Enhancing the Antioxidant Ability of Trametes versicolor Polysaccharopeptides by an Enzymatic Hydrolysis Process
Abstract
:1. Introduction
2. Results and Discussion
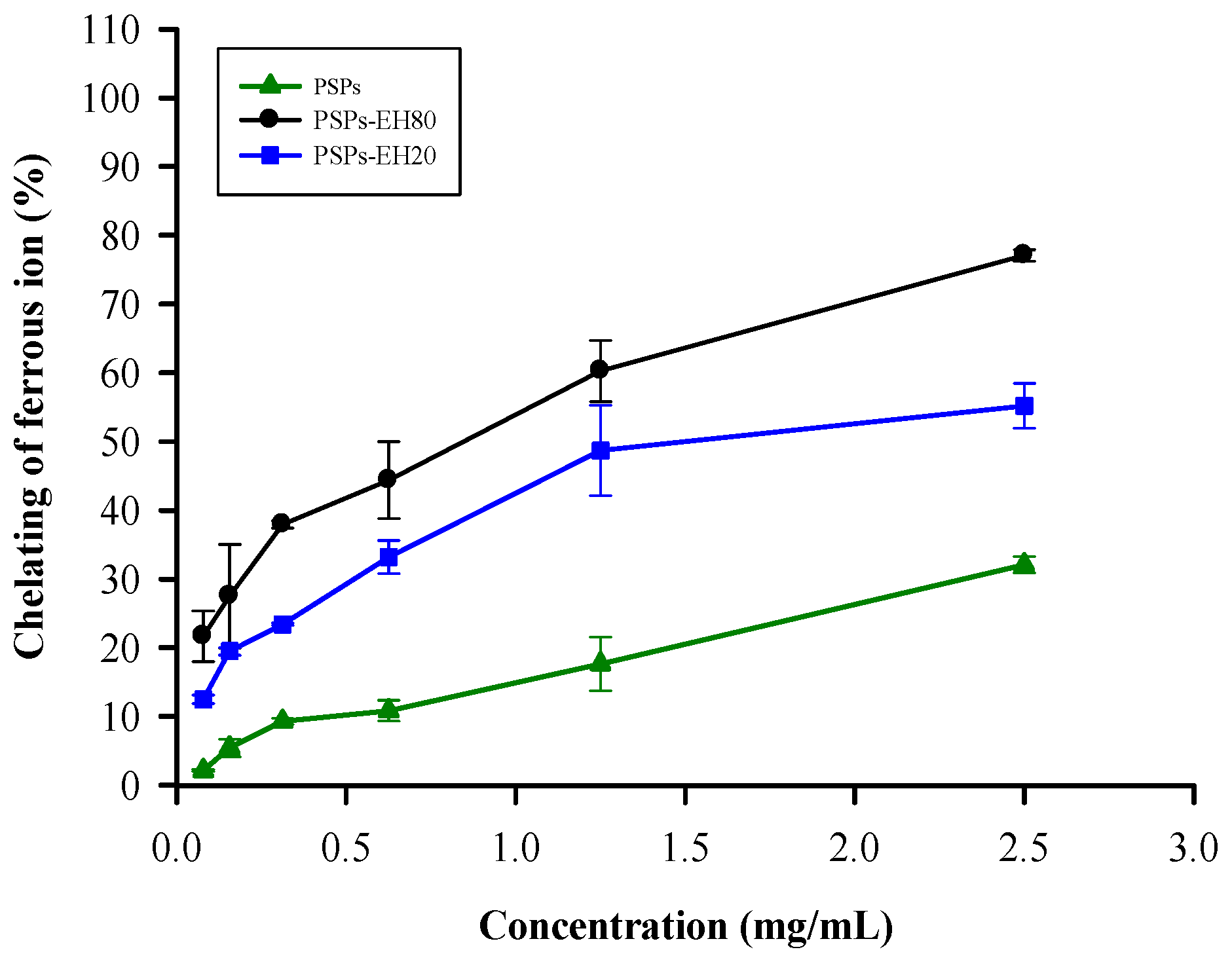
2.1. Metal Chelating Activity
2.2. ABTS Radical Scavenging Activity
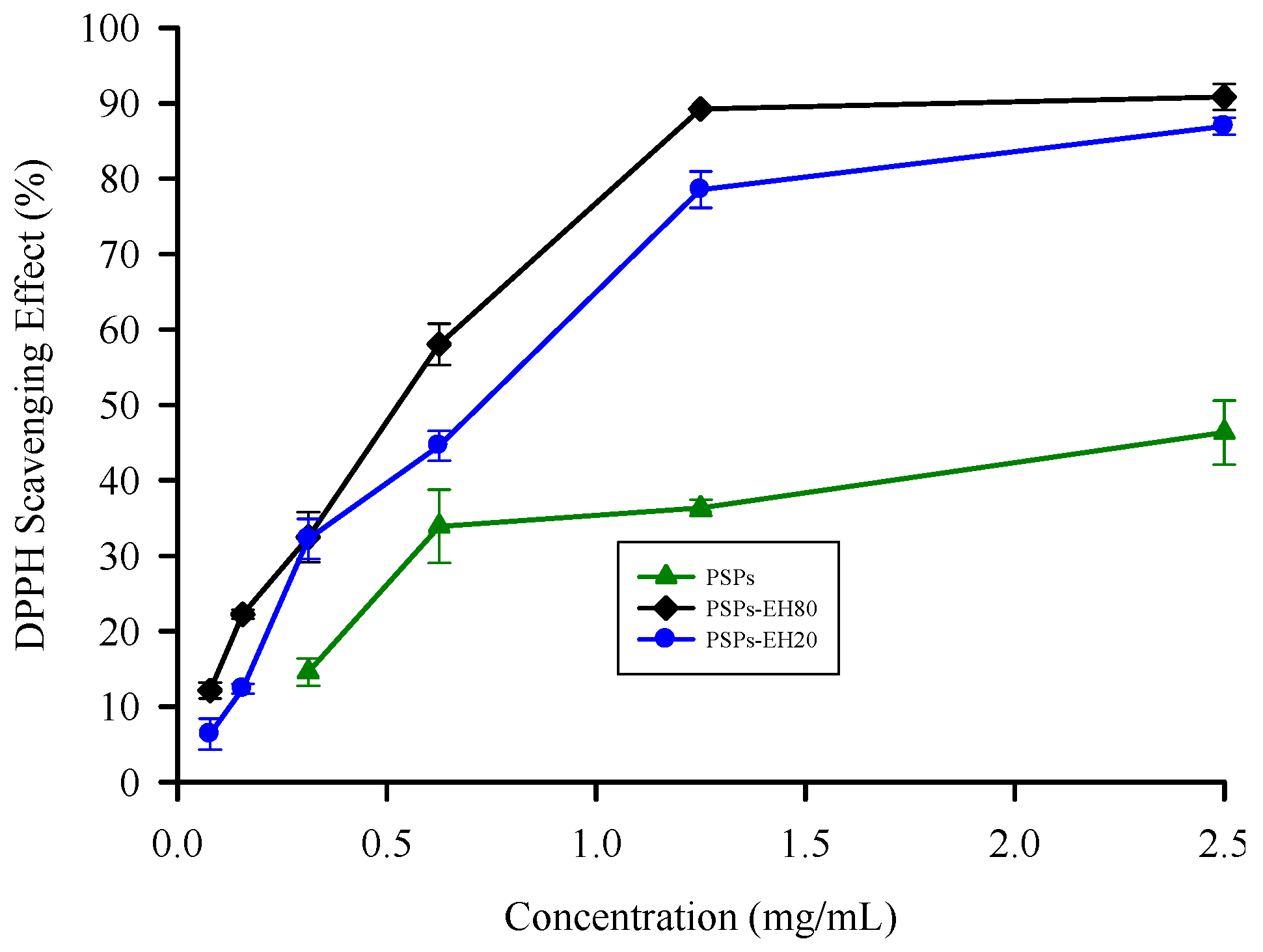
2.3. DPPH Radical Scavenging Activity
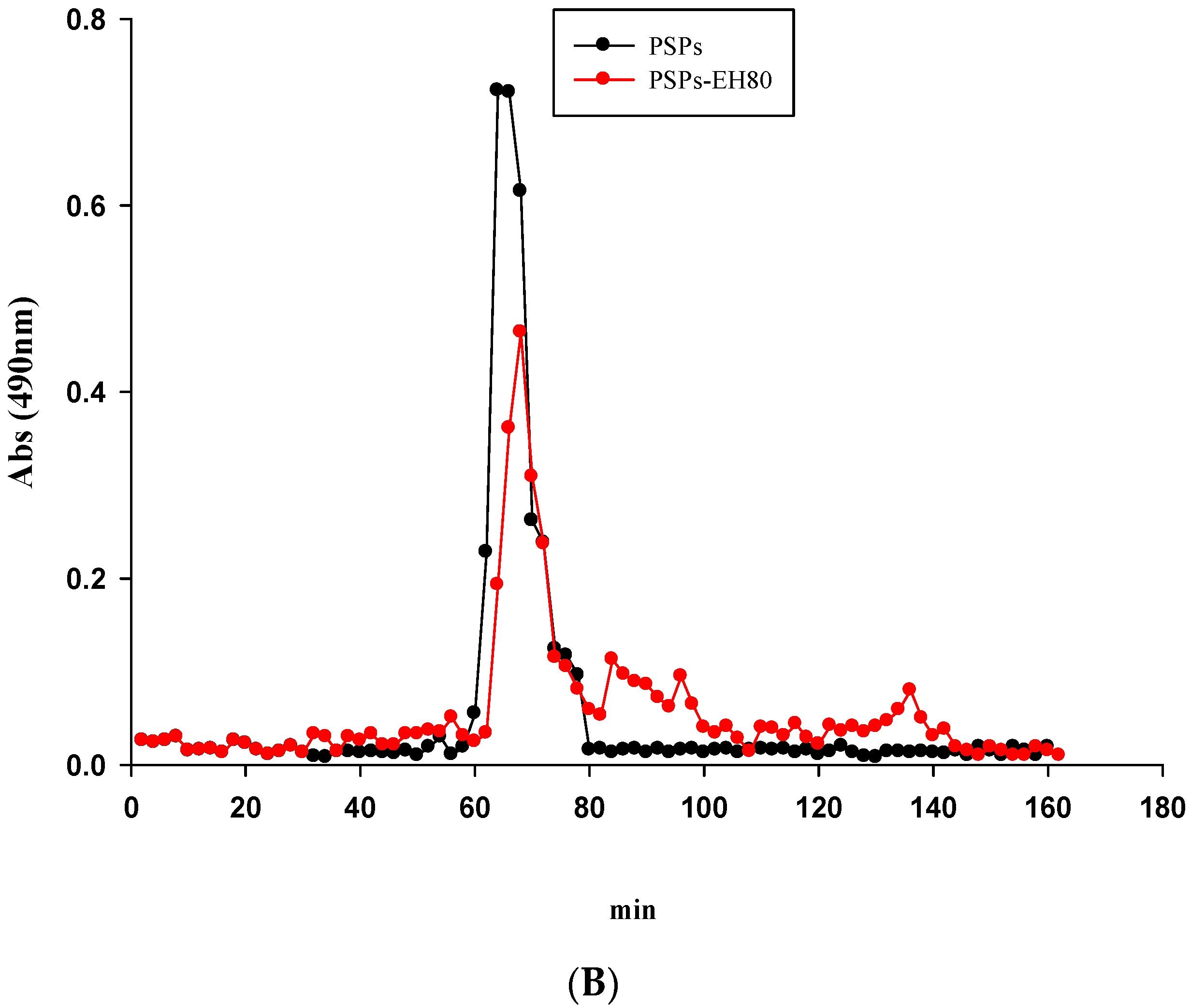
2.4. Molecular Weight and FT-IR Spectroscopy
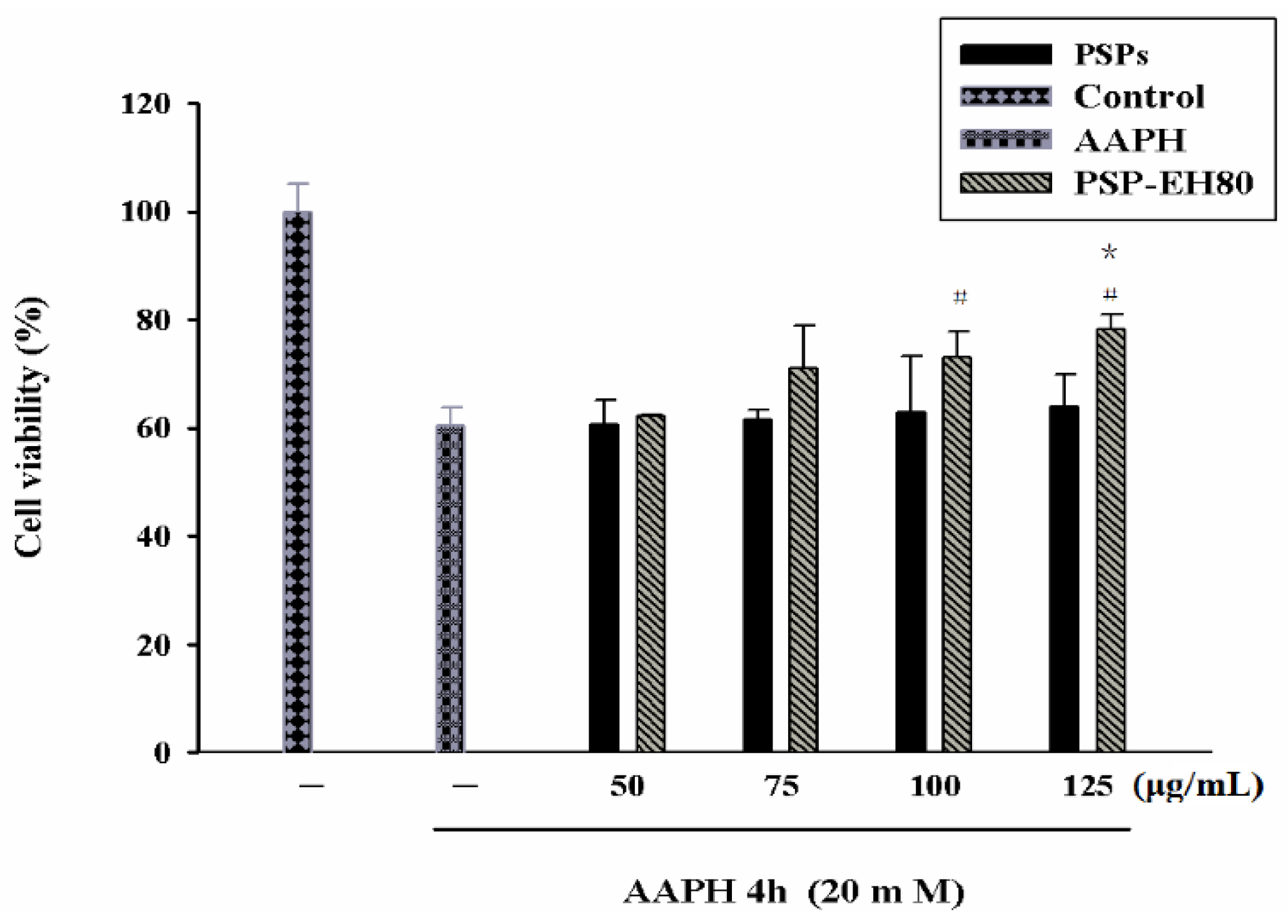
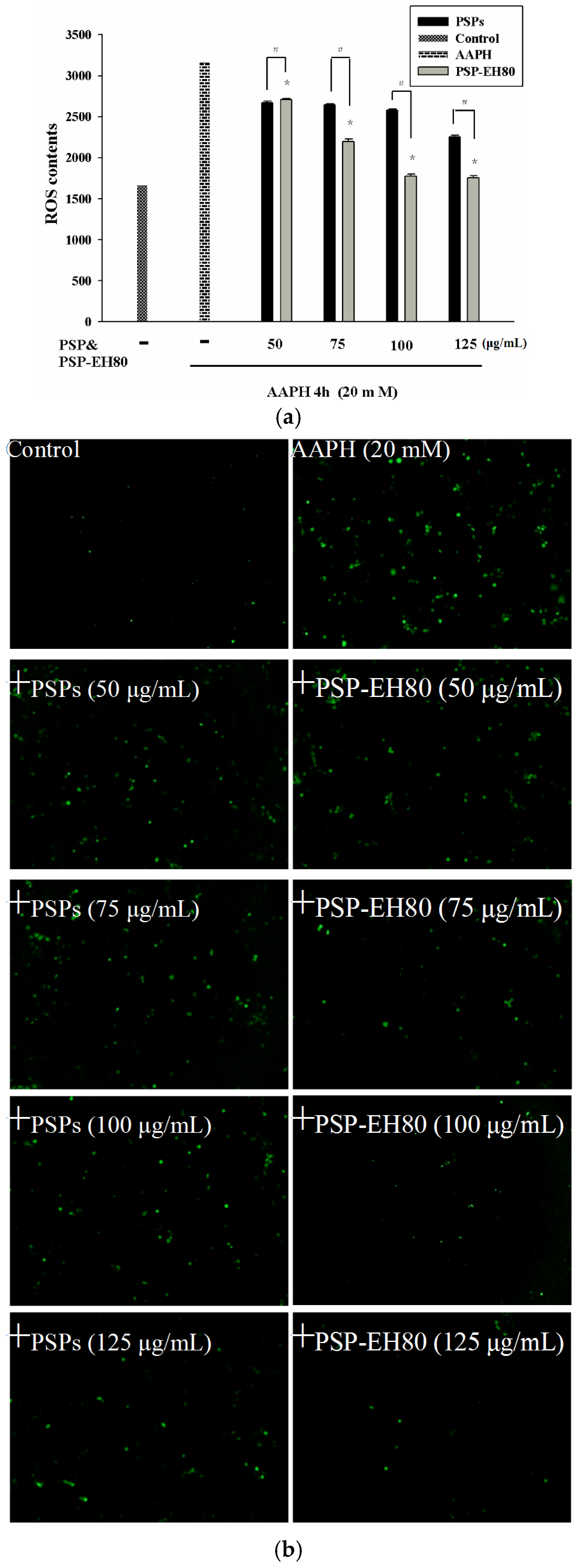
2.5. Effect of PSPs or PSP-EH80 on Cell Viability and ROS Assay in HaCaT Cells
3. Materials and Methods
3.1. Materials and Components
3.2. Preparation of PSPs from T. versicolor
3.3. Enzymatic Hydrolysis of PSPs
3.4. Metal Chelating Activity Assay
3.5. ABTS Radical Scavenging Assay
3.6. DPPH Radical Scavenging Assay
3.7. Determination of Molecular Weight
3.8. FT-IR Spectroscopy
3.9. Cell Culture
3.10. MTT Assay
3.11. Intracellular ROS Detection
3.12. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hor, S.Y.; Ahmad, M.; Farsi, E.; Lim, C.P.; Asmawi, M.Z.; Yam, M.F. Acute and subchronic oral toxicity of Coriolus versicolor standardized water extract in Sprague-Dawley rats. J. Ethnopharmacol. 2011, 137, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sun, Y.; Zhang, Q.; Zhang, H.; Yang, B.; Wang, Z.; Zhu, W.; Li, B.; Wang, Q.; Kuang, H. Screening and comparison of antioxidant activities of polysaccharides from Coriolus versicolor. Int. J. Biol. Macromol. 