Screening of Potential Xanthine Oxidase Inhibitors in Gnaphalium hypoleucum DC. by Immobilized Metal Affinity Chromatography and Ultrafiltration-Ultra Performance Liquid Chromatography-Mass Spectrometry
Abstract
:1. Introduction
2. Results
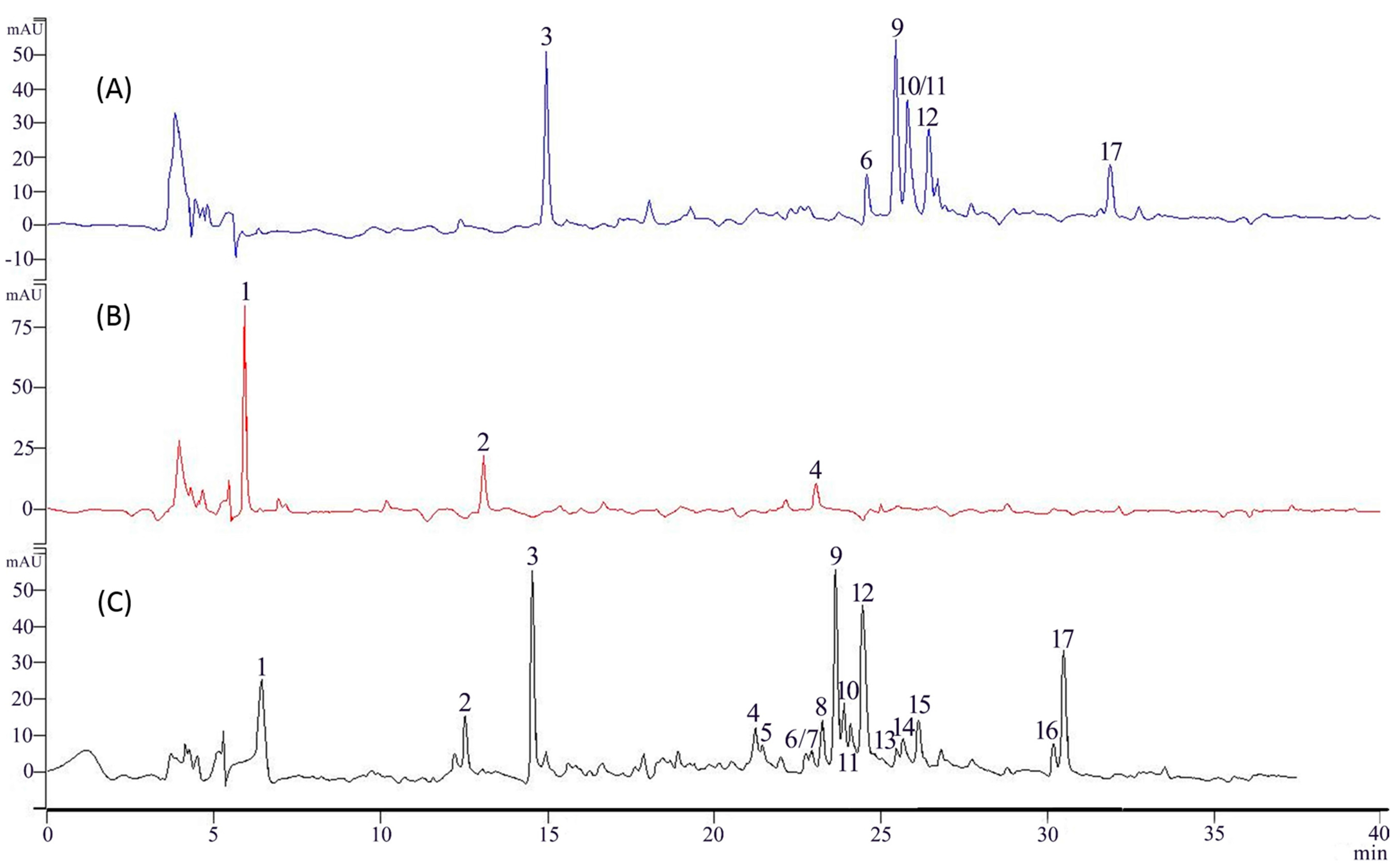
2.1. Binding and Elution of Flavonoids from IMAC Columns
2.2. Validation of XO Inhibitory Activities
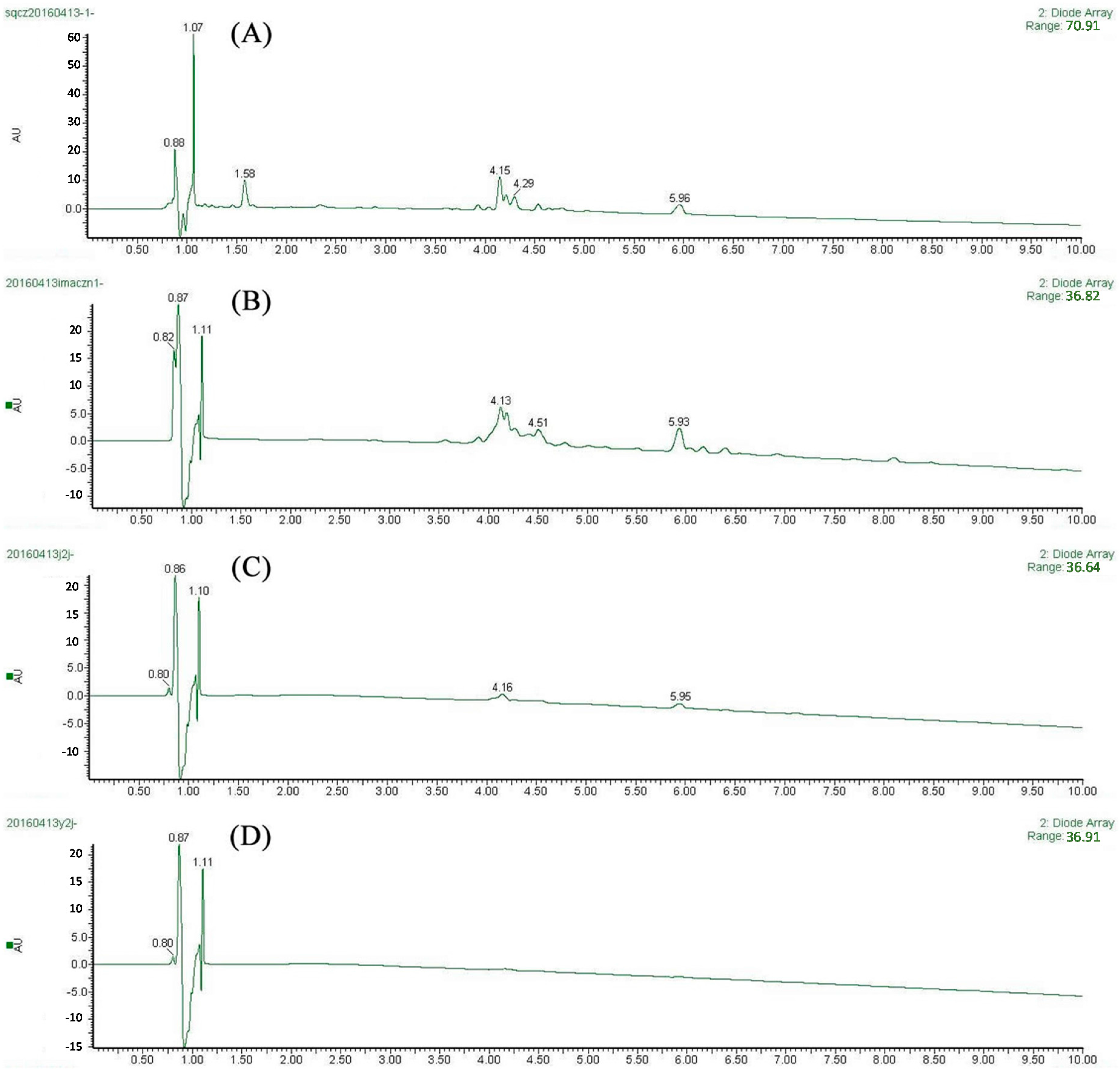
2.3. Screening of XO Ligands by IMAC-UF-UPLC-MS
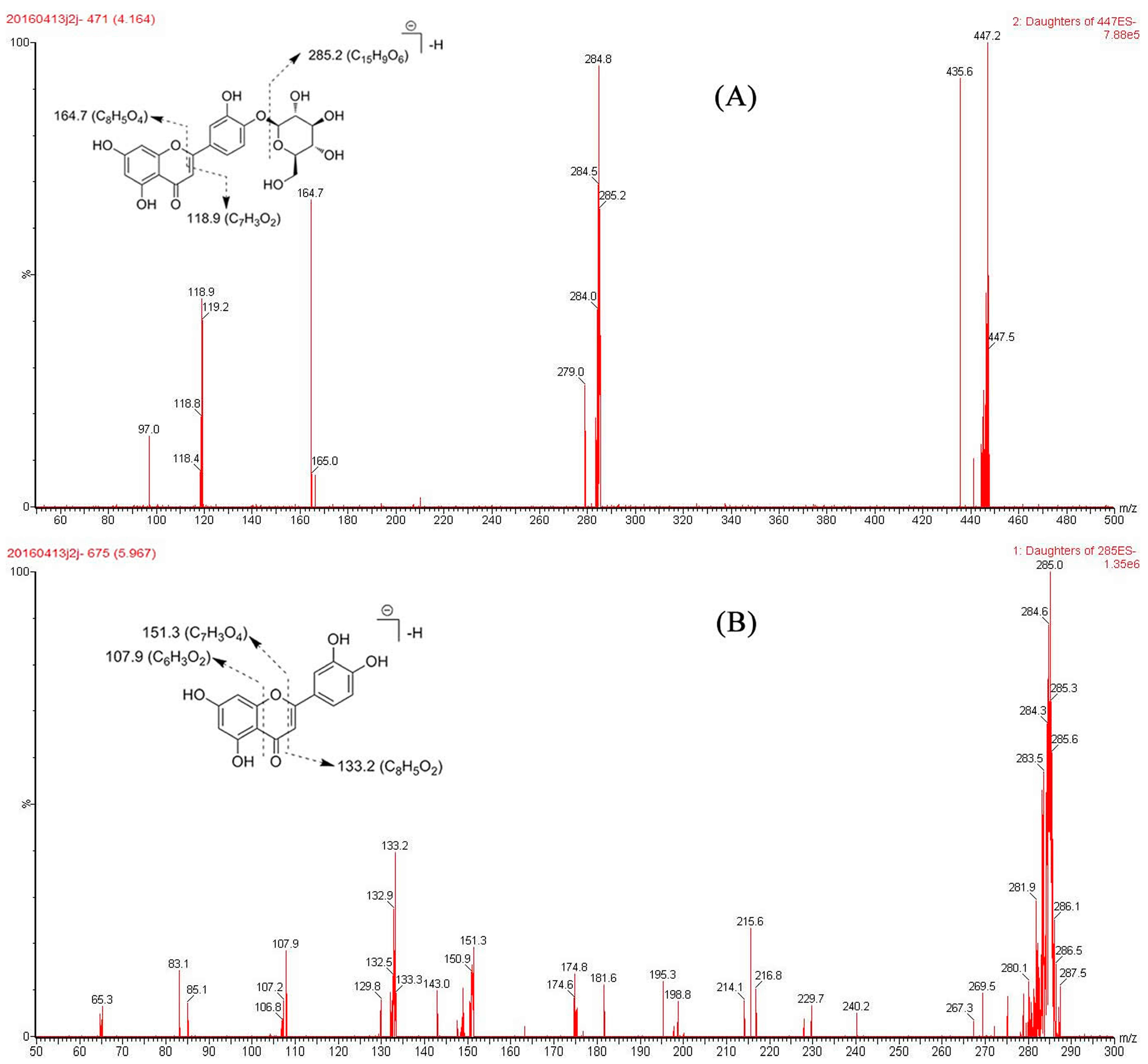
2.4. Identification of Active Compounds
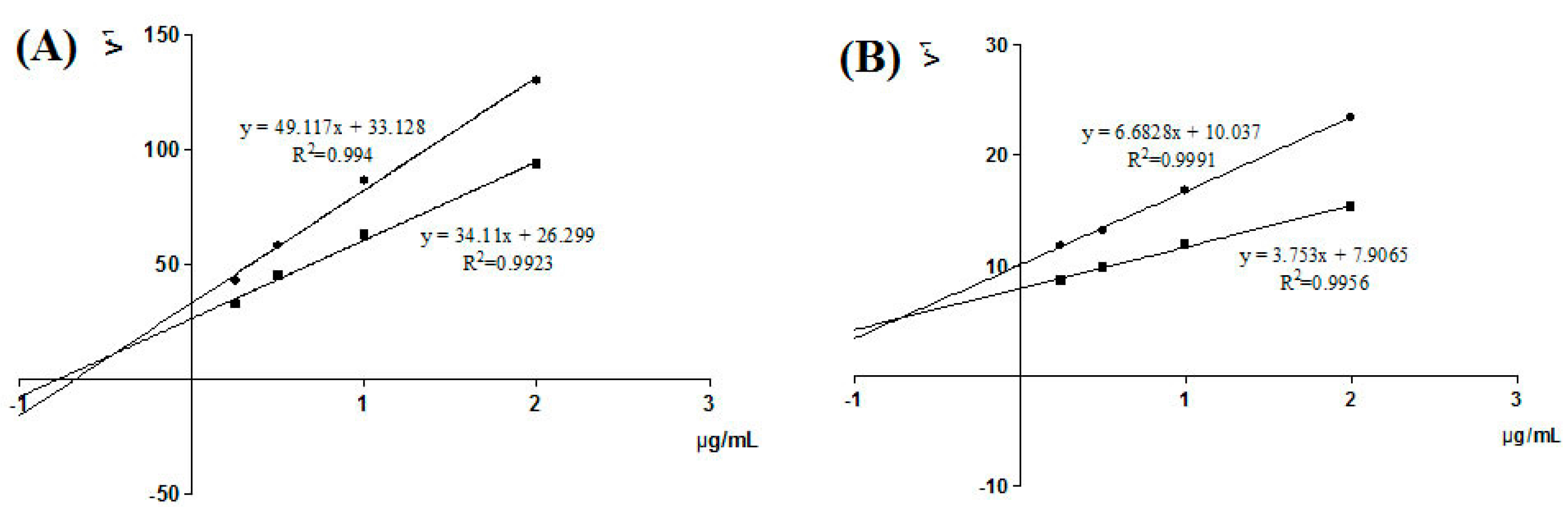
2.5. Inhibition Active and Type of Screen Compounds on XO Activity
2.6. Computational Docking of Luteolin-4′-O-glucoside and Luteolin on XO
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Apparatus
4.3. Preparation of Extract of G. hypoleucum
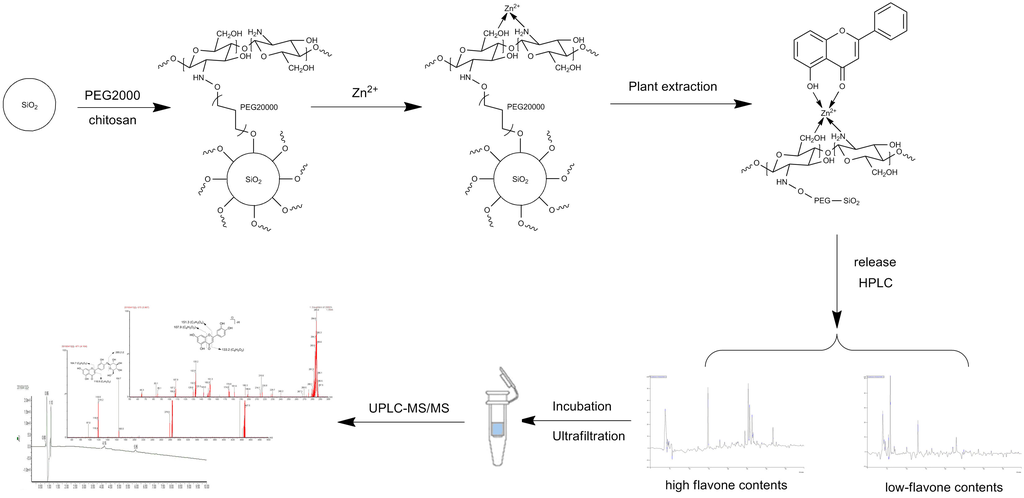
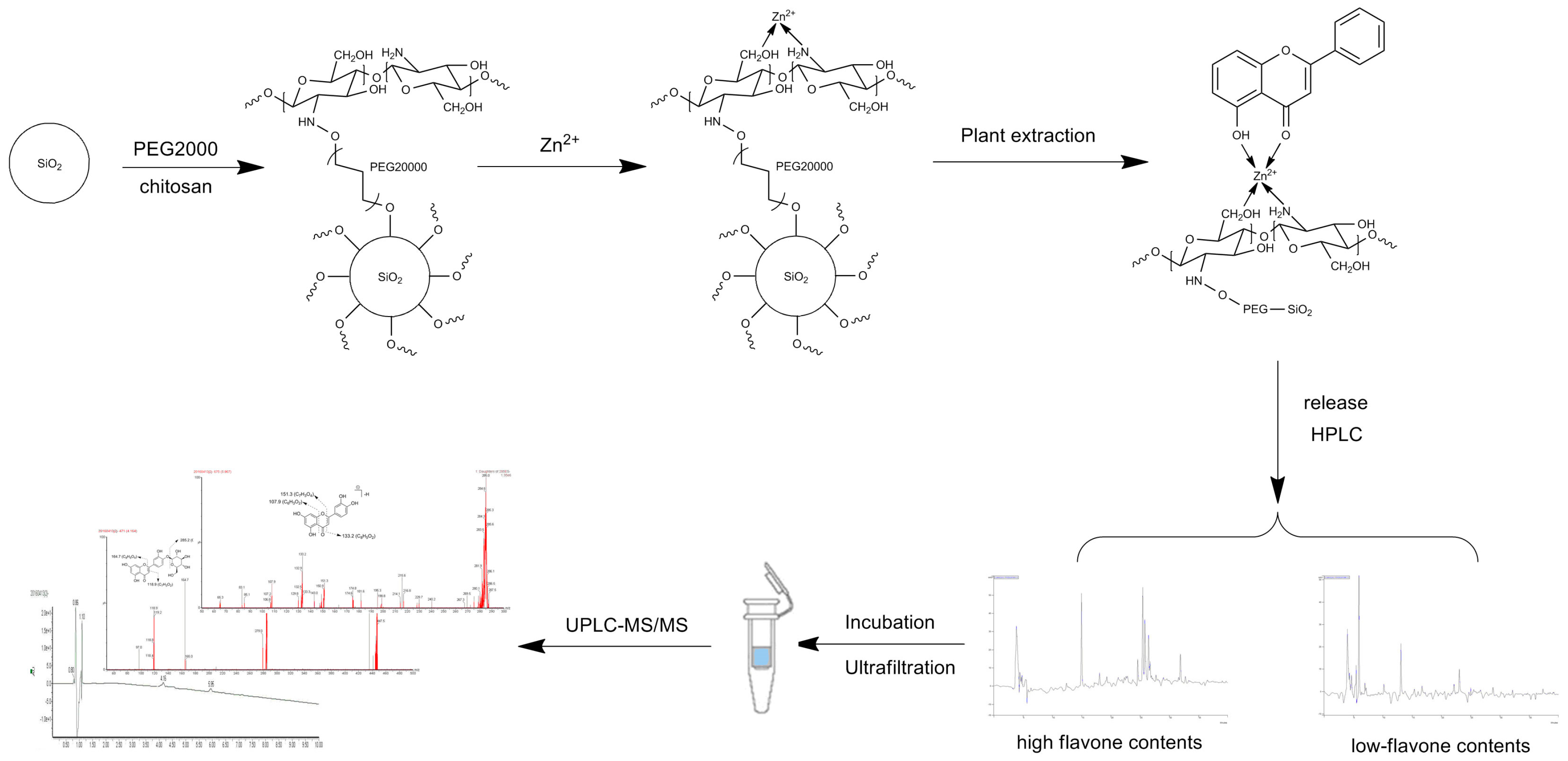
4.4. Preparation of IMAC Matrix (Metal-CTS-SiO2)
4.5. Preparation of High Flavonoid Content Fraction Measurements and Characterizations
