Comparison of the Essential Oil Composition of Selected Impatiens Species and Its Antioxidant Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Essential Oils from Impatiens L.
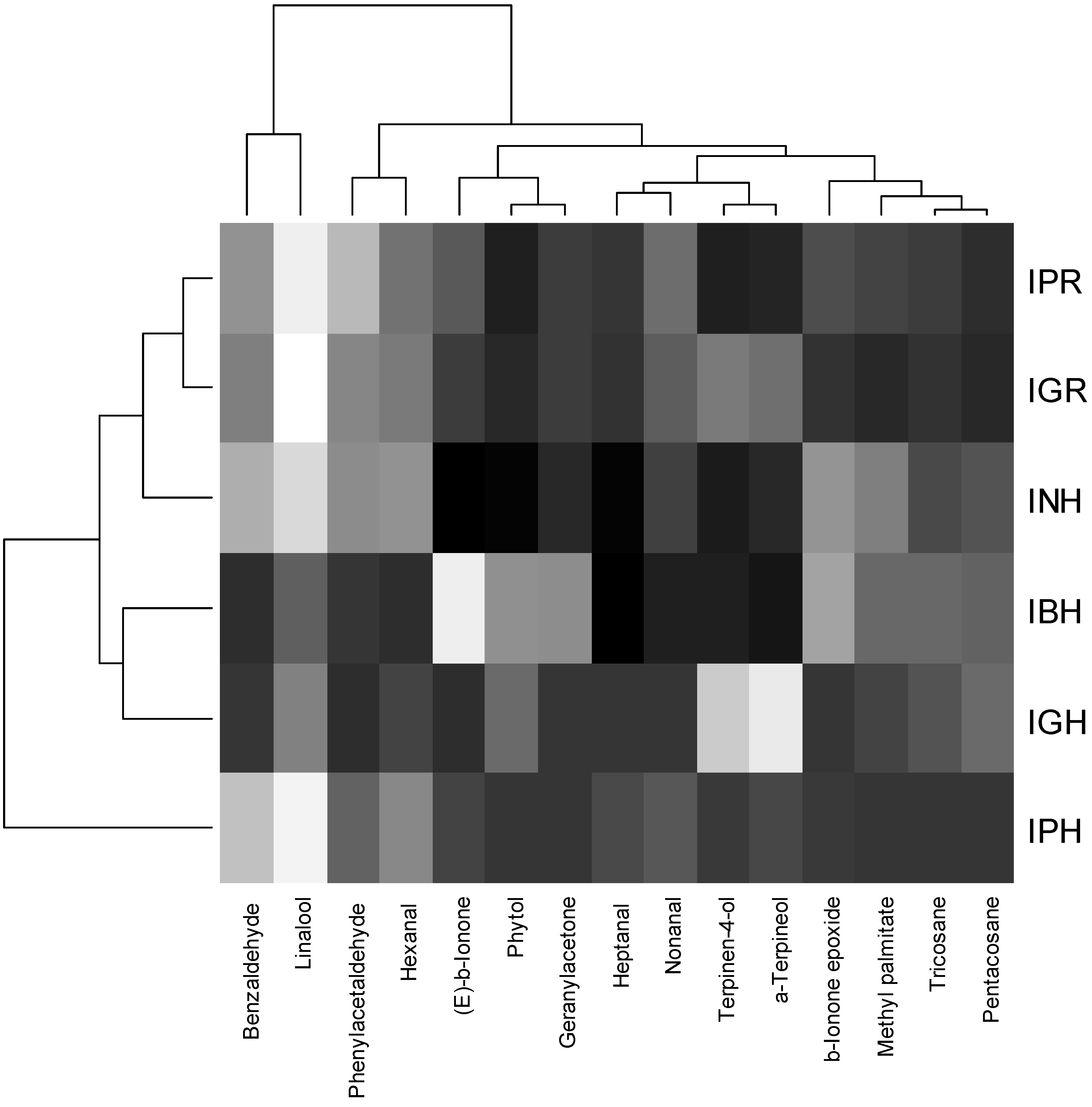
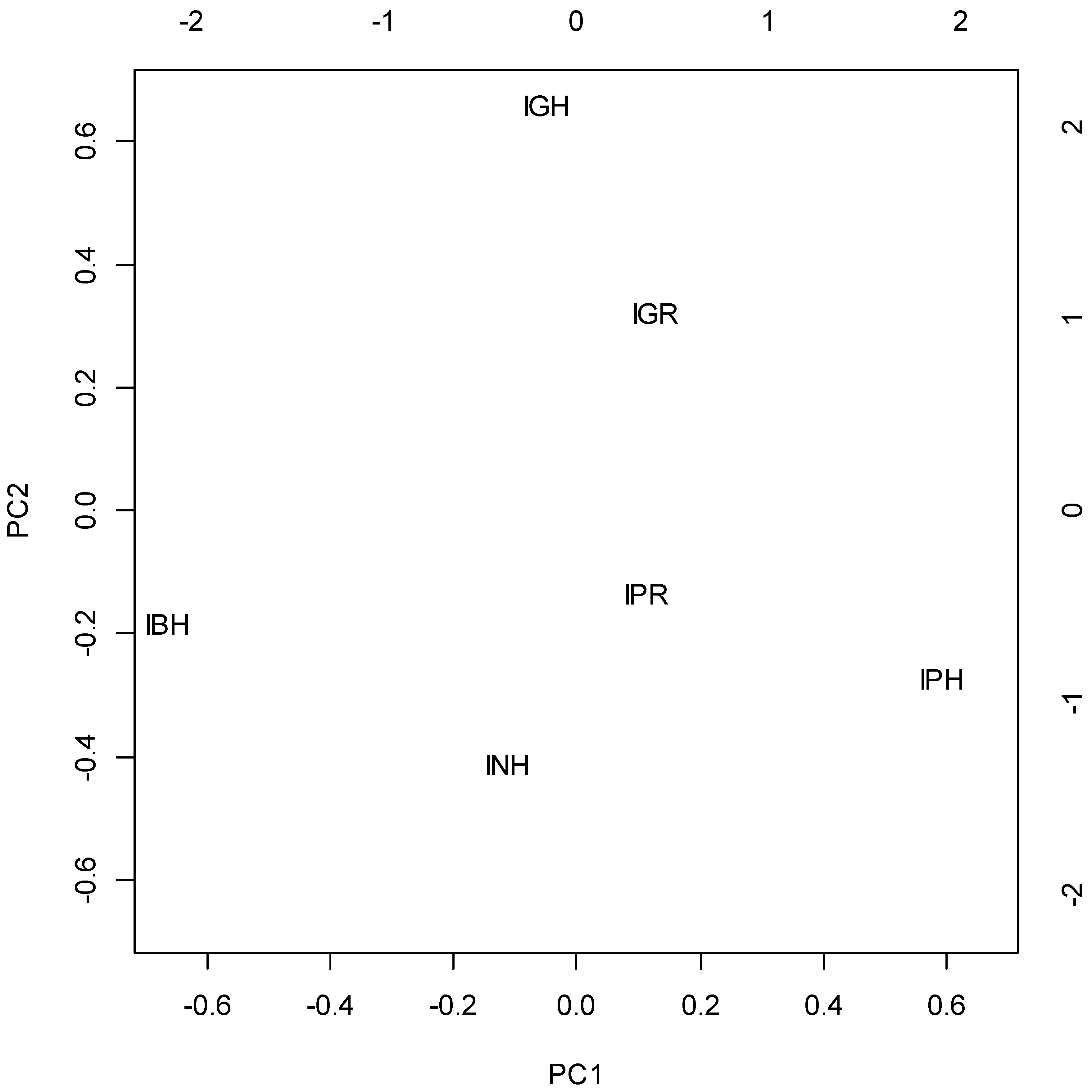
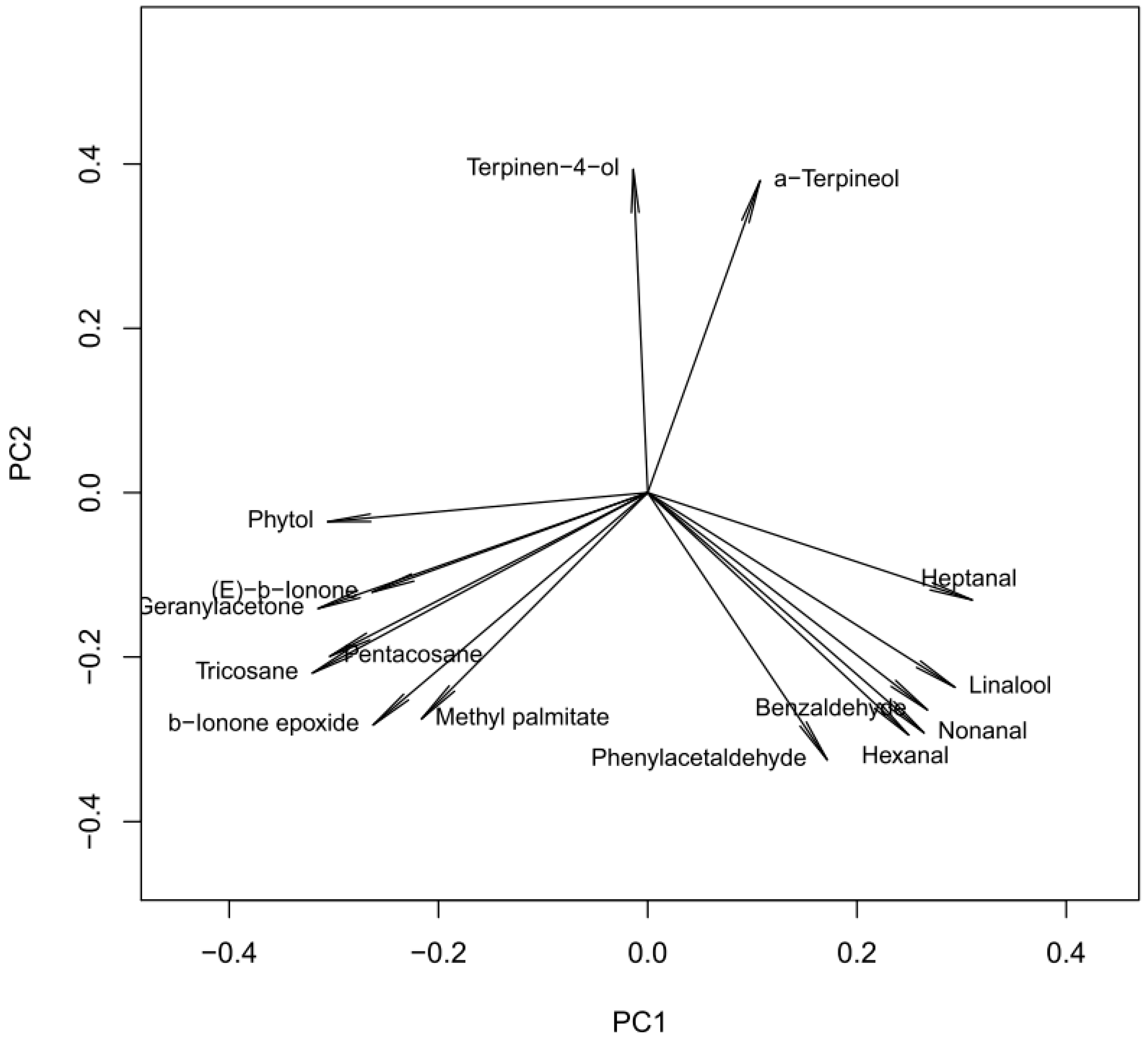
2.2. Chemometric Analysis
- Terpinen-4-ol and α-terpineol, explained mainly with PC2 and weakly (reversely) correlated with other compounds;
- Hexanal, nonanal, linalool, heptanal and benzaldehyde, located mainly in PC1, intercorrelated and reversely correlated with Group 3;
- Geranyl-acetone, β-ionone-epoxide, methyl-palmitate, phytol, tricosane and pentacosane, explained by PC1 and negatively correlated with Group 2.