2014, 69, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, L.-M. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr. Polym. 2009, 76, 349–361. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Kim, M.C.; Kim, J.S.; Balasundaram, C.; Heo, M.S. Effect of Coriolus versicolor supplemented diet on innate immune response and disease resistance in kelp grouper Epinephelus bruneus against Listonella anguillarum. Fish Shellfish Immunol. 2012, 32, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Yeung, J.H.K.; Or, P.M.Y. Polysaccharide peptides from Coriolus versicolor competitively inhibit model cytochrome p450 enzyme probe substrates metabolism in human liver microsomes. Phytomedicine 2012, 19, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Volman, J.J.; Helsper, J.P.; Wei, S.; Baars, J.J.; van Griensven, L.J.; Sonnenberg, A.S.; Mensink, R.P.; Plat, J. Effects of mushroom-derived beta-glucan-rich polysaccharide extracts on nitric oxide production by bone marrow-derived macrophages and nuclear factor-kappab transactivation in Caco-2 reporter cells: Can effects be explained by structure? Mol. Nutr. Food Res. 2010, 54, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, H.; Ma, L.; Zhang, Y. Physical modifications of polysaccharide from Inonotus obliquus and the antioxidant properties. Int. J. Biol. Macromol. 2013, 54, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Sheng, J.; Li, Z.; Qi, H.; Sun, Y.; Duan, Y.; Zhang, W. Antioxidant activity of polysaccharide fractions extracted from Athyrium multidentatum (Doll.) Ching. Int. J. Biol. Macromol. 