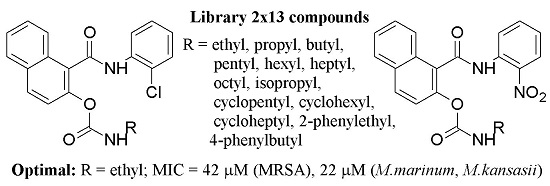
Synthesis and Antimicrobial Evaluation of 1-[(2-Substituted phenyl)carbamoyl]naphthalen-2-yl Carbamates †
Abstract
:1. Introduction
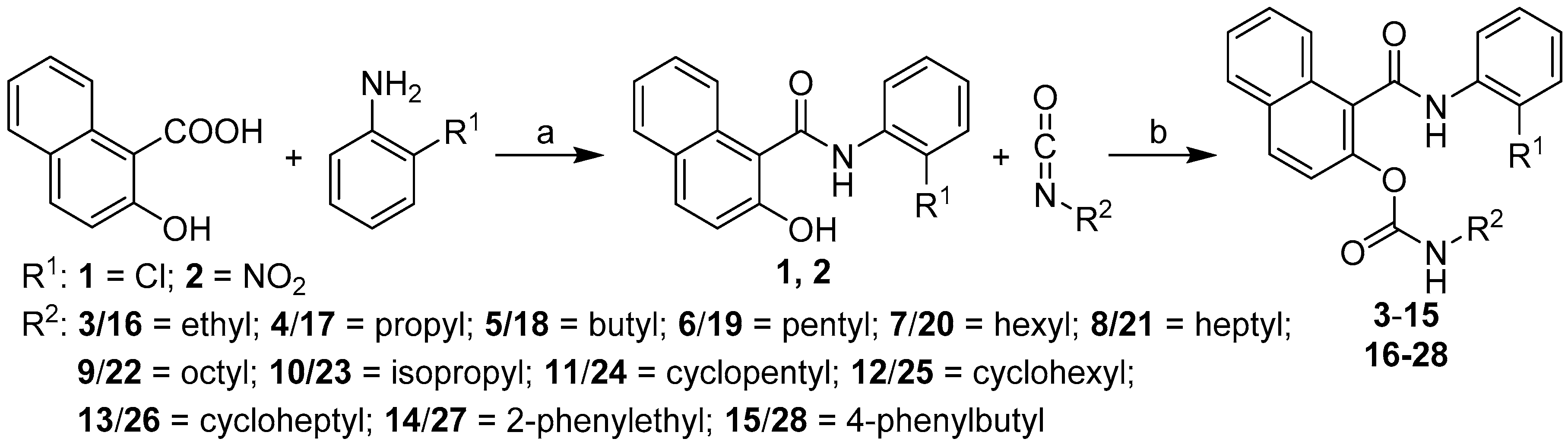
2. Results and Discussion
2.1. Chemistry
2.2. In Vitro Antibacterial Susceptibility Testing
2.3. In Vitro Antimycobacterial Evaluation
2.4. In Vitro Cytotoxicity Assay
3. Experimental Section
3.1. General Information
3.2. Synthesis
General Procedure for Synthesis of Carbamates 3–28
3.3. In Vitro Antibacterial Susceptibility Testing
3.4. In Vitro Antimycobacterial Evaluation
3.5. In Vitro Cytotoxicity Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. World Health Statistics 2016: Monitoring Health for the SDGs; WHO Press: Geneva, Switzerland, 2016. [Google Scholar]
- World Health Organization. Global Tuberculosis Report 2015; WHO Press: Geneva, Switzerland, 2015. [Google Scholar]
- World Health Organization. Global Antimicrobial Resistance Surveillance System 2015; WHO Press: Geneva, Switzerland, 2015. [Google Scholar]
- European Centre for Disease Prevention and Control. Available online: http://ecdc.europa.eu/en/Pages/home.aspx (accessed on 30 July 2016).