2.3. Antioxidant Activity
3. Experimental Section
3.1. Reagents and Materials
3.2. Isolation Procedure
3.3. GC-MS Analysis
3.4. Free Radical Scavenging Activity
3.5. Inhibition of Linoleic Acid Peroxidation
3.6. Data Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Grey-Wilson, C. Introduction to the genus Impatiens. In Impatiens of Africa: Morphology, Pollination and Pollinators, Phytogeography, Hybridisation, Keys and a Systematic Treatment of All African Species, 6th ed.; A.A. Balkema: Rotterdam, The Netherlands, 1980; p. 3. [Google Scholar]
- Utami, N.; Shimizu, T. Seed morphology and classification of Impatiens (Balsaminaceae). Blumea-Biodivers. Evol. Biogeogr. Plants 2005, 50, 447–456. [Google Scholar] [CrossRef]
- Vieira, M.N.; Winterhalter, P.; Jerz, G. Flavonoids from the flowers of Impatiens glandulifera Royle isolated by high performance countercurrent chromatography. Phytochem. Anal. 2016, 27, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Vrchotová, N.; Šerá, B.; Krejčová, J. Allelopathic activity of extracts from Impatiens species. Plant Soil. Environ. 2011, 57, 57–60. [Google Scholar]
- Tříska, J.; Vrchotová, N.; Sýkora, J.; Moos, M. Separation and Identification of 1,2,4-Trihydroxynaphthalene-1-O-glucoside in Impatiens glandulifera Royle. Molecules 2013, 18, 8429–8439. [Google Scholar] [CrossRef] [PubMed]
- Chmura, D.; Sierka, E. Relation between invasive plant and species richness of forest floor vegetation: A study of Impatiens parviflora DC. Pol. J. Ecol. 2006, 54, 417–428. [Google Scholar]
- Glushakova, A.M.; Kachalkin, A.V.; Chernov, I.Y. Effect of invasive herb species on the structure of soil yeast complexes in mixed forests exemplified by Impatiens parviflora DC. Microbiology (Russ. Fed.) 2015, 84, 717–721. [Google Scholar] [CrossRef]
- Akanksha, K.C.; Rahuja, N.; Mishra, D.P.; Srivastava, A.K.; Maurya, R. Major alkaloidal constituent from Impatiens niamniamensis seeds as antihyperglycemic agent. Med. Chem. Res. 2011, 20, 1505–1508. [Google Scholar]
- Nisar, M.; Qayum, M.; Shah, M.; Zia-Ul-Haq, M.; Khan, I.; Ahmad, K.; Qayum, Z. Chemical constituents and antioxidant activity of n-hexane extract of Impatiens bicolor. Chem. Nat. Compd. 2012, 48, 143–146. [Google Scholar] [CrossRef]
- Sakunphueak, A.; Tansakul, P.; Umehara, K.; Noguchi, H.; Panichayupakaranant, P. Effect of methionine on production of naphthoquinones in Impatiens balsamina root cultures and detection of some secondary metabolites. Pharm. Biol. 2013, 51, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, K.; Zidorn, C.; Biernasiuk, A.; Komsta, Ł.; Granica, S. Polyphenols from Impatiens (Balsaminaceae) and their antioxidantand antimicrobial activities. Ind. Crop. Prod. 2016, 86, 262–272. [Google Scholar] [CrossRef]
- Tatsuzawa, F.; Saito, N.; Mikanagi, Y.; Shinoda, K.; Toki, K.; Shigihara, A.; Honda, T. An unusual acylated malvidin 3-glucoside from flowers of Impatiens textori Miq. (Balsaminaceae). Phytochemistry 2009, 70, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Li, W.Y.; Wu, D.C.; Wang, J.J.; Wu, C.H.; Liao, J.J.; Lin, C.K. In vitro activity of 2-methoxy-1,4-naphthoquinone and stigmasta-7,22-diene-3β-ol from Impatiens balsamina L. against multiple antibiotic–resistant Helicobacter pylori. Evid. Based Complement. Altern. Med. 2011, 2011, 704–721. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Summerhurst, D.K.; Koval, S.F.; Ficker, C.; Smith, M.L.; Bernards, M.A. Isolation of an antimicrobial compound from Impatiens balsamina using bioassay-guided fractionation. Phytother. Res. 2001, 15, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.F.; Tang, L.; Zhang, Y.H.; Pi, H.F.; Ruan, H.L.; Wu, J.Z. Nitrogenous compounds from Impatiens pritzellii var. hupehensis (Balsaminaceae). Biochem. Syst. Ecol. 2007, 35, 308–310. [Google Scholar]
- Zhou, X.F.; Zhao, X.Y.; Tang, L.; Ruan, H.L.; Zhang, Y.H.; Pi, H.F.; Xiao, W.L.; Sun, H.D.; Wu, J.Z. Three new triterpenoid saponins from the rhizomes of Impatiens pritzellii var hupehensis. J. Asian. Nat. Prod. Res. 2007, 9, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, H.; Yamaki, M.; Isoi, I.; Ishiguro, K. Antianaphylactic effects of the principal compounds from the white petals of Impatiens balsamina L. Phytother. Res. 1996, 10, 202–206. [Google Scholar] [CrossRef]
- Zeng, R.; Zhang, A.; Chen, J.; Fu, Y. Impact of carboxymethyl cellulose coating enriched with extract of Impatiens balsamina stems on preservation of ‘Newhall’ navel orange. Sci. Hortic. 2013, 160, 44–48. [Google Scholar] [CrossRef]
- Gomes, A.; Das, R.; Sarkhel, S.; Mishra, R.; Mukherjee, S.; Bhattacharya, S.; Gomes, A. Herbs and herbal constituents active against snake bite. Indian J. Exp. Biol. 2010, 48, 865–878. [Google Scholar] [PubMed]
- Pavela, R.; Vrchotová, N.; Šerá, B. Repellency and toxicity of three Impatiens species (Balsaminaceae) extracts on Myzus persicae Sulzer (Homoptera: Aphididae). J. Biopestic. 2009, 2, 48–51. [Google Scholar]
- Thaler, K.; Kaminski, A.; Chapman, A.; Langley, T.; Gartlehner, G. Bach Flower Remedies for psychological problems and pain: A systematic review. BMC Complement. Altern. Med. 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhao, X.; Tang, L.; Zhang, Y.; Ruan, H.; Pi, H.; Qiu, J.; Wu, J. Immunomodulatory activity of the rhizomes of Impatiens pritzellii var. hupehensis on collagen-induced arthritis mice. J. Ethnopharmacol. 2007, 109, 505–509. [Google Scholar] [PubMed]
- Ueda, Y.; Oku, H.; Iinuma, M.; Ishiguro, K. Effects on blood pressure decrease in response to PAF of Impatiens textori Miq. Biol. Pharm. Bull. 2003, 26, 1505–1507. [Google Scholar] [CrossRef] [PubMed]
- Peana, A.T.; Moretti, M.D.L. Linalool in essential plant oils: Pharmacological effects. In Botanical Medicine in Clinical Practice, 1st ed.; Preedy, V.R., Watson, R.R., Eds.; Cromwell Press: Trowbridge, UK, 2008; pp. 716–724. [Google Scholar]
- Chang, M.Y.; Shen, Y.L. Linalool exhibits cytotoxic effects by activating antitumor immunity. Molecules 2014, 19, 6694–6706. [Google Scholar] [CrossRef] [PubMed]
- Ridenour, W.M.; Callaway, R.M. The relative importance of allelopathy in interference: The effects of an invasive weed on a native bunchgrass. Oecologia 2001, 126, 444–450. [Google Scholar] [CrossRef]
- Amri, I.; Hamrouni, L.; Hanana, M.; Jamoussi, B. Reviews on phytotoxic effects of essential oils and their individual components: News approach for weed management. Int. J. Appl. Biol. Pharm. 2013, 4, 96–114. [Google Scholar]
- De Martino, L.; Mancini, E.; Marandino, A.; Rolim de Almeida, L.F.; de Feo, V. Chemistry and antigerminative activity of essential oils and monoterpenoids from Mediterranean plants. Curr. Bioact. Compd. 2012, 8, 13–49. [Google Scholar] [CrossRef]
- Vokou, D.; Douvli, P.; Blionis, G.J.; Halley, J.M. Effects of monoterpenoids, acting alone or in pairs, on seed germination and subsequent seedling growth. J. Chem. Ecol. 2003, 29, 2281–2301. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, J.; Song, L.; Cao, X.; Yao, X.; Tang, F.; Yue, Y. GCxGC-TOFMS analysis of essential oils composition from leaves, twigs and seeds of Cinnamomum camphora L. Presl and their insecticidal and repellent activities. Molecules 2016, 21, 423–434. [Google Scholar] [PubMed]
- Brand-Williams, W.; Cuvelier, E.; Berset, C.M. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Kuo, J.M.; Yeh, D.B.; Pan, B. Rapid photometric assay evaluating antioxidative activity in edible part material. J. Agric. Food Chem. 1999, 47, 3206–3209. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.; Szewczyk, K.; Gawlik–Dziki, U.; Rzymowska, J.; Komsta, Ł. Antioxidative and cytotoxic potential of some Chenopodium L. species growing in Poland. Saudi J. Biol. Sci. 2016, 23, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.
