Stability, Antioxidant Capacity and Degradation Kinetics of Pelargonidin-3-glucoside Exposed to Ultrasound Power at Low Temperature
Abstract
:1. Introduction
2. Result and Discussion
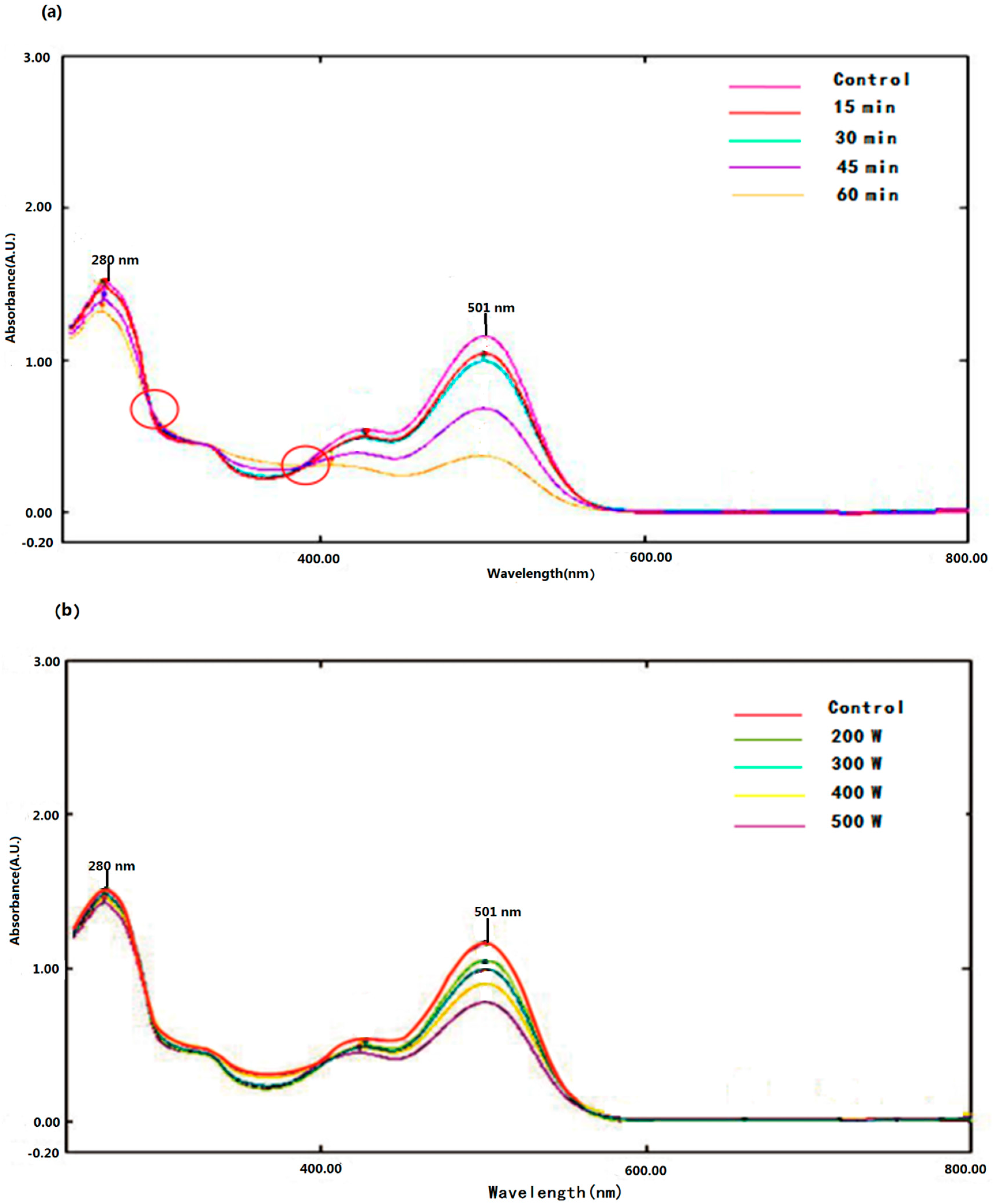
2.1. Effects of Ultrasound on the UV-Vis Spectra of Pg-3-glu
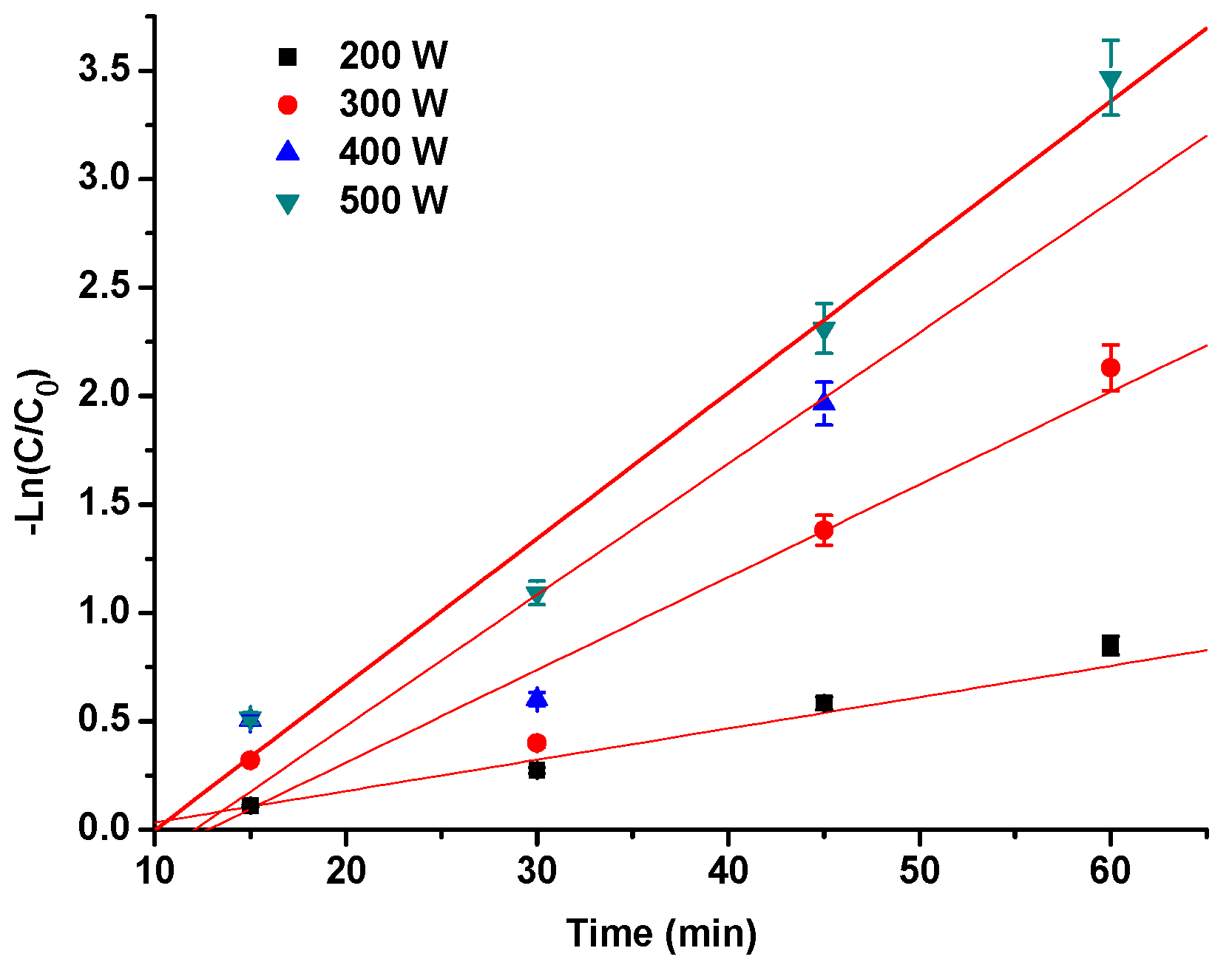