- Thampi, N.; Showler, A.; Burry, L.; Bai, A.D.; Steinberg, M.; Ricciuto, D.R.; Bell, C.M.; Morris, A.M. Multicenter study of health care cost of patients admitted to hospital with Staphylococcus aureus bacteremia: Impact of length of stay and intensity of care. Am. J. Infect. Control. 2015, 43, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Kaku, N.; Yanagihara, K.; Morinaga, Y.; Yamada, K.; Harada, Y.; Migiyama, Y.; Nagaoka, K.; Matsuda, J.; Uno, N.; Hasegawa, H.; et al. Influence of antimicrobial regimen on decreased in-hospital mortality of patients with MRSA bacteremia. J. Infect. Chemother. 2014, 20, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.S.; Silva, C.S.F.; de Souza, V.C.; da Silva, V.L.; Diniz, C.G.; Santos, M.O. Strategies and molecular tools to fight antimicrobial resistance: Resistome, transcriptome, and antimicrobial peptides. Front. Microbiol. 2013, 4, 412. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J. Potential of agricultural fungicides for antifungal drug discovery. Expert Opin. Drug Dis. 2016, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J. How Can We Bolster the Antifungal Drug Discovery Pipeline? Future Med. Chem. 2016, 8, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
- Kratky, M.; Vinsova, J. Salicylanilide ester prodrugs as potential antimicrobial agents—A review. Curr. Pharm. Des. 2011, 17, 3494–3505. [Google Scholar] [CrossRef] [PubMed]
- Imramovsky, A.; Pauk, K.; Pejchal, V.; Hanusek, J. Salicylanilides and their derivates as perspective anti-tuberculosis drugs: Synthetic routes and biological evaluations. Mini Rev. Org. Chem. 2011, 8, 211–220. [Google Scholar] [CrossRef]
- Kratky, M.; Vinsova, J. Antiviral activity of substituted salicylanilides—A review. Mini Rev. Med. Chem. 2011, 11, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Kratky, M.; Vinsova, J. Advances in mycobacterial isocitrate lyase targeting and inhibitors. Curr. Med. Chem. 2012, 19, 6126–6137. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Bobal, P.; Kollar, P.; Cizek, A.; Kralova, K.; et al. Antimycobacterial and herbicidal activity of ring-substituted 1-hydroxynaphthalene-2-carboxanilides. Bioorg. Med. Chem. 2013, 21, 6531–6541. [Google Scholar] [CrossRef] [PubMed]
- Zadrazilova, I.; Pospisilova, S.; Pauk, K.; Imramovsky, A.; Vinsova, J.; Cizek, A.; Jampilek, J. In vitro bactericidal activity of 4- and 5-chloro-2-hydroxy-N-[1-oxo-1-(phenylamino)alkan-2-yl]benzamides against MRSA. BioMed Res. Int. 2015, 2015, 349534. [Google Scholar] [CrossRef] [PubMed]
- Zadrazilova, I.; Pospisilova, S.; Masarikova, M.; Imramovsky, A.; Monreal-Ferriz, J.; Vinsova, J.; Cizek, A.; Jampilek, J. Salicylanilide carbamates: Promising antibacterial agents with high in vitro activity against methicillin-resistant Staphylococcus aureus. Eur. J. Pharm. Sci. 2015, 77, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Kushkevych, I.; Kollar, P.; Ferreira, A.L.; Palma, D.; Duarte, A.; Lopes, M.M.; Bartos, M.; Pauk, K.; Imramovsky, A.; Jampilek, J. Antimicrobial effect of salicylamide derivatives against intestinal sulfate-reducing bacteria. J. Appl. Biomed. 2016, 14, 125–130. [Google Scholar] [CrossRef]
- Steinhilber, D.; Schubert-Zsilavecz, M.; Roth, H.J. Medizinische Chemie: Targets, Arzneistoffe, Chemische Biologie; Deutscher Apotheker Verlag: Stutgart, Germany, 2010. [Google Scholar]
- US Environmental Protection Agency–Pesticide Registration: Pesticide Data Submitters List (PDSL), 2016. Available online: https://www.epa.gov/sites/production/files/2016-04/documents/dslchem_0.pdf (accessed on 30 July 2016).