2013, 56, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.K.; Li, L.; Wang, Z.M.; Leung, P.H.; Wang, W.Q.; Wu, J.Y. Acidic degradation and enhanced antioxidant activities of exopolysaccharides from Cordyceps sinensis mycelial culture. Food Chem. 2009, 117, 641–646. [Google Scholar] [CrossRef]
- Li, B.; Liu, S.; Xing, R.; Li, K.; Li, R.; Qin, Y.; Wang, X.; Wei, Z.; Li, P. Degradation of sulfated polysaccharides from Enteromorpha prolifera and their antioxidant activities. Carbohydr. Polym. 2013, 92, 1991–1996. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Mao, G.; Mao, R.; Zou, Y.; Zheng, D.; Feng, W.; Ren, Y.; Wang, W.; Zheng, W.; Song, J.; et al. Antitumor and immunomodulatory activity of a water-soluble low molecular weight polysaccharide from Schisandra chinensis (Turcz.) Baill. Food Chem. Toxicol. 2013, 55, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Considine, T.; Patel, H.A.; Anema, S.G.; Singh, H.; Creamer, L.K. Interactions of milk proteins during heat and high hydrostatic pressure treatments—A review. Innov. Food Sci. Emerg. Technol. 2007, 8, 1–23. [Google Scholar] [CrossRef]
- Meyer, K.; Dubos, R.; Smyth, E.M. The hydrolysis of the polysaccharide acids of vitreous humor, of umbilical cord, and of Streptococcus by the autolytic enzyme of Pneumococcus. J. Biol. Chem. 1937, 118, 71–78. [Google Scholar]