4.6. Determination of XO Inhibitory Activity in Vitro
4.7. Screening Protocol for Selective Inhibitors of XO
4.8. Screening Procedure of IMAC-UF-UPLC-MS
4.9. Identification of XO Ligands by UPLC-DAD-ESI-MS2
4.10. Kinetic Analysis for Identified Inhibition
4.11. Inhibitory Mechanism Study of XO Ligands
4.12. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arromdee, E.; Michet, C.J.; Crowson, C.S.; O’Fallon, W.M.; Gabriel, S.E. Epidemiology of gout: Is the incidence rising? J. Rheumatol. 2002, 29, 2403–2406. [Google Scholar] [PubMed]
- Borges, F.; Fernandes, E.; Roleira, F. Progress towards the discovery of xanthine oxidase inhibitors. Curr. Med. Chem. 2002, 9, 195–217. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; van Poel, B.; Pieters, L.; Vlietinck, A.J.; Berghe, D.V. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Terkeltaub, R. Update on gout: New therapeutic strategies and options. Nat. Rev. Rheumatol. 2010, 6, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Kunming Institute of Botany. Chinese Academy of Sciences. In Flora Yunnanica; Science Press: Beijing, China, 2004; Volume 21. [Google Scholar]
- Lin, W.; Xie, J.; Wang, H. Experimental study of the Gnaphalium affine extraction treat hyperuricemia in rats. Chin. J. Rheumatol. 2005, 9, 509–510. [Google Scholar]
- Sun, Y.; Yang, Y.; Zhu, P.; Rao, G.X. Flavonoids from Gnaphalium hypoleucum. Chem. Nat. Compd. 2016, 52, 494–496. [Google Scholar] [CrossRef]
- Sun, Q.; Lu, Y.; Wu, S.Q.; Yao, S.; Zhang, J. Study on the Chemical Constituents from Gnaphalium hypoleucum. Food Chem. 2012, 35, 566–568. [Google Scholar]
- Wang, M.C.; Li, C. AB-8 macroporous adsorption resin purification Gnaphalium affine D. Don flavonoids. Food Sci. 2011, 32, 88–91. [Google Scholar]
- Lu, Y.; Li, J.; Qiu, W.M.; Tang, Y.Q.; Zhou, X.F.; Liu, Z.H. Research of macroporous resin separation and purification of flavonoid compounds in Gnaphalium affine D. Don. J. Hunan Agric. Univ. Nat. Sci. Ed. 2009, 35, 497–500. [Google Scholar]
- Mathew, B.; Suresh, J.; Mathew, G.E.; A Rasheed, S.; Vilapurathu, J.K.; Jayaraj, P. Flavonoids: An outstanding structural core for the inhibition of xanthine oxidase enzyme. Curr. Enzyme Inhib. 2015, 11, 108–115. [Google Scholar] [CrossRef]