- Pattabiraman, V.R.; Bode, J.W. Rethinking amide bond synthesis. Nature 2011, 480, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Brindisi, M. Organic carbamates in drug design and medicinal chemistry. J. Med. Chem. 2015, 58, 2895–2940. [Google Scholar] [CrossRef] [PubMed]
- Otevrel, J.; Mandelova, Z.; Pesko, M.; Guo, J.; Kralova, K.; Sersen, F.; Vejsova, M.; Kalinowski, D.; Kovacevic, Z.; Coffey, A.; et al. Investigating the spectrum of biological activity of ring-substituted salicylanilides and carbamoylphenylcarbamates. Molecules 2010, 15, 8122–8142. [Google Scholar] [CrossRef] [PubMed]
- Imramovsky, A.; Pesko, M.; Kralova, K.; Vejsova, M.; Stolarikova, J.; Vinsova, J.; Jampilek, J. Investigating spectrum of biological activity of 4- and 5-chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]-benzamides. Molecules 2011, 16, 2414–2430. [Google Scholar] [CrossRef] [PubMed]
- Imramovsky, A.; Pesko, M.; Monreal-Ferriz, J.; Kralova, K.; Vinsova, J.; Jampilek, J. Photosynthesis-inhibiting efficiency of 4-chloro-2-(chlorophenylcarbamoyl)phenyl alkyl-carbamates. Bioorg. Med. Chem. Lett. 2011, 21, 4564–4567. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J.; Brychtova, K. Azone analogues: Classification, design, and transdermal penetration principles. Med. Res. Rev. 2012, 32, 907–947. [Google Scholar] [CrossRef] [PubMed]
- Malik, I.; Bukovsky, M.; Andriamainty, F.; Galisinova, J. Anti-microbial activity of meta-alkoxyphenylcarbamates containing substituted N-phenylpiperazine fragment. Braz. J. Microbiol. 2012, 43, 959–965. [Google Scholar] [PubMed]
- Malik, I.; Bukovsky, M.; Andriamainty, F.; Galisinova, J. Antimicrobial effect of para-alkoxyphenylcarbamic acid esters containing substituted N-phenylpiperazine moiety. Braz. J. Microbiol. 2013, 44, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Nahid, P.; Cole, S.T. Advances in the development of new tuberculosis drugs and treatment regimens. Nat. Rev. Drug Discov. 2013, 12, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Gonec, T.; Bobal, P.; Kauerova, T.; Oravec, M.; Kollar, P.; et al. Antibacterial and herbicidal activity of ring-substituted 3-hydroxynaphthalene-2-carboxanilides. Molecules 2013, 18, 7977–7997. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Govender, R.; Chambel, B.; Pereira, D.; Kollar, P.; Imramovsky, A.; O’Mahony, J.; et al. Antibacterial and herbicidal activity of ring-substituted 2-hydroxynaphthalene-1-carboxanilides. Molecules 2013, 18, 9397–9419. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J. Recent advances in design of potential quinoxaline anti-infectives. Curr. Med. Chem. 2014, 21, 4347–4373. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Kos, J.; Nevin, E.; Govender, R.; Pesko, M.; Kushkevych, I.; Oravec, M.; Kollar, P.; O’Mahony, J.; Kralova, K.; et al. Preparation and biological properties of ring-substituted naphthalene-1-carboxanilides. Molecules 2014, 19, 10386–10409. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Zadrazilova, I.; Nevin, E.; Kauerova, T.; Pesko, M.; Kos, J.; Oravec, M.; Kollar, P.; Coffey, A.; O’Mahony, J.; et al. Synthesis and biological evaluation of N-alkoxyphenyl-3-hydroxynaphthalene-2-carboxanilides. Molecules 2015, 20, 9767–9787. [Google Scholar] [CrossRef] [PubMed]