- Chesters, C.G.C.; Bull, A.T. The enzymic degradation of laminarin. 1. The distribution of laminarinase among micro-organisms. Biochem. J. 1963, 86, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Ke, C.; Hu, B.; Luo, J.; Ye, H.; Sun, Y.; Yan, X.; Zeng, X. Antioxidant activities of polysaccharides from Hyriopsis cumingii. Carbohydr. Polym. 2009, 78, 199–204. [Google Scholar] [CrossRef]
- Li, X.M.; Li, X.L.; Zhou, A.G. Evaluation of antioxidant activity of the polysaccharides extracted from Lycium barbarum fruits in vitro. Eur. Polym. J. 2007, 43, 488–497. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Pang, X.; Yao, W.; Gao, X. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int. J. Biol. Macromol. 2010, 46, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Chen, J.; Hou, R.; Liu, J.; Li, X.; Gao, Z.; Liu, C.; Wang, D.; Lu, Y.; Li, H.; et al. Effects of selenylation modification on antioxidative activities of Schisandra chinensis polysaccharide. PLoS ONE 2015, 10, e0134363. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Xu, J.L.; Zhang, J.C.; Liu, Y.; Sun, H.J.; Zha, X. Physicochemical properties and antioxidant activities of polysaccharide from floral mushrooms cultivated in Huangshan Mountain. Carbohydr. Polym. 2015, 131, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.C.; Verma, N.; Sonone, A.; Makhija, U. Evaluation of antioxidant potential of the cultured mycobiont of a lichen Usnea ghattensis. Phytother. Res. 2005, 19, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xie, M.-Y.; Nie, S.-P.; Li, C.; Wang, Y.-X. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008, 107, 231–241. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Shao, P.; Chen, X.; Sun, P. Improvement of antioxidant and moisture-preserving activities of Sargassum horneri polysaccharide enzymatic hydrolyzates. Int. J. Biol. Macromol. 2015, 74, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; He, X.; Zhou, S.; Fan, Y.; Luo, A.; Chun, Z. Purification, composition analysis and antioxidant activity of the polysaccharides from Dendrobium nobile Lindl. Carbohydr. Polym. 2010, 79, 1014–1019. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Černá, M.; Barros, A.S.; Nunes, A.; Rocha, S.l.M.; Delgadillo, I.; Čopı́ková, J.; Coimbra, M.A. Use of FT-IR spectroscopy as a tool for the analysis of polysaccharide food additives. Carbohydr. Polym. 2003, 51, 383–389. [Google Scholar] [CrossRef]
- Huang, S.Q.; Li, J.W.; Li, Y.Q.; Wang, Z. Purification and structural characterization of a new water-soluble neutral polysaccharide GLP-F1–1 from Ganoderma lucidum. Int. J. Biol. Macromol. 2011, 48, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Kim, D.S.; Park, S.H.; Yoon, J.A.; Kim, S.K.; Kwon, S.B.; Park, K.C. Involvement of ERK and p38 map kinase in AAPH-induced Cox-2 expression in HaCat cells. Chem. Phys. Lipids 2004, 129, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Roche, M.; Tarnus, E.; Rondeau, P.; Bourdon, E. Effects of nutritional antioxidants on AAPH- or AGES-induced oxidative stress in human SW872 liposarcoma cells. Cell. Biol. Toxicol. 