholar] [CrossRef]
- Abouzid, K.; Bekhit, S.A. Novel anti-inflammatory agents based on pyridazinone scaffold; design, synthesis and in vivo activity. Bioorg. Med. Chem. 2008, 16, 5547–5556. [Google Scholar] [CrossRef] [PubMed]
- Faidallah, H.M.; Rostom, S.A.; Basaif, S.A.; Makki, M.S.; Khan, K.A. Synthesis and biological evaluation of some novel urea and thiourea derivatives of isoxazolo[4,5-d]pyridazine and structurally related thiazolo[4,5-d]pyridazine as antimicrobial agents. Arch. Pharmacal Res. 2013, 36, 1354–1368. [Google Scholar] [CrossRef] [PubMed]
- Faidallah, H.M.; Khan, K.A.; Makki, M.S.I. Synthesis and Biological Evaluation of New Fused Isoxazolo[4,5-d] Pyridazine Derivatives. J. Chin. Chem. Soc. 2011, 58, 191–198. [Google Scholar] [CrossRef]
- El-Mariah, F.; Hosny, M.; Deeb, A. Pyridazine Derivatives and Related Compounds, Part 17: The Synthesis of Some 3-Substituted Pyridazino[3′,4′:3,4]pyrazolo[5,1-c]-1,2,4-triazines and Their Antimicrobial Activity. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 809–818. [Google Scholar] [CrossRef]
- Yue, Q.; Zhao, Y.; Hai, L.; Zhang, T.; Guo, L.; Wu, Y. First synthesis of novel 3,3′-bipyridazine derivatives as new potent antihepatocellular carcinoma agents. Tetrahedron 2015, 71, 7670–7675. [Google Scholar] [CrossRef]
- Asif, M. The anticancer potential of various substituted pyridazines and related compounds. Int. J. Adv. Chem. 2014, 2, 148–161. [Google Scholar] [CrossRef]
- Zhou, S.; Liao, H.; He, C.; Dou, Y.; Jiang, M.; Ren, L.; Zhao, Y.; Gong, P. Design, synthesis and structure–activity relationships of novel 4-phenoxyquinoline derivatives containing pyridazinone moiety as potential antitumor agents. Eur. J. Med. Chem. 2014, 83, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Abouzid, K.A.; Khalil, N.A.; Ahmed, E.M.; Mohamed, K.O. 3-[(6-Arylamino)pyridazinylamino]benzoic acids: Design, synthesis and in vitro evaluation of anticancer activity. Arch. Pharm. Res. 2013, 36, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Csókás, D.; Zupkó, I.; Károlyi, B.I.; Drahos, L.; Holczbauer, T.; Palló, A.; Czugler, M.; Csámpai, A. Synthesis, spectroscopy, X-ray analysis and in vitro antiproliferative effect of ferrocenylmethylene-hydrazinylpyridazin-3(2H)-ones and related ferroceno[d]pyridazin-1(2H)-ones. J. Organomet. Chem. 2013, 743, 130–138. [Google Scholar] [CrossRef]
- Rathish, I.G.; Javed, K.; Ahmad, S.; Bano, S.; Alam, M.S.; Akhter, M.; Pillai, K.K.; Ovais, S.; Samim, M. Synthesis and evaluation of anticancer activity of some novel 6-aryl-2-(p-sulfamylphenyl)-pyridazin-3-(2H)-ones. Eur. J. Med. Chem. 2012, 49, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Murty, M.; Rao, B.R.; Ram, K.R.; Yadav, J.; Antony, J.; Anto, R.J. Synthesis and preliminary evaluation activity studies of novel 4-(aryl/heteroaryl-2-ylmethyl)-6-phenyl-2-[3-(4-substituted-piperazine-1-yl)propyl]pyridazin-3(2H)-one derivatives as anticancer agents. Med. Chem. Res. 2012, 21, 3161–3169. [Google Scholar] [CrossRef]
- Al-Tel, T.H. Design and synthesis of novel tetrahydro-2H-Pyrano[3,2-c]pyridazin-3(6H)-one derivatives as potential anticancer agents. Eur. J. Med. Chem. 2010, 45, 5724–5731. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Verma, A.; Sharma, U.K.; Prajapati, S. Efficient synthesis, anticonvulsant and muscle relaxant activities of new 2-((5-amino-1,3,4-thiadiazol-2-yl)methyl)-6-phenyl-4,5-dihydropyridazin-3(2H)-one derivatives. Med. Chem. Res. 2014, 23, 146–157. [Google Scholar] [CrossRef]
