Recent Advances in Neurogenic Small Molecules as Innovative Treatments for Neurodegenerative Diseases
Abstract
:1. Introduction
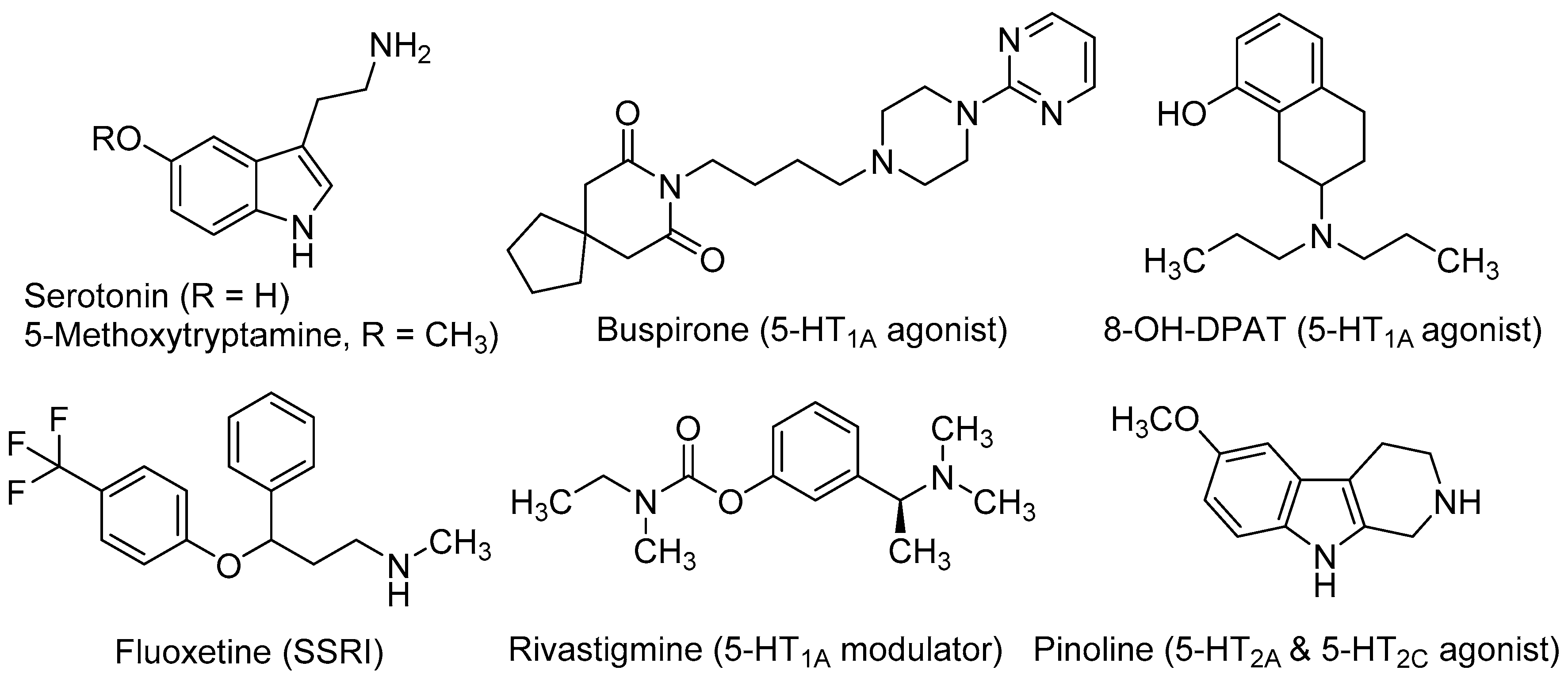
2. Role of Serotonergic System in Neurogenesis
3. Melatonergic System Role in Neurogenesis
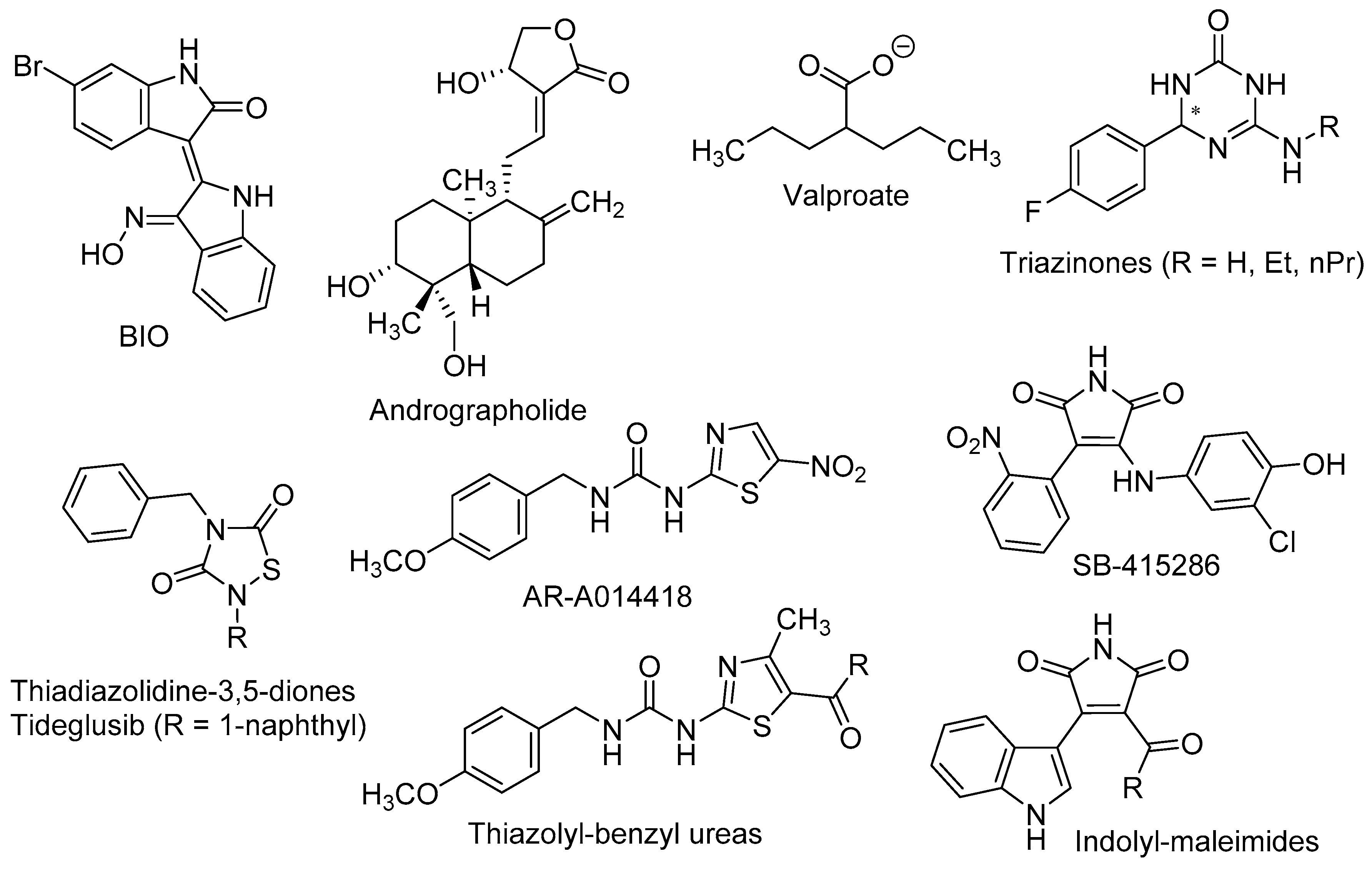
4. Effects of the Wnt/β-Catenin Pathway in Neurogenesis
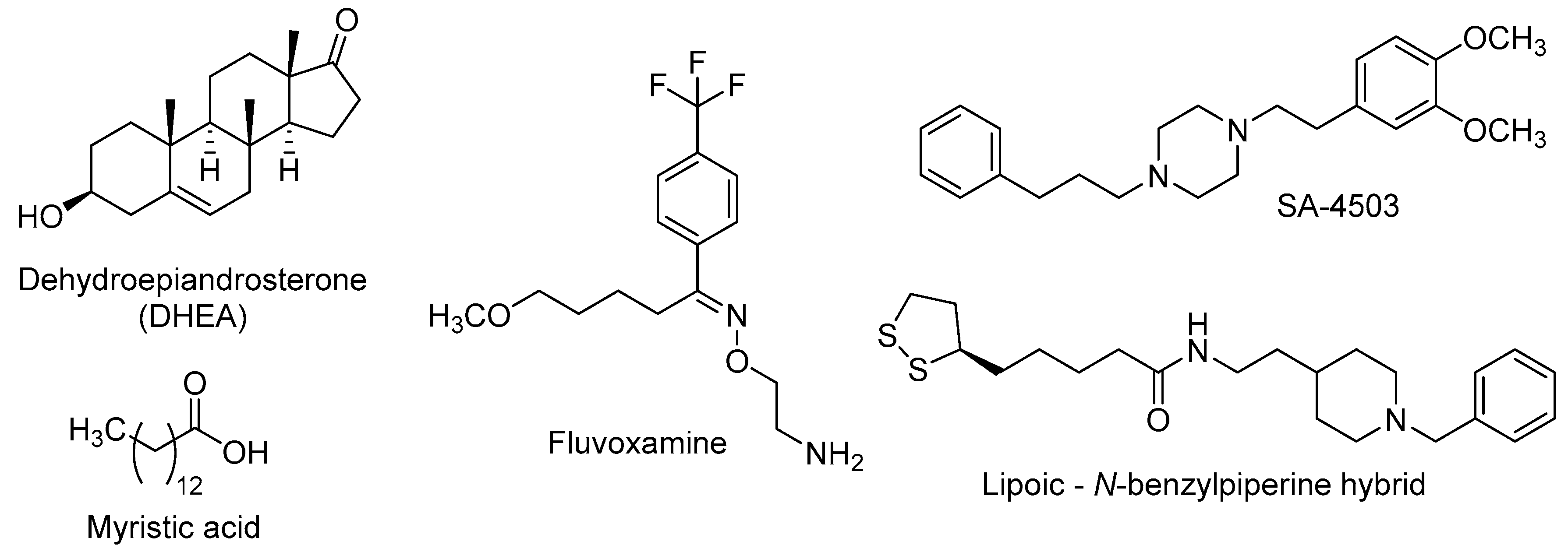
5. Sigma Receptors Role in Neurogenesis
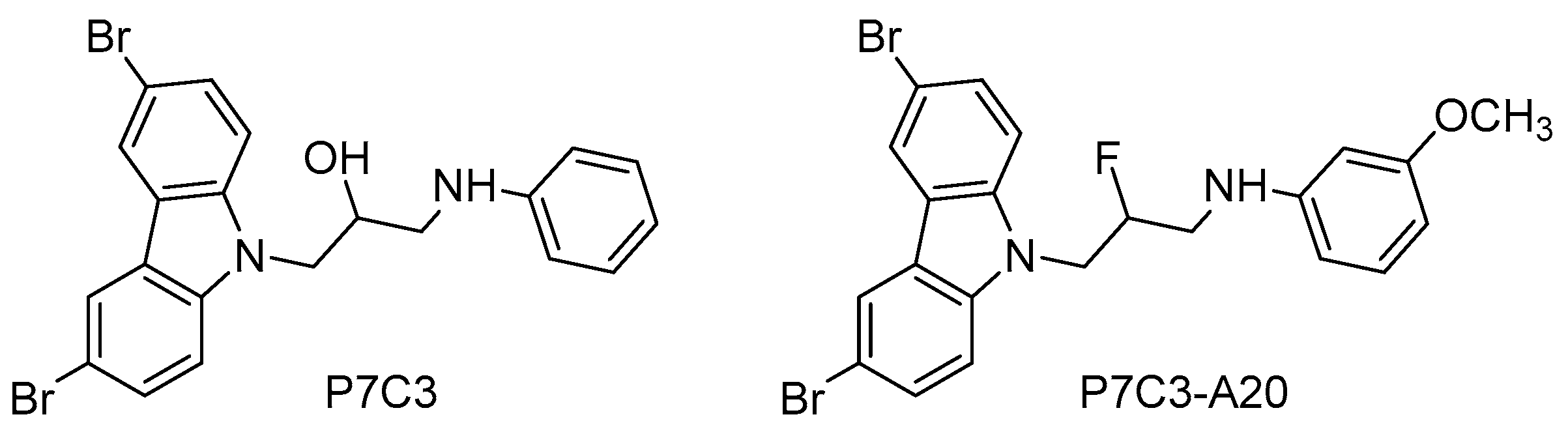
6. Role of NAMPT-NAD in Neurogenesis
7. Role of the Transcription Factor Nrf2 in Neurogenesis
8. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations and IUPAC Names of Cited Drugs
| Aβ | beta-amyloid peptide |
| N-Acetylserotonin | N-(2-(5-hydroxy-1H-indol-3-yl)ethyl)acetamide |
| AChE | Acetylcholinesterase |
| AD | Alzheimer’s disease |
| Andrographolide | 3-[2-(Decahydro-6-hydroxy-5-hydroxymethyl-5,8a-dimethyl-2-methylene-1-napthalenyl)ethylidene]dihydro-4-hydroxy-2(3H)-furanone |
| Agomelatine | N-[2-(7-Methoxynaphthalen-1-yl)ethyl]acetamide |
| ALS | Amyotrophic lateral sclerosis |
| AR-A014418 | 1-(4-Methoxybenzyl)-3-(5-nitrothiazol-2-yl)urea |
| AREs | Antioxidant response elements |
| BDNF | Brain-derived neurotrophic factor |
| BIO | 6-Bromoindirubin-3’-oxime |
| BrdU | Bromodeoxyuridine |
| BuChE | Butylcholinesterase |
| Buspirone | 8-[4-(4-Pyrimidin-2-ylpiperazin-1-yl)butyl]-8-azaspiro[4,5]decane-7,9-dione |
| CaMKIV | Calcium/calmodulin-dependent protein kinase IV |
| CNS | Central nervous system |
| Curcumin | (1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione |
| DG | Dentate gyrus |
| DHEA | Dehydroepiandrosterone |
| ERK | Extracellular signal-regulated kinase |
| Fluoxetine | N-Methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]propan-1-amine |
| Fluvoxamine | 2-{[(E)-{5-Methoxy-1-[4-(trifluoromethyl)phenyl]pentylidene}amino]oxy}ethanamine |
| GDNF | Glial cell-derived neurotrophic factor |
| GPCRs | G-protein-coupled receptors |
| GSK-3β | Glycogen synthase kinase-3β |
| HO-1 | Heme oxygenase-1 |
| 5-HT | Serotonin |
| 5-HTRs | Serotonergic receptors |
| Ketanserin | 3-{2-[4-(4-Fluorobenzoyl)piperidin-1-yl]ethyl}quinazoline-2,4(1H,3H)-dione |
| LTP | Long-term potentiation |
| Luzindole | N-(2-(2-Benzyl-1H-indol-3-yl)ethyl)acetamide |
| MeHg | Methylmercury |
| Melatonin | N-(2-(5-Methoxy-1H-indol-3-yl)ethyl)acetamide |
| 5-MeOT | 5-Methoxytryptamine |
| MTRs | Melatonergic receptors |
| Myristic acid | Tetradecanoic acid |
| NAD | Nicotinamide adenine dinucleotide |
| NAMPT | Nicotinamide phosphoribosyl transferase |
| NE-100 | 4-Methoxy-3-(2-phenylethoxy)-N,N-dipropylbenzeneethanamine |
| NGF | Nerve-growth factor |
| NQO1 | NAD(P)H-quinone oxidoreductase 1 |
| Nrf2 | Nuclear erythroid 2-related factor |
| NSCs | Neural stem-cells |
| OBX | Olfactory bulbectomized (mouse) |
| OGD/R | Oxygen-glucose deprivation/reoxygenation |
| 8-OH-DPAT | 8-Hydroxy-2-(di-n-propylamino)tetralin |
| P7C3 | 1-(3,6-Dibromo-9H-carbazol-9-yl)-3-(phenylamino)propan-2-ol |
| P7C3-A20 | N-(3-(3,6-Dibromo-9H-carbazol-9-yl)-2-fluoropropyl)-3-methoxyaniline |
| Pinoline | 6-Methoxy-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole |
| PKB | Protein kinase B |
| Resveratrol | trans-3,5,4′-Trihydroxystilbene |
| Rivastigmine | (S)-3-[1-(Dimethylamino)ethyl]phenyl N-ethyl-N-methylcarbamate |
| S32006 | N-(Pyridin-3-yl)-1H-benzo[e]indole-3(2H)-carboxamide |
| SA-4503 | 1-[2-(3,4-Dimethoxyphenyl)ethyl]-4-(3-phenylpropyl)piperazine |
| SB-415286 | 3-((3-Chloro-4-hydroxyphenyl)amino)-4-(2-nitrophenyl)-1H-pyrrole-2,5-dione |
| SGZ | Subgranular zone |
| σRs | Sigma receptors |
| SSRIs | Selective serotonin reuptake inhibitors |
| SVZ | Subventricular zone |
| Tideglusib | 4-Benzyl-2-(naphthalen-1-yl)-1,2,4-thiadiazolidine-3,5-dione |
| TrkB | Tyrosine receptor kinase |
| Valproate | 2-Propylpentanoate |
| WAY100635 | N-[2-[4-(2-Methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridyl)cyclohexane-carboxamide |
References
- Mason, C.; Dunnill, P. A brief definition of regenerative medicine. Regen. Med. 2008, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Thiel, G.T. 40 projects in stem cell research, tissue engineering, tolerance induction and more (NRP46 “Implants and Transplants” 1999–2006). Swiss Med. Wkly. 2007, 137 (Suppl. 155), 3S–8S. [Google Scholar] [PubMed]
- Altman, J. Are new neurons formed in the brains of adult mammals? Science 1962, 135, 1127–1128. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.A.; Nottebohm, F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc. Natl. Acad. Sci. USA 1983, 80, 2390–2394. [Google Scholar] [CrossRef] [PubMed]
- Ponti, G.; Peretto, P.; Bonfanti, L. Genesis of neuronal and glial progenitors in the cerebellar cortex of peripuberal and adult rabbits. PLoS ONE 2008, 3, e2366. [Google Scholar] [CrossRef] [PubMed]