- Kralova, K.; Perina, M.; Waisser, K.; Jampilek, J. Structure-activity relationships of N-benzylsalicylamides for inhibition of photosynthetic electron transport. Med. Chem. 2015, 11, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Kauerova, T.; Kos, J.; Gonec, T.; Jampilek, J.; Kollar, P. Antiproliferative and pro-apoptotic effect of novel nitro-substituted hydroxynaphthanilides on human cancer cell lines. Int. J. Mol. Sci. 2016, 17, 1219. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Pospisilova, S.; Kauerova, T.; Kos, J.; Dohanosova, J.; Oravec, M.; Kollar, P.; Coffey, A.; Liptaj, T.; Cizek, A.; et al. N-alkoxyphenylhydroxynaphthalenecarboxamides and their antimycobacterial activity. Molecules 2016, 21, 1068. [Google Scholar] [CrossRef] [PubMed]
- Pauk, K.; Zadrazilova, I.; Imramovsky, A.; Vinsova, J.; Pokorna, M.; Masarikova, M.; Cizek, A.; Jampilek, J. New derivatives of salicylamides: Preparation and antimicrobial activity against various bacterial species. Bioorg. Med. Chem. 2013, 21, 6574–6581. [Google Scholar] [CrossRef] [PubMed]
- Alnimr, A.M. Dormancy models for Mycobacterium tuberculosis: A mini review. Braz. J. Microbiol. 2015, 46, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Van Ingen, J.; Boeree, M.J.; van Soolingen, D.; Mouton, J.W. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist. Updates 2012, 15, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Matveychuk, A.; Fuks, L.; Priess, R.; Hahim, I.; Shitrit, D. Clinical and radiological features of Mycobacterium kansasii and other NTM infections. Resp. Med. 2012, 106, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- National Committee for Clinical Laboratory Standards. M24-A2 Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes; Approved Standard, 2nd ed.; NCCLS: Wayne, PA, USA, 2011. [Google Scholar]
- Tengler, J.; Kapustikova, I.; Pesko, M.; Govender, R.; Keltosova, S.; Mokry, P.; Kollar, P.; O’Mahony, J.; Coffey, A.; Kralova, K.; et al. Synthesis and biological evaluation of 2-hydroxy-3-[(2-aryloxyethyl)amino]propyl 4-[(alkoxycarbonyl)amino]benzoates. Sci. World J. 2013, 2013, 274570. [Google Scholar] [CrossRef] [PubMed]
- Kratky, M.; Vinsova, J. Salicylanilide N-monosubstituted carbamates: Synthesis and in vitro antimicrobial activity. Bioorg. Med. Chem. 2016, 24, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Nevin, E.; Soral, M.; Kushkevych, I.; Gonec, T.; Bobal, P.; Kollar, P.; Coffey, A.; O’Mahony, J.; Liptaj, T.; et al. Synthesis and antimycobacterial properties of ring-substituted 6-hydroxynaphthalene-2-carboxanilides. Bioorg. Med. Chem. 2015, 23, 2035–2043. [Google Scholar] [CrossRef] [PubMed]
- Suffness, M.; Douros, J. Current status of the NCI plant and animal product program. J. Nat. Prod. 1982, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard, 5th ed.; CLSI Document M7-A5; NCCLS: Wayne, PA, USA, 2000. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing; 12th Informational Supplement M100-S12; NCCLS: Wayne, MI, USA, 2002. [Google Scholar]
- Schwalbe, R.; Steele-Moore, L.; Goodwin, A.C. Antimicrobial Susceptibility Testing Protocols; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Clinical and Laboratory Standards Institute. Available online: http://www.clsi.org (accessed on 5 September 2016).
- Sample Availability: Samples of compounds 1–28 are available from authors T. Gonec, J. Stranik, J. Kos, J. Jampilek.