| No. | Constituent | RIexp | RIlit | IGH | IGR | IPH | IPR | IBH | INH | Class of Compound |
|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | ||||||||||
| 1 | Hexanal | 776 | 771 | 0.2 | 1.7 | 5.3 | 1.6 | 0.7 | 3.6 | AO |
| 2 | Furfural | 803 | 795 | - | 0.8 | 0.7 | 1.4 | - | - | O |
| 3 | (E)-Hex-2-enal | 826 | 822 | - | 0.3 | 2.1 | 1.4 | 2.9 | - | AO |
| 4 | (E)-Hex-3-en-1-ol | 838 | 838 | 0.6 | - | 16.8 | 0.3 | 0.7 | - | AO |
| 5 | (Z)-Hex-3-en-1-ol | 848 | 851 | 0.2 | - | - | - | 0.2 | 9.5 | AO |
| 6 | Hexanol | 852 | 856 | - | 1.7 | 4.9 | 0.3 | - | - | AO |
| 7 | Heptan-2-one | 868 | 871 | - | 0.2 | 0.2 | 0.1 | - | 0.2 | AO |
| 8 | Heptanal | 878 | 882 | 0.1 | 0.4 | 1.2 | 0.5 | 0.1 | 0.1 | AO |
| 9 | 2-Butoxyethanol | 888 | 888 | - | 0.2 | 0.1 | 0.6 | - | - | AO |
| 10 | Heptan-3-ol | 893 | 884 | - | 0.1 | - | - | - | - | AO |
| 11 | Butyraldehyde diethyl acetal | 896 | 880 | 2.4 | - | - | - | - | - | AO |
| 12 | Nonane | 900 | 900 | - | - | - | 0.1 | - | - | AH |
| 13 | 1-Butoxypropan-2-ol | 926 | 936 | - | 0.4 | - | - | - | AO | |
| 14 | Benzaldehyde | 929 | 928 | 0.1 | 1.8 | 10.2 | 2.3 | 0.7 | 4.7 | Ar |
| 15 | Dimethyl trisulfide | 942 | 942 | - | 0.4 | - | - | - | - | O |
| 16 | Benzonitrile | 945 | 940 | - | 0.5 | - | - | - | - | Ar |
| 17 | Isovaleraldehyde diethyl acetal | 946 | 930 | 0.7 | - | - | - | - | - | AO |
| 18 | Hept-1-en-3-one | 954 | 956 | - | 0.1 | - | - | - | - | AO |
| 19 | Heptanol | 956 | 952 | - | 0.3 | 0.9 | 0.3 | - | - | AO |
| 20 | Octane-2,3-dione | 961 | 959 | - | 0.3 | 0.4 | 0.5 | - | - | AO |
| 21 | Oct-1-en-3-ol | 964 | 962 | - | 1.3 | 0.9 | 0.7 | 0.3 | - | AO |
| 22 | 6-Methylhept-5-en-2-one | 964 | 962 | - | - | 0.3 | - | - | - | AO |
| 23 | Octan-3-one | 965 | 963 | - | 1.2 | - | - | - | - | AO |
| 24 | β-Pinene | 969 | 970 | - | 0.1 | - | - | - | - | MH |
| 25 | (E,E)-Hepta-2,4-dienal | 969 | 967 | - | - | 0.6 | 0.2 | - | - | AO |
| 26 | 2-Pentylfurane | 979 | 981 | - | 0.3 | 1.2 | 0.8 | 0.4 | 1.4 | O |
| 27 | Octanal | 980 | 982 | - | 0.3 | - | - | - | - | AO |
| 28 | Hexanoic acid | 981 | 973 | - | - | - | - | 0.6 | - | AO |
| 29 | Hepta-2,4-dienal (isomer) | 981 | - | - | - | 4.2 | 1.2 | - | 1.2 | AO |
| 30 | (E)-2-(Pent-2-enyl)furan | 986 | 984 | - | - | 0.5 | 0.2 | t | - | O |
| 31 | α-Phellandrene | 997 | 997 | 0.5 | - | - | - | - | - | MH |
| 32 | Decane | 1000 | 1000 | - | - | - | 0.1 | - | - | AH |
| 33 | Phenylacetaldehyde | 1009 | 1012 | t | 2.0 | 2.7 | 3.3 | 0.8 | 3.4 | Ar |
| 34 | α-Terpinene | 1009 | 1013 | 0.2 | - | - | - | - | - | MH |
| 35 | Salicylaldehyde | 1011 | 1013 | - | 1.0 | 0.1 | 0.4 | - | - | Ar |
| 36 | p-Cymene | 1012 | 1015 | 0.7 | - | - | - | - | - | MH |
| 37 | 2,2,6-Trimethylcyclohexanone | 1013 | 1013 | - | - | t | - | t | - | O |
| 38 | 2-Ethylhexan-1-ol | 1015 | 1011 | - | 0.1 | 0.3 | 0.5 | 0.1 | - | AO |
| 39 | β-Phellandrene | 1022 | 1023 | 7.4 | - | - | - | - | - | MH |
| 40 | Limonene | 1025 | 1025 | 0.3 | - | - | - | - | - | MH |
| 41 | (Z)-β-Ocimene | 1028 | 1029 | 0.6 | - | - | - | - | - | MH |
| 42 | (E)-Oct-2-enal | 1032 | 1034 | - | 0.1 | 0.2 | 0.3 | - | 0.2 | AO |
| 43 | Acetophenone | 1033 | 1030 | - | 0.2 | - | 0.2 | - | 0.1 | Ar |
| 44 | 2,6,6-Trimethylcyclohex-2-enone | 1037 | 1045 | - | - | 0.1 | - | - | - | O |
| 45 | Octa-3,5-dien-2-one (isomer) | 1043 | - | - | - | 0.3 | 0.4 | - | - | AO |
| 46 | γ-Terpinene | 1048 | 1059 | 0.1 | t | - | - | - | 0.1 | MH |
| 47 | (E)-Oct-2-en-1-ol | 1052 | 1052 | - | 0.2 | 0.6 | 0.1 | - | - | AO |
| 48 | Octanol | 1055 | 1054 | 0.2 | - | - | - | 0.1 | - | AO |
| 49 | trans-Linalool oxide (F) | 1058 | 1058 | - | 0.1 | 2.3 | 0.4 | - | 1.0 | MO |
| 50 | Guaiacol | 1061 | 1059 | 0.1 | - | 0.1 | 0.1 | - | - | Ar |
