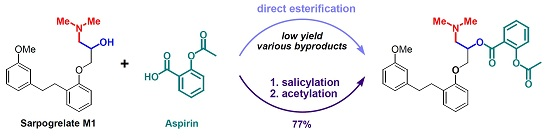
Aspirination of α-Aminoalcohol (Sarpogrelate M1)
Abstract
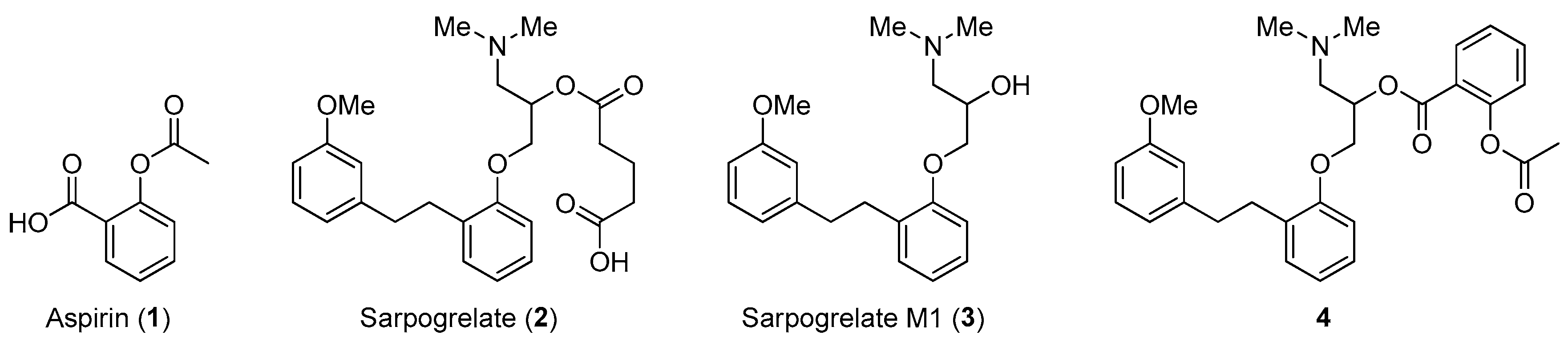
:1. Introduction
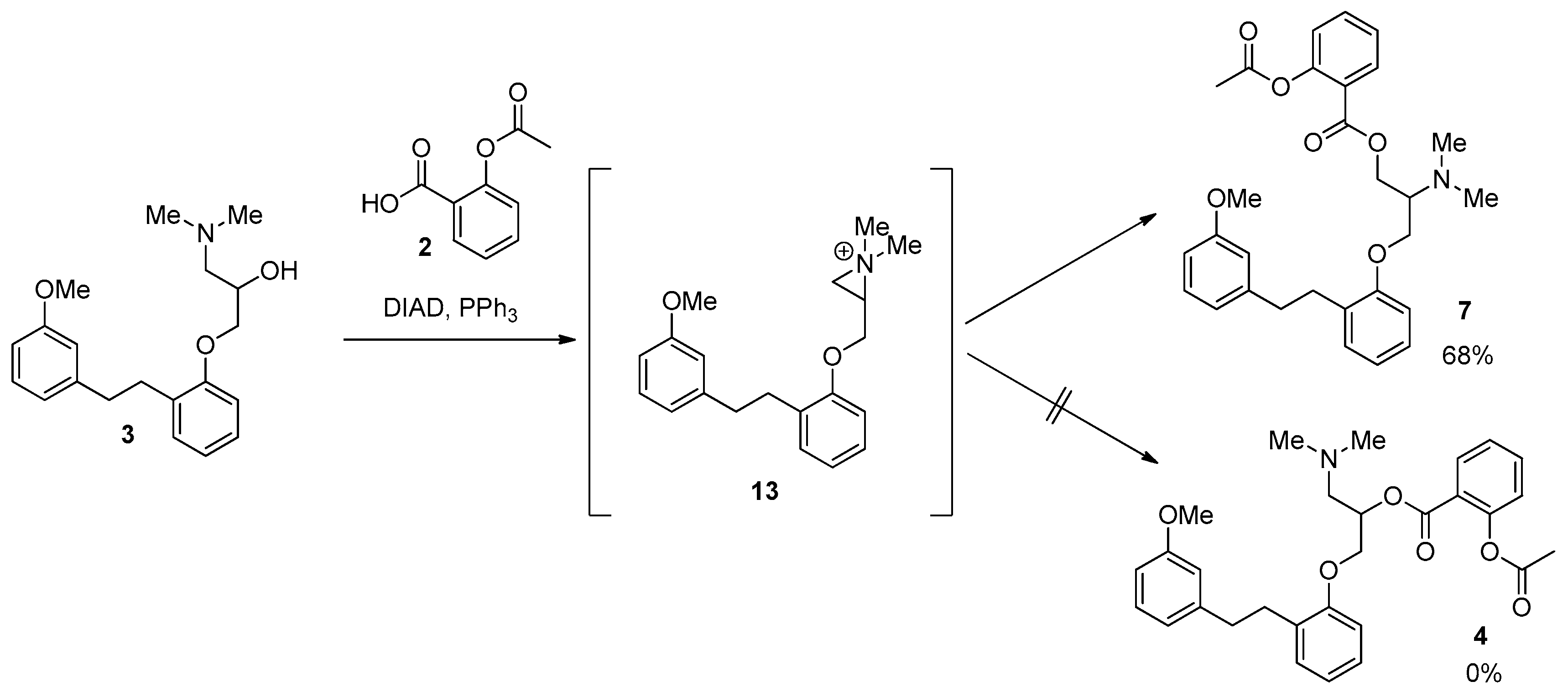
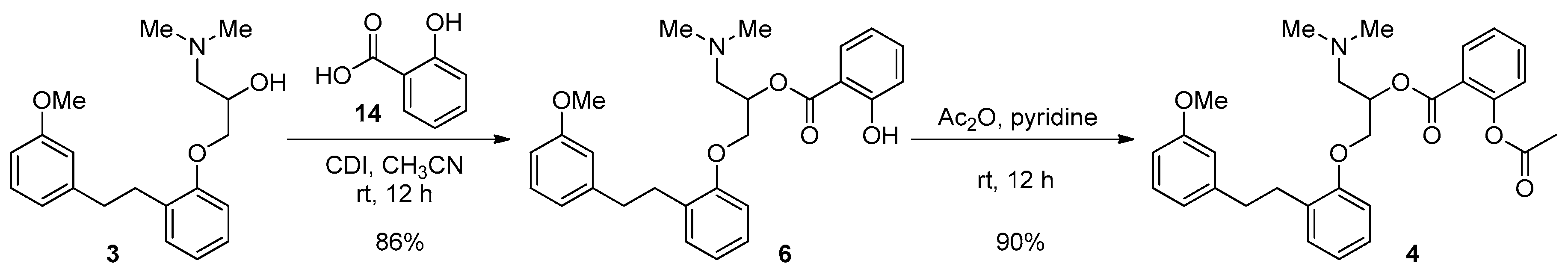
2. Results and Discussion
3. Experimental Section
3.1. General Information
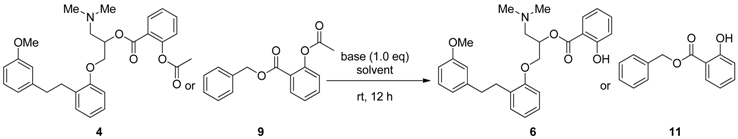
3.2. General Procedures of Esterification and Acetylation of 6
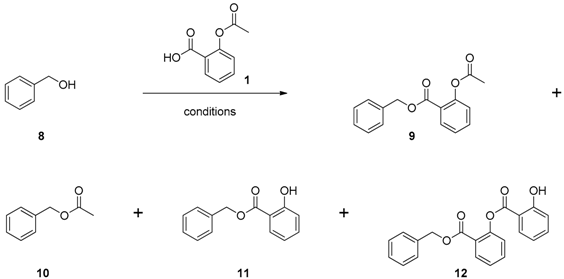
3.1.1. DCC Coupling Conditions
3.1.2. CDI Activation Conditions
3.1.3. Mitsunobu Conditions
3.1.4. Aspirinyl Chloride Coupling Conditions
3.1.5. Acetylation of 6
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abordo, E.A.; Bowden, K.; Huntington, A.P.; Powell, S.L. Prodrugs Part 3. 2-Formylphenyl esters of indomethacin, ketoprofen and ibuprofen and 6-substituted 2-formyl and 2-acylphenyl esters of aspirin. Farmaco 1998, 53, 95–101. [Google Scholar] [CrossRef]
- Velázquez, C.A.; Chen, Q.-H.; Citro, M.L.; Keefer, L.K.; Knaus, E.E. Second-generation aspirin and indomethacin prodrugs possessing an O2-(acetoxymethyl)-1-(2-carboxypyrrolidin-1-yl)diazenium-1,2-diolate nitric oxide donor moiety: Design, synthesis, biological evaluation, and nitric oxide release studies. J. Med. Chem. 2008, 51, 1954–1961. [Google Scholar] [CrossRef] [PubMed]
- Rimoli, M.G.; Calignano, A.; Cuomo, R.; Boatto, G.; Abignente, E.; Melisi, D.; Curcio, A.; Luongo, E.; la Rana, G.; Sasso, O.; et al. Galactosylated pRo-Drugs of Non-Steroidal Anti-Inflammatories with Improved Pharmacokinetic Characteristics and Reduced Toxicity of the Starting Drug. US Patent 0 212 904, 1 September 2011. [Google Scholar]
- Cai, J.; Duan, Y.; Yu, J.; Chen, J.; Chao, M.; Ji, M. Bone-targeting glycol and NSAIDS ester prodrug of rhein: Synthesis, hydroxyapatite affinity, stability, anti-inflammatory, ulcerogenicity index and pharmacokinetic studies. Eur. J. Med. Chem. 2012, 55, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Yue, Y.; Zhang, K.; Chen, Q.; Wang, H.; Lu, Y.; Huang, M.-T.; Zheng, X.; Du, Z. Synthesis and biological evaluation of curcumin derivatives containing NSAIDs for their anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2015, 25, 3044–3051. [Google Scholar] [CrossRef] [PubMed]