- Harman, A.; Meyer, P.; Ahmat, A. Neurogenesis in the hippocampus of an adult marsupial. Brain Behav. Evol. 2003, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Reeves, A.J.; Graziano, M.S.; Gross, C.G. Neurogenesis in the neocortex of adult primates. Science 1999, 286, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Richards, L.J.; Kilpatrick, T.J.; Bartlett, P.F. De novo generation of neuronal cells from the adult mouse brain. Proc. Natl. Acad. Sci. USA 1992, 89, 8591–8595. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, B.A.; Weiss, S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 1992, 255, 1707–1710. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H.; Coates, P.W.; Palmer, T.D.; Kuhn, H.G.; Fisher, L.J.; Suhonen, J.O.; Peterson, D.A.; Suhr, S.T.; Ray, J. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc. Natl. Acad. Sci. USA 1995, 92, 11879–11883. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T.D.; Takahashi, J.; Gage, F.H. The adult rat hippocampus contains primordial neural stem cells. Mol. Cell. Neurosci. 1997, 8, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Jessberger, S.; Steiner, B.; Kronenberg, G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004, 27, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.S.; Perfilieva, E.; Bjork-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Gianni, L.; Kinsella, T.J.; Klecker, R.W., Jr.; Jenkins, J.; Rowland, J.; Glatstein, E.; Mitchell, J.B.; Collins, J.; Myers, C. Pharmacological evaluation of intravenous delivery of 5-bromodeoxyuridine to patients with brain tumors. Cancer Res. 1984, 44, 1702–1705. [Google Scholar] [PubMed]
- Decimo, I.; Bifari, F.; Krampera, M.; Fumagalli, G. Neural stem cell niches in health and diseases. Curr. Pharm. Des. 2012, 18, 1755–1783. [Google Scholar] [CrossRef] [PubMed]
- Gotz, M.; Nakafuku, M.; Petrik, D. Neurogenesis in the developing and adult brain—Similarities and key differences. Cold Spring Harb. Perspect. Biol. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Urbán, N.; Guillemot, F. Neurogenesis in the embryonic and adult brain: same regulators, different roles. Front. Cell. Neurosci. 2014, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Kintner, C. Neurogenesis in embryos and in adult neural stem cells. J. Neurosci. 2002, 22, 639–643. [Google Scholar] [PubMed]
- Ohira, K.; Furuta, T.; Hioki, H.; Nakamura, K.C.; Kuramoto, E.; Tanaka, Y.; Funatsu, N.; Shimizu, K.; Oishi, T.; Hayashi, M.; et al. Ischemia-induced neurogenesis of neocortical layer 1 progenitor cells. Nat. Neurosci. 2010, 13, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Balu, D.T.; Lucki, I. Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neurosci. Biobehav. Rev. 2009, 33, 232–252. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Wiskott, L.; Gage, F.H. Functional significance of adult neurogenesis. Curr. Opin. Neurobiol. 2004, 14, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Sprick, U. Functional aspects of the involvement of the hippocampus in behavior and memory functions. Behav. Brain Res. 1995, 66, 61–64. [Google Scholar] [CrossRef]
- Mongiat, L.A.; Schinder, A.F. A price to pay for adult neurogenesis. Science 2014, 344, 594–595. [Google Scholar] [CrossRef] [PubMed]
- Rolando, C.; Taylor, V. Neural stem cell of the hippocampus: Development, physiology regulation, and dysfunction in disease. Curr. Top. Dev. Biol. 2014, 107, 183–206. [Google Scholar] [PubMed]
- Duman, R.S.; Malberg, J.; Nakagawa, S. Regulation of adult neurogenesis by psychotropic drugs and stress. J. Pharmacol. Exp. Ther. 2001, 299, 401–407. [Google Scholar] [PubMed]
- Abdipranoto, A.; Wu, S.; Stayte, S.; Vissel, B. The role of neurogenesis in neurodegenerative diseases and its implications for therapeutic development. CNS Neurol. Disord. Drug Targets 2008, 7, 187–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Song, N. Molecular mechanism of adult neurogenesis and its association with human brain diseases. J. Cent. Nerv. Syst. Dis. 2016, 8, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Winner, B.; Winkler, J. Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2015, 7, a021287. [Google Scholar] [CrossRef] [PubMed]
- Prenderville, J.A.; Kelly, A.M.; Downer, E.J. The role of cannabinoids in adult neurogenesis. Br. J. Pharmacol. 2015, 172, 3950–3963. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, K.M.; Candelario, K.M.; Steindler, D.A.; Borchelt, D.R. Regenerative medicine in Alzheimer’s disease. Transl. Res. 2014, 163, 432–438. [Google Scholar] [CrossRef]
- Huart, C.; Rombaux, P.; Hummel, T. Plasticity of the human olfactory system: The olfactory bulb. Molecules 2013, 18, 11586. [Google Scholar] [CrossRef] [PubMed]
- Doze, V.A.; Perez, D.M. G-Protein-coupled receptors in adult neurogenesis. Pharmacol. Rev. 2012, 64, 645–675. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, M.S.; Sanders-Bush, E. Serotonin and brain development. Int. Rev. Neurobiol. 2004, 59, 111–174. [Google Scholar] [PubMed]
- Oberlander, T.F. Fetal serotonin signaling: Setting pathways for early childhood development and behavior. J. Adolesc. Health 2012, 51, S9–S16. [Google Scholar] [CrossRef] [PubMed]
- Brezun, J.M.; Daszuta, A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience 1999, 89, 999–1002. [Google Scholar] [CrossRef]