- Márquez-García, B.; Fernández, M.Á.; Córdoba, F. Phenolics composition in Erica sp. differentially exposed to metal pollution in the Iberian Southwestern Pyritic Belt. Bioresour. Technol. 2009, 100, 446–451. [Google Scholar] [PubMed]
- Wang, H.L.; Cao, J.; Xu, S.Q.; Gu, D.Z.; Wang, Y.P.; Xiao, S.Y. Depletion of high-abundance flavonoids by metal complexation and identification of low-abundance flavonoids in Scutellaria baicalensis Georgi. J. Chromatogr. A 2013, 1315, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Xi, F.N.; Wu, J.M. Macroporous chitosan layer coated on non-porous silica gel as a support for metal chelate affinity chromatographic adsorbent. J. Chromatogr. A 2004, 1057, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Song, H.P.; Zhang, H.; Fu, Y.; Mo, H.Y.; Zhang, M.; Chen, J.; Li, P. Screening for selective inhibitors of xanthine oxidase from Flos Chrysanthemum using ultrafiltration LC-MS combined with enzyme channel blocking. J. Chromatogr. B 2014, 961, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Kammerer, D.R.; Carle, R.; Schieber, A. Characterization of phenolic acids and flavonoids in dandelion (Taraxacum officinale WEB. ex WIGG.) root and herb by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 179–186. [Google Scholar] [PubMed]
- Yan, J.K.; Zhang, G.W.; Hu, Y.T.; Ma, Y.D. Effect of luteolin on xanthine oxidase: Inhibition kinetics and interaction mechanism merging with docking simulation. Food Chem. 2013, 141, 3766–3773. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.D.; Cai, Y.; Huang, W.W.; Cheng, C.H.K.; Tan, R.X. Inhibition of xanthine oxidase by some Chinese medicinal plants used to treat gout. J. Ethnopharmacol. 2000, 73, 199–207. [Google Scholar] [CrossRef]
- Enroth, C.; Eger, B.T.; Okamoto, K.; Nishino, T.; Nishino, T.; Pai, E.F. Crystal structures of bovine milk xanthine dehydrogenase and xanthine oxidase: Structure-based mechanism of conversion. Proc. Natl. Acad. Sci. USA 2000, 97, 10723–10728. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Tsai, S.H.; Chen, C.S.; Chang, Y.C.; Lee, C.M.; Lai, Z.Y.; Lin, C.M. Structure-activity relationship of coumarin derivatives on xanthine oxidase-inhibiting and free radical-scavenging activities. Biochem. Pharmacol. 2008, 75, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the Gnaphalium hypoleucum DC. fractions and standard compounds are available from the authors.

| Samples | IC50 (µg/mL) |
|---|---|
| G. hypoleucum crude extract | 14.36 |
| IMAC-Zn 1% HCl-MeOH eluent | 3.43 |
| IMAC-Zn Water eluent | 23.58 |
| Allopurinol | 0.75 |
| DMSO (Control) | - |
| Rt (min) | UV | MS | Elem Comp | Fragmentation Pathways | Identification |
|---|---|---|---|---|---|
| 4.16 | 367.1 | 447.2 | C21H19O11 | [M − H]− | |
| 284.8 | C15H9O6 | [M – H − Glc]− | Luteolin-4′-O-glucoside | ||
| 164.7 | C8H5O4 | [M – H – Glc − C7H4O2]− | |||
| 118.9 | C7H4O2 | [M − H − Glc − C8H5O4]− | |||
| 5.95 | 347.1 | 285.2 | C15H9O6 | [M − H]− | |
| 253.1 | 151.3 | C7H3O4 | [M − H − C8H6O2]− | Luteolin | |
| 133.2 | C8H5O2 | [M − H − C7H4O4]− | |||