- Rosseto, L.A.; Pires, M.E.L.; Melchior, A.C.B.; Bosquesi, P.L.; Pavan, A.R.; Marcondes, S.; Chung, M.C.; dos Santos, J.L. Synthesis and preliminary evaluation of N-oxide derivatives for the prevention of atherothrombotic events. Molecules 2015, 20, 18185–18200. [Google Scholar]
- Hulsman, N.; Medema, J.P.; Bos, C.; Jongejan, A.; Leurs, R.; Smit, M.J.; de Esch, I.J.P.; Richel, D.; Wijtmans, M. Chemical insights in the concept of hybrid Drugs: The antitumor effect of nitric oxide-donating aspirin involves a quinone methide but not nitric oxide nor aspirin. J. Med. Chem. 2007, 50, 2424–2431. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.N.; Tazawa, M.J. Glucose-aspirin: Synthesis and in vitro anti-cancer activity studies. Bioorg. Med. Chem. Lett. 2012, 22, 3168–3171. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Kaur, J.; Sharma, S.K.; Huang, Z.; Wuest, F.; Knaus, E.E. Hybrid fluorescent conjugates of COX-2 inhibitors: Search for a COX-2 isozyme imaging cancer biomarker. Bioorg. Med. Chem. Lett. 2013, 23, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Bapat, A.S.; Mahesh, G.; Gokhale, R.S.; Shah, S.S.; Sengupta, S.; Prasad, S.; Ghosh, S.; Chawrai, S.R.; Arora, N.; Reddy, D.S.; et al. Conjugate-based antifungal and antibacterial prodrugs. US Patent 0 364 595, 11 December 2014. [Google Scholar]
- Zhang, Y.; Tortorella, M.D.; Liao, J.; Qin, X.; Chen, T.; Luo, J.; Guan, J.; Talley, J.J.; Tu, Z. Synthesis and evaluation of novel erlotinib–NSAID conjugates as more comprehensive anticancer agents. ACS Med. Chem. Lett. 2015, 6, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Kikumoto, R.; Hara, H.; Ninomiya, K.; Osakabe, M.; Sugano, M.; Fukami, H.; Tamao, Y. Syntheses and platelet aggregation inhibitory and antithrombotic properties of [2-[(ω-aminoalkoxy)phenyl]benzenes. J. Med. Chem. 1990, 33, 1818–1823. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, I.; Soejima, H.; Miyamoto, S.; Ogawa, H. Effects of additional treatment of sarpogrelate to aspirin therapy on platelet aggregation and plasma plasminogen activator inhibitor activity in patients with stable effort angina. Thromb. Res. 2011, 128, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Noh, Y.; Lee, J.; Shin, S.; Lim, H.-S.; Bae, S.K.; Oh, E.; Kim, G.J.; Kim, J.H.; Lee, S. Antiplatelet therapy of cilostazol or sarpogrelate with aspirin and clopidogrel after percutaneous coronary intervention: A retrospective cohort study using the korean national health insurance claim database. PLoS ONE 2016, 11, e0150475. [Google Scholar] [CrossRef] [PubMed]
- Farcet, J.-B.; Himmelbauer, M.; Mulzer, J. Photochemical and thermal [2 + 2] cycloaddition to generate the bicyclo[3.2.0]heptane core of bielschowskysin. Eur. J. Org. Chem. 2013, 2013, 4379–4398. [Google Scholar] [CrossRef]
- Ishizuka, M.; Shiro, M.; Makisumi, Y. (R)- and (S)-4-Methylaminomethyl-2,3,4,9-Tetrahydrothiopyrano[2,3-b]indole: Synthesis, absolute configuration, conformation, and analgesic activity. J. Chem. Soc. Perkin Trans. 1 1990, 827–837. [Google Scholar] [CrossRef]
- Montes-Gil, A.C.; Zanfolin, M.; Okuyama, C.E.; Lilla, S.; Alves, D.P.; Santagada, V.; Perissutti, E.; Lavecchia, A.; Fiorino, F.; Severino, B.; et al. Pharmacokinetic profile of atenolol aspirinate. Arch. Pharm. Chem. Life Sci. 2007, 340, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Press, J.B.; Falotico, R.; Hajos, Z.G.; Sawyers, R.A.; Kanojia, R.M.; Williams, L.; Haertlein, B.; Kauffman, J.A.; Lakas-Weiss, C.; Salata, J.J. Synthesis and SAR of 6-substituted purine derivatives as novel selective positive inotropes. J. Med. Chem. 1992, 35, 4509–4515. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Huang, F.; Li, Z.; Ma, J.; Li, H.; Fang, L. Synthesis and bioactive of quercetin aspirinates. Bull. Korean Chem. Soc. 2014, 35, 518–520. [Google Scholar] [CrossRef]