- Mazer, C.; Muneyyirci, J.; Taheny, K.; Raio, N.; Borella, A.; Whitaker-Azmitia, P. Serotonin depletion during synaptogenesis leads to decreased synaptic density and learning deficits in the adult rat: A possible model of neurodevelopmental disorders with cognitive deficits. Brain Res. 1997, 760, 68–73. [Google Scholar] [CrossRef]
- Xing, L.; Son, J.H.; Stevenson, T.J.; Lillesaar, C.; Bally-Cuif, L.; Dahl, T.; Bonkowsky, J.L. A serotonin circuit acts as an environmental sensor to mediate midline axon crossing through EphrinB2. J. Neurosci. 2015, 35, 14794–14808. [Google Scholar] [CrossRef] [PubMed]
- Benninghoff, J.; Gritti, A.; Rizzi, M.; Lamorte, G.; Schloesser, R.J.; Schmitt, A.; Robel, S.; Genius, J.; Moessner, R.; Riederer, P.; et al. Serotonin depletion hampers survival and proliferation in neurospheres derived from adult neural stem cells. Neuropsychopharmacology 2010, 35, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Grabiec, M.; Turlejski, K.; Djavadian, R. Reduction of the number of new cells reaching olfactory bulbs impairs olfactory perception in the adult opossum. Acta Neurobiol. Exp. (Wars.) 2009, 69, 168–176. [Google Scholar] [PubMed]
- Grabiec, M.; Turlejski, K.; Djavadian, R.L. The partial 5-HT1A receptor agonist buspirone enhances neurogenesis in the opossum (Monodelphis domestica). Eur. Neuropsychopharmacol. 2009, 19, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Guidi, S.; Stagni, F.; Bianchi, P.; Ciani, E.; Giacomini, A.; de Franceschi, M.; Moldrich, R.; Kurniawan, N.; Mardon, K.; Giuliani, A.; et al. Prenatal pharmacotherapy rescues brain development in a Down’s syndrome mouse model. Brain 2014, 137, 380–401. [Google Scholar] [CrossRef] [PubMed]
- Stagni, F.; Giacomini, A.; Guidi, S.; Ciani, E.; Ragazzi, E.; Filonzi, M.; de Iasio, R.; Rimondini, R.; Bartesaghi, R. Long-term effects of neonatal treatment with fluoxetine on cognitive performance in Ts65Dn mice. Neurobiol. Dis. 2015, 74, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Papp, M.; Gruca, P.; Lason-Tyburkiewicz, M.; Willner, P. Antidepressant, anxiolytic and procognitive effects of rivastigmine and donepezil in the chronic mild stress model in rats. Psychopharmacology 2016, 233, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Moriguchi, S.; Tagashira, H.; Fukunaga, K. Rivastigmine improves hippocampal neurogenesis and depression-like behaviors via 5-HT1A receptor stimulation in olfactory bulbectomized mice. Neuroscience 2014, 272, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Moriguchi, S.; Tagashira, H.; Fukunaga, K. Rivastigmine restores 5-HT1A receptor levels in the hippocampus of olfactory bulbectomized mice. Adv. Alzheimer’s Dis. 2014, 3, 128–136. [Google Scholar] [CrossRef]
- De la Fuente Revenga, M.; Pérez, C.; Morales-García, J.A.; Alonso-Gil, S.; Pérez-Castillo, A.; Caignard, D.H.; Yáñez, M.; Gamo, A.M.; Rodríguez-Franco, M.I. Neurogenic potential assessment and pharmacological characterization of 6-methoxy-1,2,3,4-tetrahydro-beta-carboline (pinoline) and melatonin-pinoline hybrids. ACS Chem. Neurosci. 2015, 6, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Zlotos, D.P.; Jockers, R.; Cecon, E.; Rivara, S.; Witt-Enderby, P.A. MT1 and MT2 melatonin receptors: ligands, models, oligomers, and therapeutic potential. J. Med. Chem. 2014, 57, 3161–3185. [Google Scholar] [CrossRef] [PubMed]
- Pala, D.; Lodola, A.; Bedini, A.; Spadoni, G.; Rivara, S. Homology models of melatonin receptors: challenges and recent advances. Int. J. Mol. Sci. 2013, 14, 8093–8121. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Manchester, L.C.; Tan, D.X. Neurotoxins: free radical mechanisms and melatonin protection. Curr. Neuropharmacol. 2010, 8, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; BaHammam, A.S.; Brown, G.M.; Spence, D.W.; Bharti, V.K.; Kaur, C.; Hardeland, R.; Cardinali, D.P. Melatonin antioxidative defense: therapeutical implications for aging and neurodegenerative processes. Neurotox. Res. 2013, 23, 267–300. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Huang, Q.X.; Yang, S.S.; Chu, J.; Wang, J.Z.; Tian, Q. Melatonin in Alzheimer’s disease. Int. J. Mol. Sci. 2013, 14, 14575–14593. [Google Scholar] [CrossRef] [PubMed]
- Olcese, J.M.; Cao, C.; Mori, T.; Mamcarz, M.B.; Maxwell, A.; Runfeldt, M.J.; Wang, L.; Zhang, C.; Lin, X.; Zhang, G.; et al. Protection against cognitive deficits and markers of neurodegeneration by long-term oral administration of melatonin in a transgenic model of Alzheimer disease. J. Pineal Res. 2009, 47, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Iguichi, H.; Kato, K.I.; Ibayashi, H. Age-dependent reduction in serum melatonin concentrations in healthy human subjects. J. Clin. Endocrinol. Metab. 1982, 55, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Tu, Y.; Chen, J.; Tan, D.; Liu, X.; Pi, R. Effects of melatonin and its analogues on neural stem cells. Mol. Cell. Endocrinol. 2016, 420, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Sarlak, G.; Jenwitheesuk, A.; Chetsawang, B.; Govitrapong, P. Effects of melatonin on nervous system aging: neurogenesis and neurodegeneration. J. Pharmacol. Sci. 2013, 123, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Manda, K.; Reiter, R.J. Melatonin maintains adult hippocampal neurogenesis and cognitive functions after irradiation. Prog. Neurobiol. 2010, 90, 60–68. [Google Scholar] [CrossRef] [PubMed]