- Asif, M.; Anita, S. Anticonvulsant Activity of 4-(Substituted Benzylidene)-6-(3-nitrophenyl)-4, 5-dihydroPyridazin-3(2H)-ones against Maximal Electro Shock Induced Seizure. Middle-East J. Sci. Res. 2011, 9, 481–485. [Google Scholar]
- Banerjee, P.; Sharma, P.; Nema, R. Synthesis and anticonvulsant activity of pyridazinone derivatives. Int. J. ChemTech Res 2009, 1, 522–525. [Google Scholar]
- Sivakumar, R.; Gnanasam, S.K.; Ramachandran, S.; Leonard, J.T. Pharmacological evaluation of some new 1-substituted-4-hydroxy-phthalazines. Eur. J. Med. Chem. 2002, 37, 793–801. [Google Scholar] [CrossRef]
- Asif, M.; Singh, D.; Singh, A. Analgesic activity of some 6-phenyl-4-substituted benzylidene tetrahydro pyridazin-3(2H)-ones. Glob. J. Pharmacol. 2011, 5, 18–22. [Google Scholar]
- Biancalani, C.; Giovannoni, M.P.; Pieretti, S.; Cesari, N.; Graziano, A.; Vergelli, C.; Cilibrizzi, A.; Di Gianuario, A.; Colucci, M.; Mangano, G. Further Studies on Arylpiperazinyl Alkyl Pyridazinones: Discovery of an Exceptionally Potent, Orally Active, Antinociceptive Agent in Thermally Induced Pain. J. Med. Chem. 2009, 52, 7397–7409. [Google Scholar] [CrossRef] [PubMed]
- Tan, O.U.; Ozadali, K.; Yogeeswari, P.; Sriram, D.; Balkan, A. Synthesis and antimycobacterial activities of some new N-acylhydrazone and thiosemicarbazide derivatives of 6-methyl-4,5-dihydropyridazin-3(2H)-one. Med. Chem. Res. 2012, 21, 2388–2394. [Google Scholar]
- Husain, A.; Ahmad, A.; Bhandari, A.; Ram, V. Synthesis and antitubercular activity of pyridazinone derivatives. J. Chil. Chem. Soc. 2011, 56, 778–780. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Islam, M.; Kumar, S. Synthesis and antitubeculostic activity of 5-{3′-oxo-6′-(substituted phenyl)-2′,3′,4′,5′-tetrahyropyridazin-2′-yl} methyl-2-substituted 1,3,4-oxadiazole. Der Pharm. Lett. 2010, 2, 319–327. [Google Scholar]
- Abouzid, K.; Abdel Hakeem, M.; Khalil, O.; Maklad, Y. Pyridazinone derivatives: Design, synthesis, and in vitro vasorelaxant activity. Bioorg. Med. Chem. 2008, 16, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Rathish, I.G.; Javed, K.; Bano, S.; Ahmad, S.; Alam, M.S.; Pillai, K.K. Synthesis and blood glucose lowering effect of novel pyridazinone substituted benzenesulfonylurea derivatives. Eur. J. Med. Chem. 2009, 44, 2673–2678. [Google Scholar] [CrossRef] [PubMed]
- Boukharsa, Y.; Meddah, B.; Tiendrebeogo, R.Y.; Ibrahimi, A.; Taoufik, J.; Cherrah, Y.; Benomar, A.; Faouzi, M.E.A.; Ansar, M.H. Synthesis and antidepressant activity of 5-(benzo[b]furan-2-ylmethyl)-6-methylpyridazin-3(2H)-one derivatives. Med. Chem. Res. 2016, 25, 494–500. [Google Scholar] [CrossRef]
- Wermuth, C.G.; Schlewer, G.; Bourguignon, J.J.; Maghioros, G.; Bouchet, M.J.; Moire, C.; Kan, J.P.; Worms, P.; Biziere, K. 3-aminopyridazine derivatives with atypical antidepressant, serotonergic, and dopaminergic activities. J. Med. Chem. 1989, 32, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Fang, H.; Wang, J.; Meng, Q.; Dai, X.; Wu, S.; Luo, J.; Pu, D.; Chen, L.; Minick, D. Discovery of a novel 2,3,11,11a-tetrahydro-1H-pyrazino[1,2-b]isoquinoline-1,4(6H)-dione series promoting neurogenesis of human neural progenitor cells. Bioorg. Med. Chem. Lett. 2015, 25, 3748–3753. [Google Scholar] [CrossRef] [PubMed]
- Contreras, J.-M.; Parrot, I.; Sippl, W.; Rival, Y.M.; Wermuth, C.G. Design, synthesis, and structure-activity relationships of a series of 3-[2-(1-benzylpiperidin-4-yl)ethylamino]pyridazine derivatives as acetylcholinesterase inhibitors. J. Med. Chem. 2001, 44, 2707–2718. [Google Scholar] [CrossRef] [PubMed]