2009, 25, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, T.; Jiang, J.; Wong, Y.S.; Yang, F.; Zheng, W. Selenium-containing allophycocyanin purified from selenium-enriched Spirulina platensis attenuates AAPH-induced oxidative stress in human erythrocytes through inhibition of ROS generation. J. Agric. Food Chem. 2011, 59, 8683–8690. [Google Scholar] [CrossRef] [PubMed]
- Lopez, E.; Arce, C.; Oset-Gasque, M.J.; Canadas, S.; Gonzalez, M.P. Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture. Free Radic. Boil. Med. 2006, 40, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Lekounougou, S.; Mounguengui, S.; Dumarçay, S.; Rose, C.; Courty, P.E.; Garbaye, J.; Gérardin, P.; Jacquot, J.P.; Gelhaye, E. Initial stages of Fagus sylvatica wood colonization by the white-rot basidiomycete Trametes versicolor: Enzymatic characterization. Int. Biodeterior. Biodegrad. 2008, 61, 287–293. [Google Scholar] [CrossRef]
- Hung, M.C.; Tsai, C.C.; Hsu, T.H.; Liang, Z.C.; Lin, F.Y.; Chang, S.L.; Ho, W.J.; Hsieh, C.W. Biological activities of the polysaccharides produced from different sources of Xylaria nigripes (Ascomycetes), a Chinese medicinal fungus. Int. J. Med. Mushrooms 2015, 17, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Almirall, M.; Esteve-Garcia, E. In vitro stability of a β-glucanase preparation from Trichoderma longibrachiatum and its effect in a barley based diet fed to broiler chicks. Anim. Feed Sci. Technol. 1995, 54, 149–158. [Google Scholar] [CrossRef]