- López, L.C.; Escames, G.; López, A.; García, J.A.; Doerrier, C.; Acuña-Castroviejo, D. Melatonin, neurogenesis, and aging brain. Open Neuroendocrinol. J. 2010, 3, 121–133. [Google Scholar]
- Ramírez-Rodríguez, G.; Klempin, F.; Babu, H.; Benítez-King, G.; Kempermann, G. Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacology 2009, 34, 2180–2191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Liu, L.L.; Jiang, S.X.; Zhong, Y.M.; Yang, X.L. Activation of the zeta receptor 1 suppresses NMDA responses in rat retinal ganglion cells. Neuroscience 2011, 177, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rodríguez, G.; Vega-Rivera, N.M.; Benítez-King, G.; Castro-García, M.; Ortíz-López, L. Melatonin supplementation delays the decline of adult hippocampal neurogenesis during normal aging of mice. Neurosci. Lett. 2012, 530, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Poeggeler, B. Melatonin, aging, and age-related diseases. Endocrine 2005, 27, 201–212. [Google Scholar] [CrossRef]
- Tocharus, C.; Puriboriboon, Y.; Junmanee, T.; Tocharus, J.; Ekthuwapranee, K.; Govitrapong, P. Melatonin enhances adult rat hippocampal progenitor cell proliferation via ERK signaling pathway through melatonin receptor. Neuroscience 2014, 275, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Somera-Molina, K.C.; Hudson, R.L.; Dubocovich, M.L. Melatonin potentiates running wheel-induced neurogenesis in the dentate gyrus of adult C3H/HeN mice hippocampus. J. Pineal Res. 2013, 54, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodriguez, G.; Ortiz-Lopez, L.; Dominguez-Alonso, A.; Benitez-King, G.A.; Kempermann, G. Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J. Pineal Res. 2011, 50, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.W.; Liu, X.; Pradoldej, S.; Tosini, G.; Chang, Q.; Iuvone, P.M.; Ye, K. N-Acetylserotonin activates TrkB receptor in a circadian rhythm. Proc. Natl. Acad. Sci. USA 2010, 107, 3876–3881. [Google Scholar] [CrossRef] [PubMed]
- Iuvone, P.M.; Boatright, J.H.; Tosini, G.; Ye, K. N-Acetylserotonin: Circadian activation of the BDNF receptor and neuroprotection in the retina and brain. Adv. Exp. Med. Biol. 2014, 801, 765–771. [Google Scholar] [PubMed]
- Huang, E.J.; Reichardt, L.F. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Simmons, M.S.; Perry, R.T.; Wiener, H.W.; Harrell, L.E.; Go, R.C. Genetic association of neurotrophic tyrosine kinase receptor type 2 (NTRK2) With Alzheimer’s disease. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008, 147, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Tosini, G.; Ye, K.; Iuvone, P.M. N-Acetylserotonin: Neuroprotection, neurogenesis, and the sleepy brain. Neuroscientist 2012, 18, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Guardiola-Lemaitre, B.; de Bodinat, C.; Delagrange, P.; Millan, M.J.; Munoz, C.; Mocaer, E. Agomelatine: Mechanism of action and pharmacological profile in relation to antidepressant properties. Br. J. Pharmacol. 2014, 171, 3604–3619. [Google Scholar] [CrossRef] [PubMed]
- Pompili, M.; Serafini, G.; Innamorati, M.; Venturini, P.; Fusar-Poli, P.; Sher, L.; Amore, M.; Girardi, P. Agomelatine, a novel intriguing antidepressant option enhancing neuroplasticity: A critical review. World J. Biol. Psychiatry 2013, 14, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Banasr, M.; Soumier, A.; Hery, M.; Mocaer, E.; Daszuta, A. Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol. Psychiatry 2006, 59, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Soumier, A.; Banasr, M.; Lortet, S.; Masmejean, F.; Bernard, N.; Kerkerian-Le-Goff, L.; Gabriel, C.; Millan, M.J.; Mocaer, E.; Daszuta, A. Mechanisms contributing to the phase-dependent regulation of neurogenesis by the novel antidepressant, agomelatine, in the adult rat hippocampus. Neuropsychopharmacology 2009, 34, 2390–2403. [Google Scholar] [CrossRef] [PubMed]
- López-Iglesias, B.; Pérez, C.; Morales-García, J.A.; Alonso-Gil, S.; Pérez-Castillo, A.; Romero, A.; López, M.G.; Villarroya, M.; Conde, S.; Rodríguez-Franco, M.I. New melatonin-N,N-dibenzyl(N-methyl)amine hybrids: Potent neurogenic agents with antioxidant, cholinergic, and neuroprotective properties as innovative drugs for Alzheimer’s disease. J. Med. Chem. 2014, 57, 3773–3785. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente Revenga, M.; Fernández-Sáez, N.; Herrera-Arozamena, C.; Morales-García, J.A.; Alonso-Gil, S.; Pérez-Castillo, A.; Caignard, D.H.; Rivara, S.; Rodríguez-Franco, M.I. Novel N-acetyl bioisosteres of melatonin: melatonergic receptor pharmacology, physicochemical studies, and phenotypic assessment of their neurogenic potential. J. Med. Chem. 2015, 58, 4998–5014. [Google Scholar] [CrossRef] [PubMed]