- Biagini, P.; Biancalani, C.; Graziano, A.; Cesari, N.; Giovannoni, M.P.; Cilibrizzi, A.; Dal Piaz, V.; Vergelli, C.; Crocetti, L.; Delcanale, M. Functionalized pyrazoles and pyrazolo[3,4-d]pyridazinones: Synthesis and evaluation of their phosphodiesterase 4 inhibitory activity. Bioorg. Med. Chem. 2010, 18, 3506–3517. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, M.P.; Vergelli, C.; Biancalani, C.; Cesari, N.; Graziano, A.; Biagini, P.; Gracia, J.; Gavaldà, A.; Dal Piaz, V. Novel pyrazolopyrimidopyridazinones with potent and selective phosphodiesterase 5 (PDE5) inhibitory activity as potential agents for treatment of erectile dysfunction. J. Med. Chem. 2006, 49, 5363–5371. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.A.; Mishra, R.; Shaharyar, M.; Husain, A.; Rashid, M.; Pal, P. Triazole incorporated pyridazinones as a new class of antihypertensive agents: Design, synthesis and in vivo screening. Bioorg. Med. Chem. Lett. 2011, 21, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Thota, S.; Bansal, R. Synthesis of new pyridazinone derivatives as platelet aggregation inhibitors. Med. Chem. Res. 2010, 19, 808–816. [Google Scholar] [CrossRef]
- Kumar, D.; Carron, R.; La Calle, C.; Jindal, D.; Bansal, R. Synthesis and evaluation of 2-substituted-6-phenyl-4,5-dihydropyridazin-3(2H)-ones as potent inodilators. Acta Pharm. 2008, 58, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, E.; Fraiz, N.; Yanez, M.; Terrades, V.; Laguna, R.; Cano, E.; Raviña, E. Pyridazines. Part XXIX: Synthesis and platelet aggregation inhibition activity of 5-substituted-6-phenyl-3(2H)-pyridazinones. Novel aspects of their biological actions. Bioorg. Med. Chem. 2002, 10, 2873–2882. [Google Scholar] [CrossRef]
- Gelain, A.; Barlocco, D.; Kwon, B.-M.; Jeong, T.-S.; Im, K.-R.; Legnani, L.; Toma, L. Biphenyl versus Phenylpyridazine Derivatives: The Role of the Heterocycle in a Series of Acyl-CoA: Cholesterol Acyl Transferase Inhibitors. J. Med. Chem. 2008, 51, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
- Tomori, T.; Miyatake, Y.; Sato, Y.; Kanamori, T.; Masaki, Y.; Ohkubo, A.; Sekine, M.; Seio, K. Synthesis of Peptide Nucleic Acids Containing Pyridazine Derivatives As Cytosine and Thymine Analogs, and Their Duplexes with Complementary Oligodeoxynucleotides. Org. Lett. 2015, 17, 1609–1612. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.J.; Ray, C.G. Medical Microbiology: An Introduction to Infectious Diseases; McGraw-Hill: New York, NY, USA, 2004. [Google Scholar]
- Koff, R.S. Hepatitis A. Lancet 1998, 351, 1643–1649. [Google Scholar] [CrossRef]
- Wasley, A.; Fiore, A.; Bell, B.P. Hepatitis A in the era of vaccination. Epidemiol. Rev. 2006, 28, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, F.B.; Liang, T.J. Hepatitis B virus. Fields Virol. 2001, 4, 2971–3036. [Google Scholar]
- Rashad, A.E.; Mohamed, M.S.; Zaki, M.E.A.; Fatahala, S.S. Synthesis and Biological Evaluation of Some Pyrrolo[2,3-d]pyrimidines. Arch. Pharm. 2006, 339, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, M.T.; El-Sayed, W.A.; El-Ashry, E.-S.H. Synthesis and Antiviral Evaluation of Some Sugar Arylglycinoylhydrazones and Their Oxadiazoline Derivatives. Arch. Pharm. 2006, 339, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, A.A.H.; Wada, T. Synthesis and Antiviral Evaluation of 5-(1,2,3-Triazol-1-ylmethyl)uridine Derivatives. Z. Naturforschung Sect. C J. Biosci. 2009, 64, 163–166. [Google Scholar] [CrossRef]
- El-Sayed, A.A.; El-Shahat, M.; Rabie, S.T.; Flefel, E.M.; Abd-Elshafyc, D.N. New pyrimidine and fused pyrimidine derivatives: Synthesis and anti Hepatitis A virus (HAV) evaluation. Int. J. Pharm. 2015, 5, 69–79. [Google Scholar]