| 107.9 | C6H3O2 | [M – H − C9H6O4]− |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.-J.; Hu, Y.-J.; Xu, P.; Liang, W.-Q.; Zhou, J.; Liu, P.-G.; Cheng, L.; Pu, J.-B. Screening of Potential Xanthine Oxidase Inhibitors in Gnaphalium hypoleucum DC. by Immobilized Metal Affinity Chromatography and Ultrafiltration-Ultra Performance Liquid Chromatography-Mass Spectrometry. Molecules 2016, 21, 1242. https://doi.org/10.3390/molecules21091242
Zhang H-J, Hu Y-J, Xu P, Liang W-Q, Zhou J, Liu P-G, Cheng L, Pu J-B. Screening of Potential Xanthine Oxidase Inhibitors in Gnaphalium hypoleucum DC. by Immobilized Metal Affinity Chromatography and Ultrafiltration-Ultra Performance Liquid Chromatography-Mass Spectrometry. Molecules. 2016; 21(9):1242. https://doi.org/10.3390/molecules21091242
Chicago/Turabian StyleZhang, Hong-Jian, Yi-Juan Hu, Pan Xu, Wei-Qing Liang, Jie Zhou, Pei-Gang Liu, Lin Cheng, and Jin-Bao Pu. 2016. "Screening of Potential Xanthine Oxidase Inhibitors in Gnaphalium hypoleucum DC. by Immobilized Metal Affinity Chromatography and Ultrafiltration-Ultra Performance Liquid Chromatography-Mass Spectrometry" Molecules 21, no. 9: 1242. https://doi.org/10.3390/molecules21091242