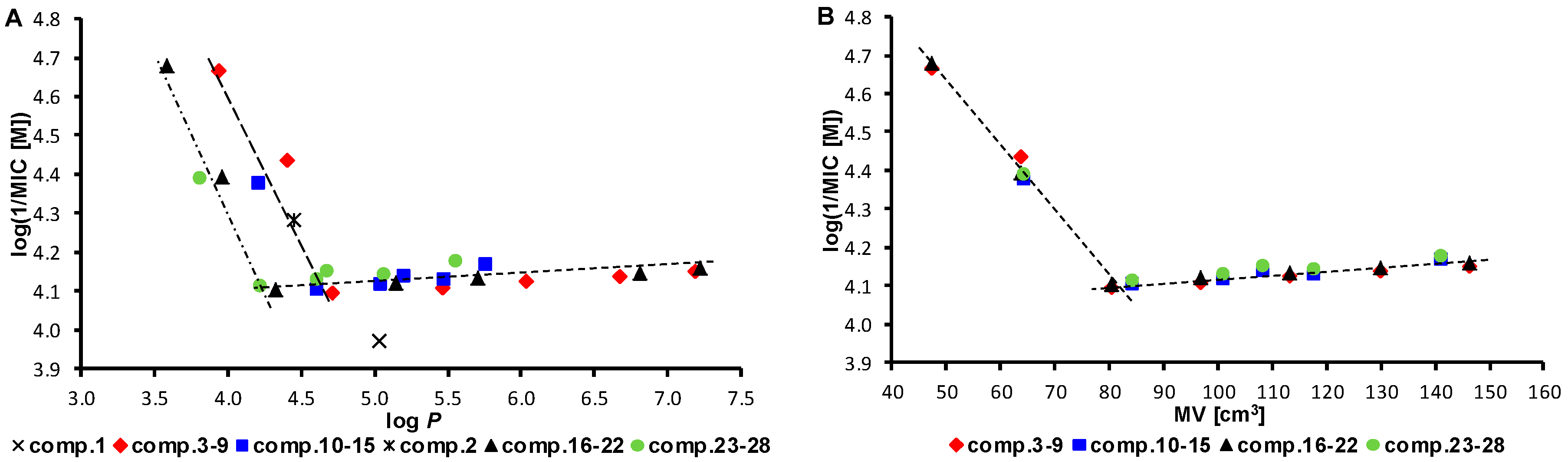

| Compd. | R1 | R2 | log P a | MVR2 a [cm3] | σ*R2 a | MIC [µM] | LD50 [µM] | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SA | MRSA SA 630 | MRSA 3202 | MM | MK | |||||||
| 1 | Cl | − | 5.03 | – | – | 215 | 860 | 860 | 107 | 107 | >30 |
| 3 | Cl | Et | 3.94 | 47.29 | −0.11 | 21.6 | 43.3 | 43.3 | 21.6 | 21.6 | ˃30 |
| 4 | Cl | Pr | 4.41 | 63.80 | −0.12 | 83.5 | 83.5 | 83.5 | 41.7 | 36.5 | ˃30 |
| 5 | Cl | Bu | 4.71 | 80.31 | −0.25 | 645 | 645 | 645 | 80.6 | 80.6 | – |
| 6 | Cl | Pen | 5.47 | 96.81 | −0.23 | 623 | 623 | 623 | 77.8 | 77.8 | – |
| 7 | Cl | Hex | 6.03 | 113.32 | −0.25 | 602 | 602 | 602 | 75.3 | 75.3 | – |
| 8 | Cl | Hep | 6.67 | 129.83 | −0.23 | 583 | 583 | 583 | 72.9 | 72.9 | – |
| 9 | Cl | Oct | 7.19 | 146.33 | −0.23 | 565 | 565 | 565 | 70.6 | 70.6 | – |
| 10 | Cl | i-Pr | 4.20 | 64.18 | −0.19 | 83.5 | 167 | 167 | 41.7 | 41.7 | ˃30 |
| 11 | Cl | c-Pent | 4.60 | 84.10 | −0.20 | 313 | 313 | 626 | 78.2 | 78.2 | – |
| 12 | Cl | c-Hex | 5.03 | 100.87 | −0.15 | 303 | 605 | 303 | 75.6 | 75.6 | – |
| 13 | Cl | c-Hep | 5.46 | 117.56 | −0.14 | 146 | 586 | 586 | 73.2 | 73.2 | – |
| 14 | Cl | PhEt | 5.19 | 108.00 | 0.08 | 288 | 575 | 575 | 71.9 | 71.9 | – |
| 15 | Cl | PhBu | 5.75 | 141.01 | −0.21 | 271 | 541 | 541 | 67.6 | 67.6 | – |
| 2 | NO2 | − | 4.45 | – | – | 26 | 104 | 52 | 104 | 51.9 | >30 |
| 16 | NO2 | Et | 3.58 | 47.29 | −0.11 | 5.27 | 42.1 | 42.1 | 21.0 | 21.0 | ˃30 |
| 17 | NO2 | Pr | 3.96 | 63.80 | −0.12 | 10.1 | 81.3 | 81.3 | 40.6 | 40.6 | ˃30 |
| 18 | NO2 | Bu | 4.32 | 80.31 | −0.25 | 628 | 628 | 628 | 78.5 | 78.5 | – |
| 19 | NO2 | Pen | 5.15 | 96.81 | −0.23 | 607 | 607 | 607 | 75.9 | 75.9 | – |
| 20 | NO2 | Hex | 5.71 | 113.32 | −0.25 | 587 | 587 | 587 | 73.4 | 73.4 | – |
| 21 | NO2 | Hep | 6.81 | 129.83 | −0.23 | 569 | 569 | 569 | 71.1 | 71.1 | – |
| 22 | NO2 | Oct | 7.22 | 146.33 | −0.23 | 552 | 552 | 552 | 69.0 | 69.0 | – |