- Mattarei, A.; Biasutto, L.; Rastrelli, F.; Garbisa, S.; Marotta, E.; Zoratti, M.; Paradisi, C. Regioselective O-derivatization of quercetin via ester intermediates. An improved synthesis of rhamnetin and development of a new mitochondriotropic derivative. Molecules 2010, 15, 4722–4736. [Google Scholar] [CrossRef] [PubMed]
- Poelert, M.; Hulshof, L.A.; Kellogg, R.M. Application of the Mitsunobu reaction to ephedrines and some related aminoalcohols, Aspects of intramolecular participation of the amino group. Recl. Trav. Chim. Pays-Bas 1994, 113, 355–364. [Google Scholar] [CrossRef]
- Knapp, S.; Morriello, G.J.; Doss, G.A. Stereoselective ring contraction diverts the Mitsunobu reaction of a 6-hydroxy-1,4-diazepan-2-one. Tetrahedron Lett. 2003, 44, 2645–2647. [Google Scholar] [CrossRef]
- Mondon, M.; Fontelle, N.; Désiré, J.; Lecornué, F.; Guillard, J.; Marrot, J.; Blérlot, Y. Acess to l- and d-Iminosugar C-Glycosides from a d-gluco-Derived 6-Azidolactol Exploiting a Ring Isomerization/Alkylation Strategy. Org. Lett. 2012, 14, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Tummatorn, J.; Albiniak, P.A.; Dudley, G.B. Synthesis of Benzyl Esters Using 2-Benzyloxy-1-methylpyridinium Triflate. J. Org. Chem. 2007, 72, 8962–8964. [Google Scholar] [CrossRef] [PubMed]
- Isley, N.A.; Hageman, M.S.; Lipshutz, B.H. Dehalogenation of functionalized alkyl halides in water at room temperature. Green Chem. 2015, 17, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Yoshida, S.; Fujita, H.; Kitamura, M.; Kunishima, M. O-Benzylation of Carboxylic Acids Using 2,4,6-Tris(benzyloxy)-1,3,5-triazine (TriBOT) under Acidic or Thermal Conditions. Eur. J. Org. Chem. 2015, 2015, 7997–8002. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 4–7 and 12 are available from the authors.