- Varela-Nallar, L.; Inestrosa, N.C. Wnt signaling in the regulation of adult hippocampal neurogenesis. Front. Cell. Neurosci. 2013, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Machon, O.; Backman, M.; Machonova, O.; Kozmik, Z.; Vacik, T.; Andersen, L.; Krauss, S. A dynamic gradient of Wnt signaling controls initiation of neurogenesis in the mammalian cortex and cellular specification in the hippocampus. Dev. Biol. 2007, 311, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Inestrosa, N.C.; Arenas, E. Emerging roles of Wnts in the adult nervous system. Nat. Rev. Neurosci. 2010, 11, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Lie, D.C.; Colamarino, S.A.; Song, H.J.; Desire, L.; Mira, H.; Consiglio, A.; Lein, E.S.; Jessberger, S.; Lansford, H.; Dearie, A.R.; et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature 2005, 437, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Jessberger, S.; Clark, R.E.; Broadbent, N.J.; Clemenson, G.D., Jr.; Consiglio, A.; Lie, D.C.; Squire, L.R.; Gage, F.H. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn. Mem. 2009, 16, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Eom, T.Y.; Jope, R.S. Blocked inhibitory serine-phosphorylation of glycogen synthase kinase-3alpha/beta impairs in vivo neural precursor cell proliferation. Biol. Psychiatry 2009, 66, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Sirerol-Piquer, M.; Gomez-Ramos, P.; Hernandez, F.; Perez, M.; Moran, M.A.; Fuster-Matanzo, A.; Lucas, J.J.; Avila, J.; Garcia-Verdugo, J.M. GSK3beta overexpression induces neuronal death and a depletion of the neurogenic niches in the dentate gyrus. Hippocampus 2011, 21, 910–922. [Google Scholar] [PubMed]
- Wexler, E.M.; Geschwind, D.H.; Palmer, T.D. Lithium regulates adult hippocampal progenitor development through canonical Wnt pathway activation. Mol. Psychiatry 2008, 13, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Dastjerdi, F.V.; Zeynali, B.; Tafreshi, A.P.; Shahraz, A.; Chavoshi, M.S.; Najafabadi, I.K.; Vardanjani, M.M.; Atashi, A.; Soleimani, M. Inhibition of GSK-3beta enhances neural differentiation in unrestricted somatic stem cells. Cell. Biol. Int. 2012, 36, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Mesquita, A.R.; Bessa, J.; Sousa, J.C.; Sotiropoulos, I.; Leao, P.; Almeida, O.F.; Sousa, N. Lithium blocks stress-induced changes in depressive-like behavior and hippocampal cell fate: The role of glycogen-synthase-kinase-3beta. Neuroscience 2008, 152, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.J.; Shi, Y.; Austin, R.C.; Werstuck, G.H. Valproate protects cells from ER stress-induced lipid accumulation and apoptosis by inhibiting glycogen synthase kinase-3. J. Cell. Sci. 2005, 118, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Boku, S.; Nakagawa, S.; Masuda, T.; Nishikawa, H.; Kato, A.; Takamura, N.; Omiya, Y.; Kitaichi, Y.; Inoue, T.; Kusumi, I. Valproate recovers the inhibitory effect of dexamethasone on the proliferation of the adult dentate gyrus-derived neural precursor cells via GSK-3beta and beta-catenin pathway. Eur. J. Pharmacol. 2014, 723, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Usuki, F. Low concentrations of methylmercury inhibit neural progenitor cell proliferation associated with up-regulation of glycogen synthase kinase 3beta and subsequent degradation of cyclin E in rats. Toxicol. Appl. Pharmacol. 2015, 288, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, A.; Rosi, M.C.; Grossi, C.; Luccarini, I.; Casamenti, F. Lithium improves hippocampal neurogenesis, neuropathology and cognitive functions in APP mutant mice. PLoS ONE 2010, 5, e14382. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tan, X.F.; Nguyen, V.S.; Yang, P.; Zhou, J.; Gao, M.; Li, Z.; Lim, T.K.; He, Y.; Ong, C.S.; et al. A quantitative chemical proteomics approach to profile the specific cellular targets of andrographolide, a promising anticancer agent that suppresses tumor metastasis. Mol. Cell. Proteom. 2014, 13, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Serrano, F.G.; Tapia-Rojas, C.; Carvajal, F.J.; Hancke, J.; Cerpa, W.; Inestrosa, N.C. Andrographolide reduces cognitive impairment in young and mature AbetaPPswe/PS-1 mice. Mol. Neurodegener. 2014, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Varela-Nallar, L.; Arredondo, S.B.; Tapia-Rojas, C.; Hancke, J.; Inestrosa, N.C. Andrographolide stimulates neurogenesis in the adult hippocampus. Neural Plast. 2015, 2015, 935403. [Google Scholar] [CrossRef] [PubMed]
- Morales-García, J.A.; Luna-Medina, R.; Alonso-Gil, S.; Sanz-Sancristóbal, M.; Palomo, V.; Gil, C.; Santos, A.; Martínez, A.; Pérez-Castillo, A. Glycogen synthase kinase 3 inhibition promotes adult hippocampal neurogenesis in vitro and in vivo. ACS Chem. Neurosci. 2012, 3, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Lovestone, S.; Boada, M.; Dubois, B.; Hull, M.; Rinne, J.O.; Huppertz, H.J.; Calero, M.; Andres, M.V.; Gomez-Carrillo, B.; Leon, T.; et al. A phase II trial of tideglusib in Alzheimer’s disease. J. Alzheimer's Dis. 2015, 45, 75–88. [Google Scholar]