- Nadaroglu, H.D.; Demır, N.; Demır, Y. Antioxidant and radical scavenging activities of capsules of caper (Capparis spinosa). Asian J. Chem. 2009, 21, 5123–5134. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, M.; Shi, J.; Yang, N.; Jiang, Y. Effect of ultrasonic treatment on the recovery and DPPH radical scavenging activity of polysaccharides from longan fruit pericarp. Food Chem. 2008, 106, 685–690. [Google Scholar] [CrossRef]
- Lee, J.S.; Kwon, J.S.; Yun, J.S.; Pahk, J.W.; Shin, W.C.; Lee, S.Y.; Hong, E.K. Structural characterization of immunostimulating polysaccharide from cultured mycelia of Cordyceps militaris. Carbohydr. Polym. 2010, 80, 1011–1017. [Google Scholar] [CrossRef]
- Zhang, L.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Bailey, T.; Longvah, T. Immunomodulatory activities of polysaccharides isolated from Taxillus chinensis and Uncaria rhyncophylla. Carbohydr. Polym. 2013, 98, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.H.; Zhao, S.; Ho, K.P.; Wu, J.Y. Chemical properties and antioxidant activity of exopolysaccharides from mycelial culture of Cordyceps sinensis fungus CS-HK1. Food Chem. 2009, 114, 1251–1256. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, M.S.; Jeong, G.S.; Yoon, J. Xanthii fructus extract inhibits TNF-α/IFN-γ-induced Th2-chemokines production via blockade of NF-κB, Stat1 and p38-MAPK activation in human epidermal keratinocytes. J. Ethnopharmacol. 