- Sayed, H.H.; Hashem, A.I.; Yousif, N.M.; El-Sayed, W.A. Conversion of 3-Arylazo-5-phenyl-2(3H)-furanones into Other Heterocycles of Anticipated Biological Activity. Arch. Pharm. 2007, 340, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Aquino, R.; de Simone, F.; Pizza, C.; Conti, C.; Stein, M. Plant Metabolites. Structure and In Vitro Antiviral Activity of Quinovic Acid Glycosides from Uncaria tomentosa and Guettarda platyipoda. J. Nat. Prod. 1989, 52, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Tebas, P.; Stabell, E.C.; Olivo, P.D. Antiviral susceptibility testing with a cell line which expresses beta-galactosidase after infection with herpes simplex virus. Antimicrob. Agents Chemother. 1995, 39, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Schuhmacher, A.; Reichling, J.; Schnitzler, P. Virucidal effect of peppermint oil on the enveloped viruses herpes simplex virus type 1 and type 2 in vitro. Phytomedicine 2003, 10, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhan, B.; Yao, X.; Gao, Y.; Song, J. Antiviral activity of tannin from the pericarp of Punica granatum L. against genital Herpes virus in vitro. China J. Chin. Mater. Med. 1995, 20, 556–558. [Google Scholar]
- Amoros, M.; Lurton, E.; Boustie, J.; Girre, L.; Sauvager, F.; Cormier, M. Comparison of the anti-herpes simplex virus activities of propolis and 3-methyl-but-2-enyl caffeate. J. Nat. Prod. 1994, 57, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
| Compounds | Concentrations (µg/mL) * | |||||
|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | 25 | 30 | |
| 2 | - | - | - | +2 | +3 | +3 |
| 3 | - | - | - | - | - | - |
| 4 | - | - | - | - | - | - |
| 5 | - | - | - | - | - | - |
| 6 | - | - | - | - | - | - |
| 8 | - | - | - | +1 | +2 | +4 |
| 9 | - | - | - | +1 | +2 | +4 |
| 10 | - | - | - | +2 | +3 | +4 |
| 11 | - | - | - | - | - | +1 |
| 12 | - | - | - | - | - | - |
| 13 | - | - | - | +1 | +2 | +3 |
| 14 | - | - | - | - | - | - |
| 15 | - | - | +1 | +3 | +4 | +4 |
| Compound | Concentration (µg/mL) | % Inhibition |
|---|---|---|
| 2 | 10 | 13 |
| 15 | 29 | |
| 3 | 20 | 19 |
| 25 | 66 | |
| 4 | 20 | 56 |
| 25 | 73 | |
| 5 | 20 | 30 |
| 25 | 62 | |
| 6 | 20 | 0 |
| 25 | 0 | |
| 8 | 10 | 26 |
| 15 | 65 | |
| 9 | 10 | 37 |
| 15 | 50 | |
| 10 | 10 | 100 |
| 15 | 100 | |
| 11 | 20 | 50 |
| 25 | 75 | |
| 12 | 20 | 42 |
| 25 | 37 | |
| 13 | 10 | 33 |
| 15 | 4 | |
| 14 | 20 | 46 |
| 25 | 76 | |
| 15 | 10 | 0 |
| 15 | 38 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flefel, E.M.; Tantawy, W.A.; El-Sofany, W.I.; El-Shahat, M.; El-Sayed, A.A.; Abd-Elshafy, D.N. Synthesis of Some New Pyridazine Derivatives for Anti-HAV Evaluation. Molecules 2017, 22, 148. https://doi.org/10.3390/molecules22010148
Flefel EM, Tantawy WA, El-Sofany WI, El-Shahat M, El-Sayed AA, Abd-Elshafy DN. Synthesis of Some New Pyridazine Derivatives for Anti-HAV Evaluation. Molecules. 2017; 22(1):148. https://doi.org/10.3390/molecules22010148
Chicago/Turabian StyleFlefel, Eman M., Waled A. Tantawy, Walaa I. El-Sofany, Mahmoud El-Shahat, Ahmed A. El-Sayed, and Dina N. Abd-Elshafy. 2017. "Synthesis of Some New Pyridazine Derivatives for Anti-HAV Evaluation" Molecules 22, no. 1: 148. https://doi.org/10.3390/molecules22010148
APA StyleFlefel, E. M., Tantawy, W. A., El-Sofany, W. I., El-Shahat, M., El-Sayed, A. A., & Abd-Elshafy, D. N. (2017). Synthesis of Some New Pyridazine Derivatives for Anti-HAV Evaluation. Molecules, 22(1), 148. https://doi.org/10.3390/molecules22010148