| 51 | (E,E)-Octa-3,5-dien-2-one | 1065 | 1063 | - | - | 0.4 | 0.4 | 0.1 | - | AO |
| 52 | Nonan-2-one | 1070 | 1072 | - | - | - | 0.1 | - | - | AO |
| 53 | cis-Linalool oxide (F) | 1072 | 1072 | t | - | 1.0 | 0.1 | - | 0.3 | MO |
| 54 | 6-Methylhepta-3,5-dien-2-one | 1077 | 1075 | - | - | 0.1 | 0.3 | - | - | AO |
| 55 | Terpinolene | 1079 | 1082 | 0.1 | - | - | - | - | - | MH |
| 56 | Nonanal | 1083 | 1086 | 0.1 | 1.1 | 1.9 | 1.5 | 0.5 | 1.2 | AO |
| 57 | Linalool | 1086 | 1087 | 0.7 | 5.3 | 15.1 | 4.9 | 1.6 | 6.5 | MO |
| 58 | Isophorone | 1092 | 1094 | - | - | 0.1 | - | t | - | MO |
| 59 | cis-Rose oxide | 1096 | 1096 | - | - | 0.2 | 0.5 | - | 0.1 | MO |
| 60 | α-Campholenal | 1104 | 1105 | t | - | - | - | - | - | MO |
| 61 | Oxoisophorone | 1105 | 1005 | - | - | t | 0.3 | t | 0.2 | MO |
| 62 | (E)-But-1-enylbenzene | 1107 | 1110 | 0.5 | - | - | - | - | - | Ar |
| 63 | trans-Rose oxide | 1113 | 1114 | - | - | 0.1 | 0.3 | - | 0.1 | MO |
| 64 | trans-Pinocarveol | 1122 | 1126 | - | 0.3 | - | - | - | - | MO |
| 65 | trans-p-Menth-2-en-1-ol | 1123 | 1123 | - | 0.7 | - | - | - | - | MO |
| 66 | Citronellal | 1126 | 1129 | - | - | 0.4 | 0.5 | - | - | MO |
| 67 | o-Acetylphenol | 1129 | 1135 | - | - | - | 0.3 | - | - | Ar |
| 68 | Hexyl isobutyrate | 1133 | 1132 | 0.1 | - | - | - | - | - | AO |
| 69 | 4-Vinylanisol | 1134 | 1134 | - | - | - | - | 0.2 | - | Ar |
| 70 | (E)-Non-2-enal | 1134 | 1139 | - | - | - | 0.5 | - | AO | |
| 71 | (E,E)-Nona-3,6-dien-1-ol | 1136 | 1145 | - | - | - | - | 0.2 | - | AO |
| 72 | Pinocarvone | 1137 | 1137 | - | 0.1 | - | - | - | - | MO |
| 73 | Pentylbenzene | 1143 | 1150 | 0.3 | - | - | - | - | - | Ar |
| 74 | Borneol | 1149 | 1150 | 0.1 | 4.9 | - | - | - | - | MO |
| 75 | cis- or trans-Linalool oxide (P) | 1153 | 1148 | - | - | 0.1 | - | - | - | MO |
| 76 | Benzoic acid | 1156 | 1157 | - | - | - | - | 2.0 | - | Ar |
| 77 | p-Cymen-9-ol | 1156 | 1157 | - | 0.4 | - | - | - | - | MO |
| 78 | Nonanol | 1157 | 1149 | - | - | - | 0.9 | - | - | AO |
| 79 | Cryptone | 1157 | 1160 | 5.7 | - | - | - | - | - | O |
| 80 | Terpinen-4-ol | 1160 | 1164 | 1.5 | 1.7 | 0.3 | 0.2 | 0.5 | 0.5 | MO |
| 81 | Octanoic acid | 1164 | 1160 | - | - | - | - | 0.1 | - | AO |
| 82 | p-Cymen-8-ol | 1166 | 1169 | - | - | - | 0.3 | 0.5 | - | MO |
| 83 | Methyl salicylate | 1168 | 1171 | - | - | - | - | 0.1 | - | Ar |
| 84 | Myrtenal | 1168 | 1172 | - | 0.3 | - | - | - | - | MO |
| 85 | α-Terpineol | 1174 | 1176 | 1.9 | 1.5 | 1.0 | 0.3 | 0.4 | 0.7 | MO |
| 86 | Safranal | 1179 | 1182 | - | - | 0.3 | t | 0.5 | t | MO |
| 87 | cis-Piperitol | 1181 | 1181 | 0.1 | - | - | - | - | - | MO |
| 88 | Myrtenol | 1181 | 1184 | - | 0.6 | - | 4.2 | 1.2 | - | MO |
| 89 | Decanal | 1184 | 1184 | - | 0.8 | 0.1 | 1.6 | 0.4 | - | AO |
| 90 | (E,E)-Nona-2,4-dienal | 1186 | 1188 | - | - | 0.1 | - | - | - | AO |
| 91 | Benzothiazole | 1188 | 1186 | - | 0.1 | - | - | - | - | O |
| 92 | 3,5,5-Trimethyl-4-methylenecyclohex-2-enone | 1190 | 1200 | - | - | 0.3 | - | - | - | O |
| 93 | trans-Piperitol | 1191 | 1193 | 0.3 | - | - | - | - | - | MO |
| 94 | Octyl acetate | 1193 | 1191 | 0.9 | - | - | - | - | - | AO |
| 95 | Carvotanacetol | 1193 | 1195 | - | - | - | - | 0.2 | - | MO |
| 96 | β-Cyclocitral | 1195 | 1195 | - | 0.1 | 0.6 | 0.1 | 0.5 | 0.4 | MO |
| 97 | trans-Carveol | 1199 | 1200 | 0.1 | - | - | - | - | - | MO |
| 98 | p-Isopropylbenzaldehyde | 1212 | 1214 | 0.9 | - | - | - | - | - | Ar |
| 99 | Citronellol | 1214 | 1213 | - | - | 2.5 | 17.9 | - | 1.9 | MO |
| 100 | Carvone | 1215 | 1214 | 0.6 | - | - | - | - | - | MO |
| 101 | Piperitone | 1226 | 1226 | 0.2 | - | - | - | - | - | MO |
| 102 | (2,6,6-Trimethylcyclohex-1-en-1-yl)acetaldehyde | 1235 | 1236 | - | - | - | - | 0.4 | - | O |