- Prati, F.; de Simone, A.; Bisignano, P.; Armirotti, A.; Summa, M.; Pizzirani, D.; Scarpelli, R.; Perez, D.I.; Andrisano, V.; Perez-Castillo, A.; et al. Multitarget drug discovery for Alzheimer's disease: Triazinones as BACE-1 and GSK-3beta inhibitors. Angew. Chem. Int., Ed. 2015, 54, 1578–1582. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; de Simone, A.; Armirotti, A.; Summa, M.; Pizzirani, D.; Scarpelli, R.; Bertozzi, S.M.; Perez, D.I.; Andrisano, V.; Perez-Castillo, A.; et al. 3,4-Dihydro-1,3,5-triazin-2(1H)-ones as the first dual BACE-1/GSK-3beta fragment hits against Alzheimer’s disease. ACS Chem. Neurosci. 2015, 6, 1665–1682. [Google Scholar] [CrossRef] [PubMed]
- Weissman, A.D.; Su, T.P.; Hedreen, J.C.; London, E.D. Sigma receptors in post-mortem human brains. J. Pharmacol. Exp. Ther. 1988, 247, 29–33. [Google Scholar] [PubMed]
- Jansen, K.L.R.; Faull, R.L.M.; Dragunow, M.; Leslie, R.A. Autoradiographic distribution of sigma receptors in human neocortex, hippocampus, basal ganglia, cerebellum, pineal and pituitary glands. Brain Res. 1991, 559, 172–177. [Google Scholar] [CrossRef]
- Sha, S.; Qu, W.J.; Li, L.; Lu, Z.H.; Chen, L.; Yu, W.F.; Chen, L. Sigma-1 receptor knockout impairs neurogenesis in dentate gyrus of adult hippocampus via down-regulation of NMDA receptors. CNS Neurosci. Ther. 2013, 19, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Mishina, M.; Ohyama, M.; Ishii, K.; Kitamura, S.; Kimura, Y.; Oda, K.; Kawamura, K.; Sasaki, T.; Kobayashi, S.; Katayama, Y.; et al. Low density of sigma1 receptors in early Alzheimer's disease. Ann. Nucl. Med. 2008, 22, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Prause, J.; Goswami, A.; Katona, I.; Roos, A.; Schnizler, M.; Bushuven, E.; Dreier, A.; Buchkremer, S.; Johann, S.; Beyer, C.; et al. Altered localization, abnormal modification and loss of function of sigma receptor-1 in amyotrophic lateral sclerosis. Hum. Mol. Genet. 2013, 22, 1581–1600. [Google Scholar] [CrossRef] [PubMed]
- Klette, K.L.; DeCoster, M.A.; Moreton, J.E.; Tortella, F.C. Role of calcium in sigma-mediated neuroprotection in rat primary cortical neurons. Brain Res. 1995, 704, 31–41. [Google Scholar] [CrossRef]
- Pal, A.; Fontanilla, D.; Gopalakrishnan, A.; Chae, Y.K.; Markley, J.L.; Ruoho, A.E. The sigma-1 receptor protects against cellular oxidative stress and activates antioxidant response elements. Eur. J. Pharmacol. 2012, 682, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Carter, E.L.; Torbey, M.T.; Martin, L.J.; Koehler, R.C. Sigma receptor ligand 4-phenyl-1-(4-phenylbutyl)-piperidine modulates neuronal nitric oxide synthase/postsynaptic density-95 coupling mechanisms and protects against neonatal ischemic degeneration of striatal neurons. Exp. Neurol. 2010, 221, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Klouz, A.; Said, D.B.; Ferchichi, H.; Kourda, N.; Ouanes, L.; Lakhal, M.; Tillement, J.P.; Morin, D. Protection of cellular and mitochondrial functions against liver ischemia by N-benzyl-N’-(2-hydroxy-3,4-dimethoxybenzyl)-piperazine (BHDP), a sigma1 ligand. Eur. J. Pharmacol. 2008, 578, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Baulieu, E.E.; Robel, P. Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) as neuroactive neurosteroids. Proc. Natl. Acad. Sci. USA 1998, 95, 4089–4091. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, A. Dehydroepiandrosterone sulfate and aging. Ann. N. Y. Acad. Sci. 1995, 774, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, T.; Merril, C.R.; Harrington, M.G.; Lawlor, B.A.; Molchan, S.E.; Martinez, R.; Murphy, D.L. Reduced plasma dehydroepiandrosterone concentrations in Alzheimer’s disease. Lancet 1989, 2, 570. [Google Scholar] [CrossRef]
- Moriguchi, S.; Yamamoto, Y.; Ikuno, T.; Fukunaga, K. Sigma-1 receptor stimulation by dehydroepiandrosterone ameliorates cognitive impairment through activation of CaM kinase II, protein kinase C and extracellular signal-regulated kinase in olfactory bulbectomized mice. J. Neurochem. 2011, 117, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, S.; Shinoda, Y.; Yamamoto, Y.; Sasaki, Y.; Miyajima, K.; Tagashira, H.; Fukunaga, K. Stimulation of the sigma-1 receptor by DHEA enhances synaptic efficacy and neurogenesis in the hippocampal dentate gyrus of olfactory bulbectomized mice. PLoS ONE 2013, 8, e60863. [Google Scholar] [CrossRef] [PubMed]
- Ishima, T.; Nishimura, T.; Iyo, M.; Hashimoto, K. Potentiation of nerve growth factor-induced neurite outgrowth in PC12 cells by donepezil: Role of sigma-1 receptors and IP3 receptors. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1656–1659. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Hayashi, T.; Harvey, B.K.; Wang, Y.; Wu, W.W.; Shen, R.F.; Zhang, Y.; Becker, K.G.; Hoffer, B.J.; Su, T.P. Sigma-1 receptors regulate hippocampal dendritic spine formation via a free radical-sensitive mechanism involving Rac1xGTP pathway. Proc. Natl. Acad. Sci. USA 2009, 106, 22468–22473. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Pokrass, M.J.; Klauer, N.R.; Nohara, H.; Su, T.P. Sigma-1 receptor regulates tau phosphorylation and axon extension by shaping p35 turnover via myristic acid. Proc. Natl. Acad. Sci. USA 2015, 112, 6742–6747. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.R.; Hen, R. The current state of the neurogenic theory of depression and anxiety. Curr. Opin. Neurobiol. 2015, 30, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Su, T.P. Sigma-1 receptor ligands: Potential in the treatment of neuropsychiatric disorders. CNS Drugs 2004, 18, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Hindmarch, I.; Hashimoto, K. Cognition and depression: the effects of fluvoxamine, a sigma-1 receptor agonist, reconsidered. Hum. Psychopharmacol. 2010, 25, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, S.; Sakagami, H.; Yabuki, Y.; Sasaki, Y.; Izumi, H.; Zhang, C.; Han, F.; Fukunaga, K. Stimulation of sigma-1 receptor ameliorates depressive-like behaviors in CaMKIV null mice. Mol. Neurobiol. 2015, 52, 1210–1222. [Google Scholar] [CrossRef] [PubMed]