| Entry | Conditions | Yield (%) 1 | |||
|---|---|---|---|---|---|
| 4 | 5 | 6 | 7 | ||
| 1 | 1 (1.1 eq), DCC (1.1 eq), DMAP (0.1 eq), CH2Cl2, 0 °C, 1 h, then rt, 18 h | 13 | 87 | 0 | 0 |
| 2 | 1 (1.1 eq), DCC (1.1 eq), DMAP (0.5 eq), CH2Cl2, 0 °C, 1 h, then rt, 18 h | 5 | 89 | 0 | 0 |
| 3 2 | 1 (1.1 eq), DCC (1.1 eq), CH2Cl2, 0 °C, 1 h, then rt, 18 h | 35 | 8 | 3 | 0 |
| 4 | 1 (1.1 eq), CDI (1.2 eq), CH2Cl2, rt, 12 h | 0 | 0 | 75 | 0 |
| 5 | 1 (1.1 eq), CDI (1.2 eq), CH3CN, rt, 12 h | 0 | 0 | 84 | 0 |
| 6 | 1 (1.5 eq), DIAD (1.5 eq), PPh3 (1.5 eq), THF, 0 °C, 1 h | 0 | 0 | 0 | 68 |
| 7 | 1 (2.0 eq), (COCl)2 (2.4 eq), DMF (0.2 eq), CH2Cl2, 0 °C to rt, 12 h; 3, pyridine (6.0 eq), CH2Cl2, rt, 12 h | 62 | 0 | 0 | 0 |
| Entry | Conditions | Yield (%) 1 | |||
|---|---|---|---|---|---|
| 9 | 10 | 11 | 12 | ||
| 1 | 1 (1.1 eq), DCC (1.1 eq), DMAP (0.1 eq), CH2Cl2, 0 °C, 1 h, then rt, 18 h | 70 | 17 | 0 | 0 |
| 2 | 1 (1.1 eq), DCC (1.1 eq), DMAP (0.5 eq), CH2Cl2, 0 °C, 1 h, then rt, 18 h | 41 | 40 | 8 | 0 |
| 3 2 | 1 (1.1 eq), DCC (1.1 eq), CH2Cl2, 0 °C, 1 h, then rt, 18 h | 36 | 0 | 0 | 0 |
| 4 | 1 (1.1 eq), CDI (1.2 eq), CH2Cl2, rt, 12 h | 0 | 15 | 55 | 7 |
| 5 | 1 (1.1 eq), CDI (1.2 eq), CH3CN, rt, 12 h | 0 | 27 | 42 | 10 |
| 6 | 1 (1.5 eq), DIAD (1.5 eq), PPh3 (1.5 eq), THF, 0 °C, 1 h | 100 | 0 | 0 | 0 |
| 7 | 1 (2.0 eq), (COCl)2 (2.4 eq), DMF (0.2 eq), CH2Cl2, 0 °C to rt, 12 h; 8, pyridine (6.0 eq), CH2Cl2, rt, 12 h | 36 | 0 | 0 | 0 |
| Entry | Aspirinate Ester | Base | Solvent | Salicylate Ester | Ratio (4/9:6/11) 1 |
|---|---|---|---|---|---|
| 1 | 4 | DMAP | CH2Cl2 | 6 | 20:1 |
| 2 | 9 | DMAP | CH2Cl2 | 11 | 30:1 |
| 3 | 4 | imidazole | CH2Cl2 | 6 | 3:1 |
| 4 | 4 | imidazole | CH3CN | 6 | 2:1 |
| 5 | 9 | imidazole | CH2Cl2 | 11 | 18:1 |
| 6 | 9 | imidazole | CH3CN | 11 | 28:1 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Lee, J.; Shin, K.J.; Seo, J.H. Aspirination of α-Aminoalcohol (Sarpogrelate M1). Molecules 2016, 21, 1126. https://doi.org/10.3390/molecules21091126
Park S, Lee J, Shin KJ, Seo JH. Aspirination of α-Aminoalcohol (Sarpogrelate M1). Molecules. 2016; 21(9):1126. https://doi.org/10.3390/molecules21091126
Chicago/Turabian StylePark, Sunhwa, Jiyun Lee, Kye Jung Shin, and Jae Hong Seo. 2016. "Aspirination of α-Aminoalcohol (Sarpogrelate M1)" Molecules 21, no. 9: 1126. https://doi.org/10.3390/molecules21091126