| 103 | 2-Phenylbut-2-enal | 1235 | 1237 | - | 0.2 | - | - | - | - | Ar |
| 104 | Geranial | 1240 | 1235 | - | - | 0.7 | 12.8 | - | - | MO |
| 105 | Phellandral | 1251 | 1250 | 3.8 | - | - | - | - | - | MO |
| 106 | 4-Ethylguaiacol | 1253 | 1257 | 0.2 | - | - | - | - | - | Ar |
| 107 | Terpinen-7-al | 1257 | 1257 | 0.3 | - | - | - | - | - | MO |
| 108 | Nonanoic acid | 1258 | 1263 | - | - | - | - | 0.2 | - | AO |
| 109 | p-Cymen-7-ol | 1266 | 1266 | 0.9 | - | - | - | - | - | MO |
| 110 | Bornyl acetate | 1268 | 1270 | 0.3 | 4.3 | - | - | - | - | MO |
| 111 | Thymol | 1271 | 1267 | - | - | - | - | - | 0.2 | MO |
| 112 | Undecan-2-one | 1273 | 1274 | - | 0.3 | - | 0.4 | - | - | AO |
| 113 | Carvacrol | 1280 | 1278 | 0.2 | - | - | - | - | - | MO |
| 114 | 4-Vinylguaiacol | 1284 | 1282 | - | - | 0.3 | 1.2 | 1.7 | - | Ar |
| 115 | Undecanal | 1286 | 1286 | - | 0.5 | - | 0.6 | - | 2.1 | AO |
| 116 | (E,E)-Deca-2,4-dienal | 1289 | 1288 | - | - | 0.1 | 0.2 | 0.1 | 0.2 | AO |
| 117 | Theaspirane A | 1290 | 1293 | - | - | 0.1 | - | - | - | O13 |
| 118 | Theaspirane B | 1304 | 1304 | - | - | 0.1 | - | - | - | O13 |
| 119 | Eugenol | 1329 | 1331 | - | - | 1.0 | - | 0.1 | 1.1 | Ar |
| 120 | 1,1,6-Trimethyl-1,2-dihydronaphthalene | 1335 | 1336 | - | - | - | - | 0.1 | - | Ar |
| 121 | α-Terpinyl acetate | 1336 | 1335 | 16.6 | - | - | - | - | - | MO |
| 122 | (E)-Undec-2-enal | 1339 | 1341 | - | - | 0.2 | 1.6 | - | - | AO |
| 123 | Neryl acetate | 1341 | 1342 | - | - | - | - | - | 0.2 | MO |
| 124 | Decanoic acid | 1352 | 1350 | - | - | - | - | 0.3 | - | AO |
| 125 | α-Longipinene | 1354 | 1360 | - | 0.3 | - | - | - | - | SH |
| 126 | Geranyl acetate | 1359 | 1362 | 0.6 | - | - | - | - | - | MO |
| 127 | (E)-β-Damascenone | 1361 | 1361 | - | - | t | 0.3 | 3.6 | t | O13 |
| 128 | Methyl eugenol | 1369 | 1369 | 0.1 | - | - | - | - | - | Ar |
| 129 | α-Copaene | 1375 | 1379 | 0.3 | - | - | - | - | - | SH |
| 130 | cis-β-Elemene | 1377 | 1381 | - | - | 0.1 | - | - | - | SH |
| 131 | Dodecan-2-one | 1385 | 1377 | - | - | - | - | 0.5 | - | AO |
| 132 | Dodecanal | 1385 | 1386 | - | - | - | 0.7 | 0.2 | - | AO |
| 133 | β-Elemene | 1386 | 1389 | 0.3 | 0.9 | - | - | - | - | SH |
| 134 | (E)-β-Damascone | 1392 | 1398 | - | - | - | - | 1.0 | - | O13 |
| 135 | 7,8-Dihydro-β-damascenone | 1396 | 1424 | - | - | - | - | 0.7 | - | O13 |
| 136 | Tetradecane | 1400 | 1400 | - | 0.1 | 0.1 | - | - | - | AH |
| 137 | (E)-α-Ionone | 1404 | 1413 | - | t | 0.1 | 0.1 | - | 0.2 | O13 |
| 138 | α-Barbatene | 1411 | 1414 | - | 0.9 | - | - | - | - | SH |
| 139 | cis-α-Bergamotene | 1412 | 1411 | - | 0.1 | - | - | - | - | SH |
| 140 | α-Santalene | 1416 | 1422 | - | 2.0 | - | - | - | - | SH |
| 141 | Geranylacetone | 1427 | 1428 | 0.1 | 0.6 | 0.1 | 0.6 | 2.7 | 0.7 | O |
| 142 | γ-Elemene | 1427 | 1429 | 0.5 | - | - | - | - | - | SH |
| 143 | trans-α-Bergamotene | 1431 | 1434 | - | 0.1 | - | - | - | - | SH |
| 144 | Isobazzanene | 1439 | 1442 | - | 0.5 | - | - | - | - | SH |
| 145 | β-Barbatene | 1444 | 1445 | - | 5.3 | - | - | - | - | SH |
| 146 | Sesquisabinene B | 1445 | 1445 | 0.6 | - | - | - | - | - | SH |
| 147 | 4-(2,4,4-Trimethyl-cyclohexa-1,5-dienyl)-but-3-en-2-one | 1456 | - | - | - | - | - | 0.8 | - | O13 |
| 148 | β-Santalene | 1458 | 1460 | - | 0.4 | - | - | - | - | SH |
| 149 | β-Ionone epoxide | 1459 | 1456 | 0.1 | 0.4 | 0.2 | 0.9 | 3.3 | 3.7 | O13 |
| 150 | (E)-β-Ionone | 1462 | 1468 | t | 0.6 | 0.8 | 1.1 | 5.7 | t | O13 |
| 151 | 4,5-di-epi-Aristolochene | 1465 | 1470 | - | 0.1 | - | - | - | - | SH |
| 152 | γ-Muurolene | 1470 | 1474 | 0.5 | - | - | - | - | - | SH |
| 153 | ar-Curcumene | 1471 | 1473 | - | 0.2 | - | - | - | - | SH |