- Estrada, M.; Pérez, C.; Soriano, E.; Laurini, E.; Romano, M.; Pricl, S.; Morales-García, J.A.; Pérez-Castillo, A.; Rodríguez-Franco, M.I. New neurogenic lipoic-based hybrids as innovative Alzheimer’s drugs with sigma-1 agonism and beta-secretase inhibition. Future Med. Chem. 2016, 8, 1191–1207. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, X.Z.; Tian, W.W.; Guan, Y.F.; Wang, P.; Miao, C.Y. Extracellular visfatin has nicotinamide phosphoribosyltransferase enzymatic activity and is neuroprotective against ischemic injury. CNS Neurosci. Ther. 2014, 20, 539–547. [Google Scholar] [CrossRef]
- Stein, L.R.; Imai, S. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 2014, 33, 1321–1340. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.N.; Xu, T.Y.; Li, W.L.; Miao, C.Y. Targeting nicotinamide phosphoribosyltransferase as a potential therapeutic strategy to restore adult neurogenesis. CNS Neurosci. Ther. 2016, 22, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Pieper, A.A.; Xie, S.; Capota, E.; Estill, S.J.; Zhong, J.; Long, J.M.; Becker, G.L.; Huntington, P.; Goldman, S.E.; Shen, C.H.; et al. Discovery of a proneurogenic, neuroprotective chemical. Cell 2010, 142, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.C.; Britt, J.K.; de Jesus-Cortes, H.; Lu, Y.; Genova, R.M.; Khan, M.Z.; Voorhees, J.R.; Shao, J.; Katzman, A.C.; Huntington, P.J.; et al. P7C3 neuroprotective chemicals block axonal degeneration and preserve function after traumatic brain injury. Cell Rep. 2014, 8, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Latchney, S.E.; Jaramillo, T.C.; Rivera, P.D.; Eisch, A.J.; Powell, C.M. Chronic P7C3 treatment restores hippocampal neurogenesis in the Ts65Dn mouse model of Down syndrome. Neurosci. Lett. 2015, 591, 86–92. [Google Scholar] [CrossRef] [PubMed]
- De Jesus-Cortes, H.; Xu, P.; Drawbridge, J.; Estill, S.J.; Huntington, P.; Tran, S.; Britt, J.; Tesla, R.; Morlock, L.; Naidoo, J.; et al. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of Parkinson disease. Proc. Natl. Acad. Sci. USA 2012, 109, 17010–17015. [Google Scholar] [CrossRef] [PubMed]
- Tesla, R.; Wolf, H.P.; Xu, P.; Drawbridge, J.; Estill, S.J.; Huntington, P.; McDaniel, L.; Knobbe, W.; Burket, A.; Tran, S.; et al. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 2012, 109, 17016–17021. [Google Scholar] [CrossRef]
- Wang, G.; Han, T.; Nijhawan, D.; Theodoropoulos, P.; Naidoo, J.; Yadavalli, S.; Mirzaei, H.; Pieper, A.A.; Ready, J.M.; McKnight, S.L. P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell 2014, 158, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.I.; Kobayashi, A.; Wakabayashi, N.; Kim, S.G.; Yamamoto, M. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc. Natl. Acad. Sci. USA 2004, 101, 2046–2051. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Johnson, D.A.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Vargas, M.R.; Chen, P.C. The Nrf2-ARE pathway: An indicator and modulator of oxidative stress in neurodegeneration. Ann. N. Y. Acad. Sci. 2008, 1147, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Y.; Zheng, H.; Fu, J.; Hou, Y.; Wang, H.; Zhang, Q.; Yamamoto, M.; Pi, J. The role of nuclear factor E2-Related factor 2 and uncoupling protein 2 in glutathione metabolism: Evidence from an in vivo gene knockout study. Biochem. Biophys. Res. Commun. 2016. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y. Genomic structure and variation of nuclear factor (erythroid-derived 2)-like 2. Oxid. Med. Cell. Longev. 2013, 2013, 286524. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wu, T.; Lau, A.; Jiang, T.; Huang, Z.; Wang, X.J.; Chen, W.; Wong, P.K.; Zhang, D.D. Nrf2 promotes neuronal cell differentiation. Free Radic. Biol. Med. 2009, 47, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Karkkainen, V.; Pomeshchik, Y.; Savchenko, E.; Dhungana, H.; Kurronen, A.; Lehtonen, S.; Naumenko, N.; Tavi, P.; Levonen, A.L.; Yamamoto, M.; et al. Nrf2 regulates neurogenesis and protects neural progenitor cells against Abeta toxicity. Stem Cells 2014, 32, 1904–1916. [Google Scholar] [CrossRef] [PubMed]
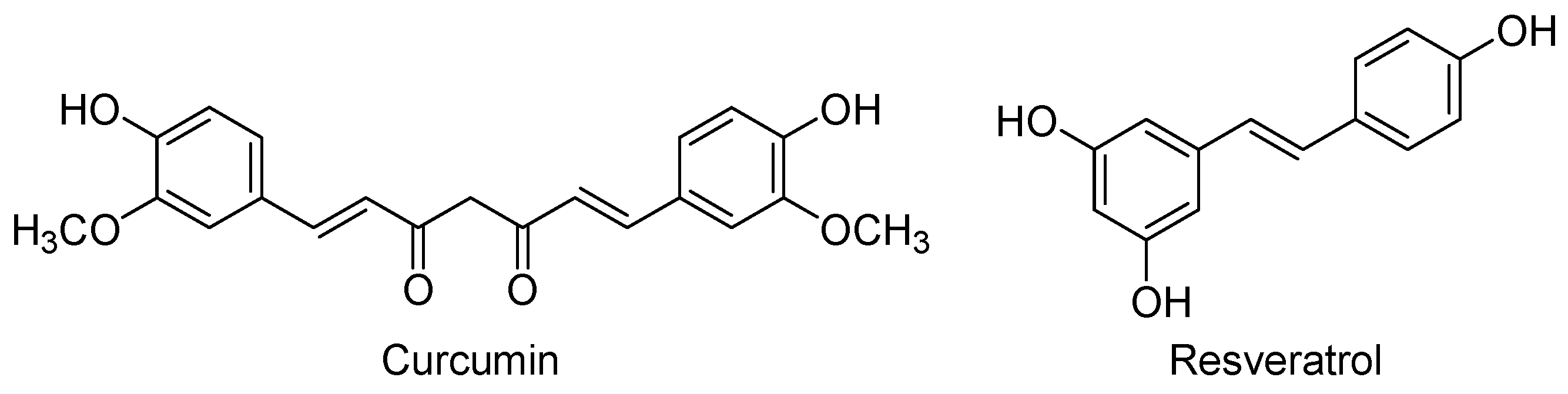