2015, 171, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.J.; Yang, H.L.; Tsai, Y.C.; Hung, P.C.; Chang, S.H.; Lo, H.W.; Shen, P.C.; Chen, S.C.; Wang, H.M.; Wang, S.Y.; et al. Lucidone protects human skin keratinocytes against free radical-induced oxidative damage and inflammation through the up-regulation of HO-1/NRF2 antioxidant genes and down-regulation of NF-κB signaling pathway. Food Chem. Toxicol. 2013, 59, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the Trametes versicolor are available from the authors.



| PSPs Fraction | Peak | Mw (kDa) | Area % | Hydrolysis Rate |
|---|---|---|---|---|
| PSPs | Peak 1 | 300 kDa | 100.0 | 38.6% |
| PSPs-EH80 | Peak 2 | 300 kDa | 61.4 | |
| Peak 3 | 190 kDa | 18.8 | ||
| Peak 4 | 140 kDa | 7.2 | ||
| Peak 5 | 50 kDa | 12.6 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jhan, M.-H.; Yeh, C.-H.; Tsai, C.-C.; Kao, C.-T.; Chang, C.-K.; Hsieh, C.-W. Enhancing the Antioxidant Ability of Trametes versicolor Polysaccharopeptides by an Enzymatic Hydrolysis Process. Molecules 2016, 21, 1215. https://doi.org/10.3390/molecules21091215
Jhan M-H, Yeh C-H, Tsai C-C, Kao C-T, Chang C-K, Hsieh C-W. Enhancing the Antioxidant Ability of Trametes versicolor Polysaccharopeptides by an Enzymatic Hydrolysis Process. Molecules. 2016; 21(9):1215. https://doi.org/10.3390/molecules21091215
Chicago/Turabian StyleJhan, Mei-Hsin, Ching-Hua Yeh, Chia-Chun Tsai, Ching-Tian Kao, Chao-Kai Chang, and Chang-Wei Hsieh. 2016. "Enhancing the Antioxidant Ability of Trametes versicolor Polysaccharopeptides by an Enzymatic Hydrolysis Process" Molecules 21, no. 9: 1215. https://doi.org/10.3390/molecules21091215