| 154 | γ-Curcumene | 1472 | 1475 | - | 0.1 | - | - | - | - | SH |
| 155 | 5-epi-Aristolochene | 1473 | 1477 | 0.1 | - | - | - | - | - | SH |
| 156 | Tridecan-2-one | 1474 | 1476 | - | 0.6 | - | 0.2 | - | - | AO |
| 157 | trans-β-Bergamotene | 1477 | 1480 | - | 0.1 | - | - | - | - | SH |
| 158 | 3,4-Dimethyl-5-pentyl-5H-furan-2-one | 1480 | 1481 | - | 1.1 | 0.1 | - | 2.4 | 1.5 | O |
| 159 | Aristolochene | 1481 | 1486 | 0.7 | - | - | - | - | - | SH |
| 160 | Dihydroactinidiolide | 1487 | 1487 | - | - | - | - | 0.8 | - | O |
| 161 | Tridecanal | 1488 | 1490 | - | 0.3 | - | 1.0 | - | - | AO |
| 162 | α-Selinene | 1490 | 1494 | 0.7 | - | - | - | - | - | SH |
| 163 | Cuparene | 1493 | 1498 | - | 1.3 | - | - | - | - | SH |
| 164 | Pentadecane | 1500 | 1500 | - | - | - | - | 0.3 | - | AH |
| 165 | Germacrene A | 1500 | 1503 | - | - | 0.7 | - | - | - | SH |
| 166 | β-Bisabolene | 1502 | 1503 | - | 0.2 | - | - | - | - | SH |
| 167 | Methyl dodecanoate | 1504 | 1507 | - | - | - | - | 0.7 | - | AO |
| 168 | γ-Cadinene | 1505 | 1507 | 0.2 | - | 0.3 | 0.3 | - | - | SH |
| 169 | trans-Calamenene | 1508 | 1517 | 0.1 | - | - | - | - | - | SH |
| 170 | Photosantalol | 1509 | 1511 | - | 0.2 | - | - | - | - | SO |
| 171 | δ-Cadinene | 1518 | 1520 | 0.7 | 0.1 | - | t | - | - | SH |
| 172 | Selina-4(15),7(11)-diene | 1528 | 1534 | 0.4 | - | - | - | - | - | SH |
| 173 | Dodecanoic acid | 1546 | 1554 | - | - | - | 1.3 | 4.1 | - | AO |
| 174 | (E,E)-Pseudoionone | 1555 | 1563 | - | - | - | - | 0.3 | - | O13 |
| 175 | (2E)-2-Methyl-4-(2,6,6-trimethylcyclohex-1-en-1-yl)but-2-enal | 1568 | 1584 | - | - | - | - | 0.4 | - | O13 |
| 176 | Maaliol | 1573 | 1565 | - | 0.5 | - | - | - | - | SO |
| 177 | Globulol | 1574 | 1578 | - | - | - | 0.6 | 1.2 | 1.7 | SO |
| 178 | Tetradecanal | 1592 | 1592 | - | 1.0 | - | 0.9 | - | - | AO |
| 179 | Hexadecane | 1600 | 1600 | - | - | - | 0.1 | 0.1 | - | AH |
| 180 | Butylphthalide | 1610 | 1616 | 0.4 | - | - | - | - | - | Ar |
| 181 | ar-Bisabolol | 1613 | 1619 | - | 0.1 | - | - | - | - | SO |
| 182 | T-Cadinol | 1624 | 1623 | - | - | - | 0.1 | - | - | SO |
| 183 | T-Muurolol | 1626 | 1633 | 0.2 | - | - | - | - | - | SO |
| 184 | Vulgarone B | 1630 | 1632 | - | 14.9 | - | - | - | - | SO |
| 185 | α-Cadinol | 1639 | 1642 | - | - | - | 0.2 | - | - | SO |
| 186 | Pogostol | 1639 | 1647 | - | - | t | - | - | 0.3 | SO |
| 187 | (Z)-Butylidenphthalide | 1641 | 1644 | 8.5 | - | - | - | - | - | Ar |
| 188 | α-Barbatenal | 1652 | 1659 | - | 0.5 | - | - | - | - | SO |
| 189 | Hexahydrofarnesol | 1659 | 1667 | - | - | - | - | 0.8 | - | O |
| 190 | Tetradecanol | 1664 | 1670 | - | - | - | 0.5 | - | - | AO |
| 191 | α-Bisabolol | 1670 | 1673 | - | - | - | 0.3 | 0.3 | 1.0 | SO |
| 192 | Acorenone | 1671 | 1681 | - | 4.0 | - | - | - | - | SO |
| 193 | Pentadecan-2-one | 1676 | 1677 | - | t | - | 0.2 | 0.2 | - | AO |
| 194 | (E)-Butylidenphthalide | 1681 | 1673 | 0.8 | - | - | - | - | - | Ar |
| 195 | Pentadecanal | 1692 | 1693 | - | 5.8 | 0.6 | - | 1.6 | 1.7 | AO |
| 196 | Heptadecane | 1700 | 1700 | - | - | - | 0.1 | 0.4 | - | AH |
| 197 | (Z)-Ligustilide | 1703 | 1732 | 11.0 | - | - | - | - | - | Ar |
| 198 | Methyl myristate | 1710 | 1713 | - | - | - | 0.1 | 0.5 | - | AO |
| 199 | Phenanthrene | 1746 | 1744 | - | 0.9 | - | - | - | - | Ar |
| 200 | Myristic acid | 1747 | 1748 | 0.3 | - | - | 0.9 | 3.3 | - | AO |
| 201 | (E)-Ligustilide | 1756 | 1782 | 0.1 | - | - | - | - | - | Ar |
| 202 | Hexadecanal | 1793 | 1782 | - | 0.1 | - | 0.3 | 0.2 | - | AO |
| 203 | Octadecane | 1800 | 1800 | - | t | - | 0.3 | 0.5 | 0.3 | AH |
| 204 | Hexahydrofarnesyl acetone | 1829 | 1832 | 1.0 | - | 0.4 | - | 13.4 | 0.3 | O |