- Tsai, Y.M.; Chien, C.F.; Lin, L.C.; Tsai, T.H. Curcumin and its nano-formulation: The kinetics of tissue distribution and blood-brain barrier penetration. Int. J. Pharm. 2011, 416, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, J.E.; Liu, K.; Yan, X.; Toldo, S.; Selden, T.; Estrada, M.; Rodríguez-Franco, M.I.; Halquist, M.S.; Ye, D.; Zhang, S. Discovery of 5-(4-hydroxyphenyl)-3-oxo-pentanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide as a neuroprotectant for Alzheimer’s disease by hybridization of curcumin and melatonin. ACS Chem. Neurosci. 2014, 5, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Cha, S.H.; Jeon, H.G. Curcumin-induced histone hypoacetylation enhances caspase-3-dependent glioma cell death and neurogenesis of neural progenitor cells. Stem Cells Dev. 2006, 15, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Son, T.G.; Park, H.R.; Park, M.; Kim, M.S.; Kim, H.S.; Chung, H.Y.; Mattson, M.P.; Lee, J. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J. Biol. Chem. 2008, 283, 14497–14505. [Google Scholar] [CrossRef] [PubMed]
- Vivar, C. Adult hippocampal neurogenesis, aging and neurodegenerative diseases: Possible strategies to prevent cognitive impairment. Curr. Top. Med. Chem. 2015, 15, 2175–2192. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alloza, M.; Borrelli, L.A.; Rozkalne, A.; Hyman, B.T.; Bacskai, B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007, 102, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.; Lam, C.W.; Cheung, S.K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Ferrieres, J. The French paradox: Lessons for other countries. Heart 2004, 90, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Sharan, S.; Nagar, S. Pulmonary metabolism of resveratrol: In vitro and in vivo evidence. Drug Metab. Dispos. 2013, 41, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.O.; Lee, J.S.; Lee, H.G. Improving solubility, stability, and cellular uptake of resveratrol by nanoencapsulation with chitosan and gamma-poly (glutamic acid). Colloids Surf. B Biointerfaces 2016, 147, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Greene, L.M.; Meegan, M.J.; Zisterer, D.M. Combretastatins: More than just vascular targeting agents? J. Pharmacol. Exp. Ther. 2015, 355, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.; Singh, S.; Pandey, V.P.; Dwivedi, U.N. Alzheimer’s disease: An overview of amyloid beta dependent pathogenesis and its therapeutic implications along with in silico approaches emphasizing the role of natural products. J. Neurol. Sci. 2016, 361, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Pandey, A.; Jahan, S.; Shukla, R.K.; Kumar, D.; Srivastava, A.; Singh, S.; Rajpurohit, C.S.; Yadav, S.; Khanna, V.K.; et al. Differential responses of trans-resveratrol on proliferation of neural progenitor cells and aged rat hippocampal neurogenesis. Sci. Rep. 2016, 6, 28142. [Google Scholar] [CrossRef] [PubMed]
- Torres-Perez, M.; Tellez-Ballesteros, R.I.; Ortiz-Lopez, L.; Ichwan, M.; Vega-Rivera, N.M.; Castro-Garcia, M.; Gomez-Sanchez, A.; Kempermann, G.; Ramirez-Rodriguez, G.B. Resveratrol enhances neuroplastic changes, including hippocampal neurogenesis, and memory in Balb/C mice at six months of age. PLoS ONE 2015, 10, e0145687. [Google Scholar] [CrossRef] [PubMed]
- Dias, G.P.; Cocks, G.; do Nascimento Bevilaqua, M.C.; Nardi, A.E.; Thuret, S. Resveratrol: A potential hippocampal plasticity enhancer. Oxid. Med. Cell. Longev. 2016, 2016, 9651236. [Google Scholar] [CrossRef] [PubMed]
- Rege, S.D.; Geetha, T.; Broderick, T.L.; Babu, J.R. Resveratrol protects beta amyloid-induced oxidative damage and memory associated proteins in H19–7 hippocampal neuronal cells. Curr. Alzheimer Res. 2015, 12, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Yu, P.; Wang, L.; Shen, C.; Song, X.; Chen, J.; Tang, F.; Yang, Q. Sonic hedgehog signaling mediates resveratrol to increase proliferation of neural stem cells after oxygen-glucose deprivation/reoxygenation injury in vitro. Cell Physiol. Biochem. 2015, 35, 2019–2032. [Google Scholar] [CrossRef]
- Ren, J.; Fan, C.; Chen, N.; Huang, J.; Yang, Q. Resveratrol pretreatment attenuates cerebral ischemic injury by upregulating expression of transcription factor Nrf2 and HO-1 in rats. Neurochem. Res. 2011, 36, 2352–2362. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S.; et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Arozamena, C.; Martí-Marí, O.; Estrada, M.; De la Fuente Revenga, M.; Rodríguez-Franco, M.I. Recent Advances in Neurogenic Small Molecules as Innovative Treatments for Neurodegenerative Diseases. Molecules 2016, 21, 1165. https://doi.org/10.3390/molecules21091165
Herrera-Arozamena C, Martí-Marí O, Estrada M, De la Fuente Revenga M, Rodríguez-Franco MI. Recent Advances in Neurogenic Small Molecules as Innovative Treatments for Neurodegenerative Diseases. Molecules. 2016; 21(9):1165. https://doi.org/10.3390/molecules21091165
Chicago/Turabian StyleHerrera-Arozamena, Clara, Olaia Martí-Marí, Martín Estrada, Mario De la Fuente Revenga, and María Isabel Rodríguez-Franco. 2016. "Recent Advances in Neurogenic Small Molecules as Innovative Treatments for Neurodegenerative Diseases" Molecules 21, no. 9: 1165. https://doi.org/10.3390/molecules21091165
APA StyleHerrera-Arozamena, C., Martí-Marí, O., Estrada, M., De la Fuente Revenga, M., & Rodríguez-Franco, M. I. (2016). Recent Advances in Neurogenic Small Molecules as Innovative Treatments for Neurodegenerative Diseases. Molecules, 21(9), 1165. https://doi.org/10.3390/molecules21091165