| 23 | NO2 | i-Pr | 3.80 | 64.18 | −0.19 | 40.6 | 162 | 162 | 40.6 | 40.6 | ˃30 |
| 24 | NO2 | c-Pent | 4.21 | 84.10 | −0.20 | 153 | 610 | 610 | 76.2 | 76.2 | – |
| 25 | NO2 | c-Hex | 4.60 | 100.87 | −0.15 | 74 | 591 | 591 | 73.8 | 73.8 | – |
| 26 | NO2 | c-Hep | 5.05 | 117.56 | −0.14 | 72 | 572 | 572 | 71.5 | 71.5 | – |
| 27 | NO2 | PhEt | 4.67 | 108.00 | 0.08 | 281 | 562 | 562 | 70.2 | 70.2 | – |
| 28 | NO2 | PhBu | 5.55 | 141.01 | −0.21 | 132 | 529 | 529 | 66.1 | 66.1 | – |
| APC | – | − | – | – | 5.72 | 45.8 | 45.8 | – | – | – | |
| INH | – | − | – | – | – | – | – | 467 | 29.2 | – | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonec, T.; Pospisilova, S.; Holanova, L.; Stranik, J.; Cernikova, A.; Pudelkova, V.; Kos, J.; Oravec, M.; Kollar, P.; Cizek, A.; et al. Synthesis and Antimicrobial Evaluation of 1-[(2-Substituted phenyl)carbamoyl]naphthalen-2-yl Carbamates. Molecules 2016, 21, 1189. https://doi.org/10.3390/molecules21091189
Gonec T, Pospisilova S, Holanova L, Stranik J, Cernikova A, Pudelkova V, Kos J, Oravec M, Kollar P, Cizek A, et al. Synthesis and Antimicrobial Evaluation of 1-[(2-Substituted phenyl)carbamoyl]naphthalen-2-yl Carbamates. Molecules. 2016; 21(9):1189. https://doi.org/10.3390/molecules21091189
Chicago/Turabian StyleGonec, Tomas, Sarka Pospisilova, Lucie Holanova, Josef Stranik, Aneta Cernikova, Valeria Pudelkova, Jiri Kos, Michal Oravec, Peter Kollar, Alois Cizek, and et al. 2016. "Synthesis and Antimicrobial Evaluation of 1-[(2-Substituted phenyl)carbamoyl]naphthalen-2-yl Carbamates" Molecules 21, no. 9: 1189. https://doi.org/10.3390/molecules21091189
APA StyleGonec, T., Pospisilova, S., Holanova, L., Stranik, J., Cernikova, A., Pudelkova, V., Kos, J., Oravec, M., Kollar, P., Cizek, A., & Jampilek, J. (2016). Synthesis and Antimicrobial Evaluation of 1-[(2-Substituted phenyl)carbamoyl]naphthalen-2-yl Carbamates. Molecules, 21(9), 1189. https://doi.org/10.3390/molecules21091189