| 205 | Farnesylacetone | 1894 | 1895 | - | - | - | - | 1.9 | - | O |
| 206 | Nonadecane | 1900 | 1900 | - | 0.2 | - | 0.5 | 0.5 | 0.7 | AH |
| 207 | Methyl palmitate | 1904 | 1904 | 0.2 | 0.3 | 0.1 | 0.7 | 1.8 | 3.0 | AO |
| 208 | Isophytol | 1939 | 1949 | - | - | - | 0.4 | 0.9 | 0.3 | O |
| 209 | Palmitic acid | 1945 | 1951 | 1.1 | - | - | 0.6 | 0.9 | - | AO |
| 210 | Ethyl palmitate | 1974 | 1954 | - | - | - | - | - | 0.3 | AO |
| 211 | Eicosane | 2000 | 2000 | - | - | - | 0.2 | - | 0.3 | AH |
| 212 | Fluoranthene | 2026 | 2020 | - | 0.9 | - | - | - | - | Ar |
| 213 | Methyl linoleate | 2067 | 2046 | - | 0.1 | - | 0.3 | 0.4 | 0.2 | AO |
| 214 | Methyl palmitate | 2071 | 2102 | 0.1 | - | - | - | - | - | AO |
| 215 | Methyl linolenate | 2072 | 2102 | - | 0.2 | - | 0.7 | 1.3 | 0.3 | AO |
| 216 | Pyrene | 2076 | 2070 | - | 0.6 | - | - | - | - | Ar |
| 217 | Methyl oleate | 2087 | 2082 | - | - | - | - | - | 0.2 | AO |
| 218 | Heneicosane | 2100 | 2100 | - | 0.3 | - | 0.3 | 0.3 | 0.2 | AH |
| 219 | Phytol | 2107 | 2104 | 0.5 | 0.3 | 0.1 | 0.2 | 2.8 | 0.1 | O |
| 220 | Docosane | 2200 | 2200 | - | - | - | - | 0.2 | 0.2 | AH |
| 221 | Tricosane | 2300 | 2300 | 0.3 | 0.4 | 0.1 | 0.6 | 1.8 | 1.4 | AH |
| 222 | Tetracosane | 2400 | 2400 | - | 0.1 | - | - | 0.2 | 0.2 | AH |
| 223 | Pentacosane | 2500 | 2500 | 0.5 | 0.3 | 0.1 | 0.4 | 1.7 | 1.7 | AH |
| 224 | Hexacosane | 2600 | 2600 | - | 0.1 | - | - | - | - | AH |
| 225 | Heptacosane | 2700 | 2700 | 0.2 | - | - | - | - | - | AH |
| 226 | Nonacosane | 2900 | 2900 | 0.5 | - | - | - | - | - | AH |
| Total identified constituents | 82.5 | 87.4 | 88.2 | 86.3 | 84.2 | 61.7 | ||||
| Aliphatic hydrocarbons AH | 1.5 | 1.5 | 0.3 | 2.7 | 6.0 | 5.0 | ||||
| Oxygenated aliphatics AO | 7.2 | 20.0 | 42.8 | 26.1 | 23.3 | 24.0 | ||||
| Monoterpene hydrocarbons MH | 9.9 | 0.1 | 0.1 | 0.4 | - | 0.1 | ||||
| Oxygenated monoterpenes MO | 28.2 | 20.3 | 24.7 | 42.8 | 5.4 | 12.1 | ||||
| Sesquiterpene hydrocarbons SH | 5.1 | 12.6 | 1.1 | 0.3 | - | - | ||||
| Oxygenated sesquiterpenes SO | 0.2 | 20.2 | - | 1.2 | 1.5 | 3.0 | ||||
| Aromatic compounds Ar | 23.0 | 8.1 | 14.4 | 7.8 | 5.7 | 9.3 | ||||
| Other O + O13 | 7.3 + 0.1 | 3.4 + 1.2 | 3.7 + 1.1 | 3.6 + 2.4 | 26.5 + 15.8 | 4.3 + 3.9 | ||||
| Identified compounds | 76 | 94 | 70 | 88 | 80 | 54 | ||||
| Oil yield | 0.22 | 0.19 | 0.24 | 0.16 | 0.10 | 0.14 | ||||
| Essential Oils | Radical Scavenging Activity DPPH (EC50, µg/mL) | Inhibition of Linoleic Acid Peroxidation (IC50, µg/mL) |
|---|---|---|
| I. balsamina herb (IBH) | 16.14 ± 0.68 g | 468.06 ± 2.03 f |
| I. glandulifera herb (IGH) | 3.96 ± 0.03 b | 102.08 ± 0.71 b |
| I. glandulifera roots (IGR) | 5.84 ± 0.03 d | 116.98 ± 0.43 c |
| I. noli-tangere herb (INH) | 4.76 ± 0.05 b,c | 123.18 ± 1.34 c |
| I. parviflora herb (IPH) | 9.06 ± 0.07 e | 190.94 ± 0.76 d |
| I. parviflora roots (IPR) | 10.38 ± 0.17 f | 323.66 ± 0.24 e |
| Ascorbic acid | 2.05 ± 0.01 a | - |
| BHT | - | 18.21 ± 0.11 a |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szewczyk, K.; Kalemba, D.; Komsta, Ł.; Nowak, R. Comparison of the Essential Oil Composition of Selected Impatiens Species and Its Antioxidant Activities. Molecules 2016, 21, 1162. https://doi.org/10.3390/molecules21091162
Szewczyk K, Kalemba D, Komsta Ł, Nowak R. Comparison of the Essential Oil Composition of Selected Impatiens Species and Its Antioxidant Activities. Molecules. 2016; 21(9):1162. https://doi.org/10.3390/molecules21091162
Chicago/Turabian StyleSzewczyk, Katarzyna, Danuta Kalemba, Łukasz Komsta, and Renata Nowak. 2016. "Comparison of the Essential Oil Composition of Selected Impatiens Species and Its Antioxidant Activities" Molecules 21, no. 9: 1162. https://doi.org/10.3390/molecules21091162