2.2. The Degradation Kinetics of Pg-3-glu Exposed to Ultrasound
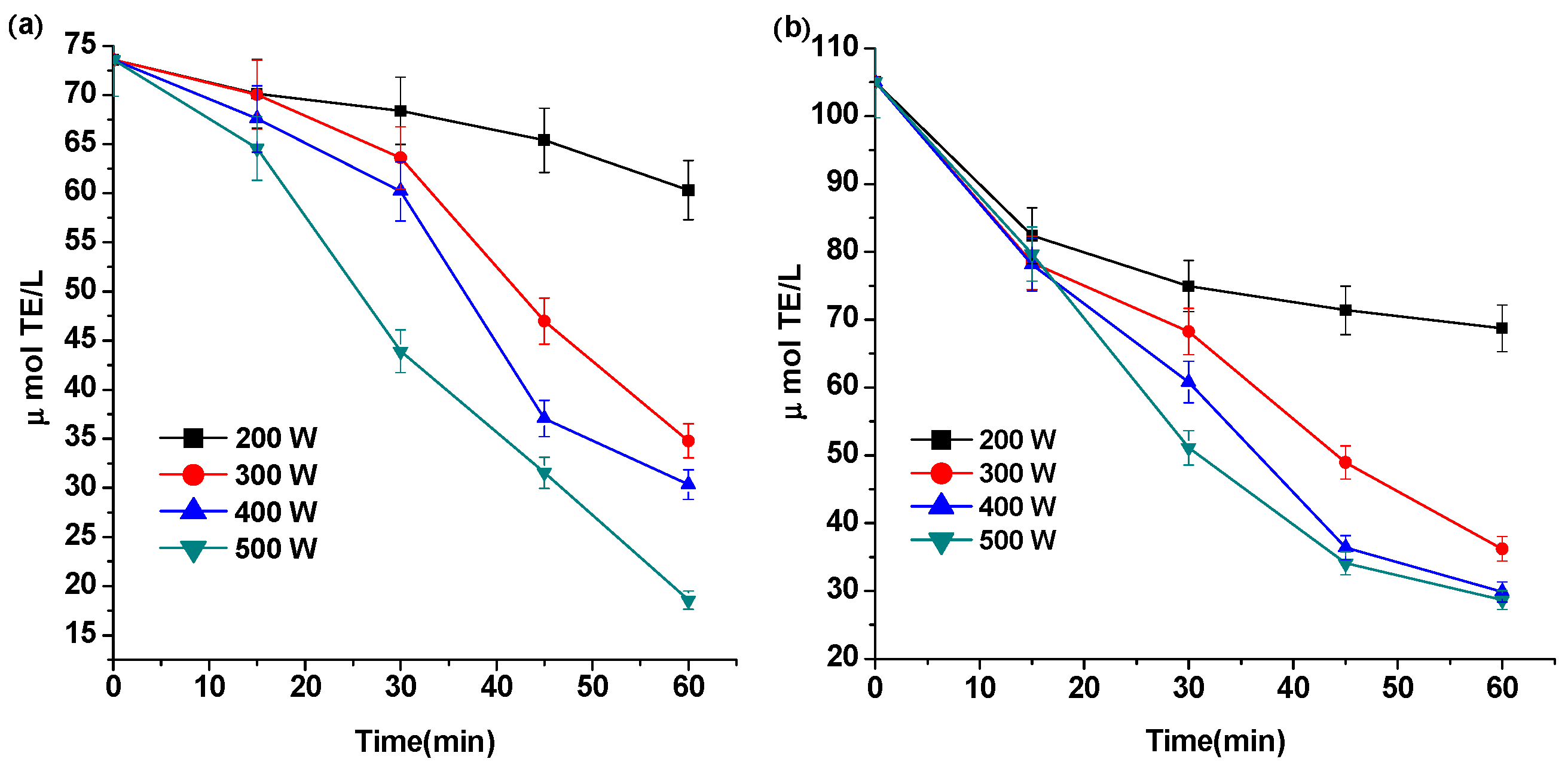
2.3. Effect of Ultrasound on the Antioxidant Capacity of Pg-3-glu
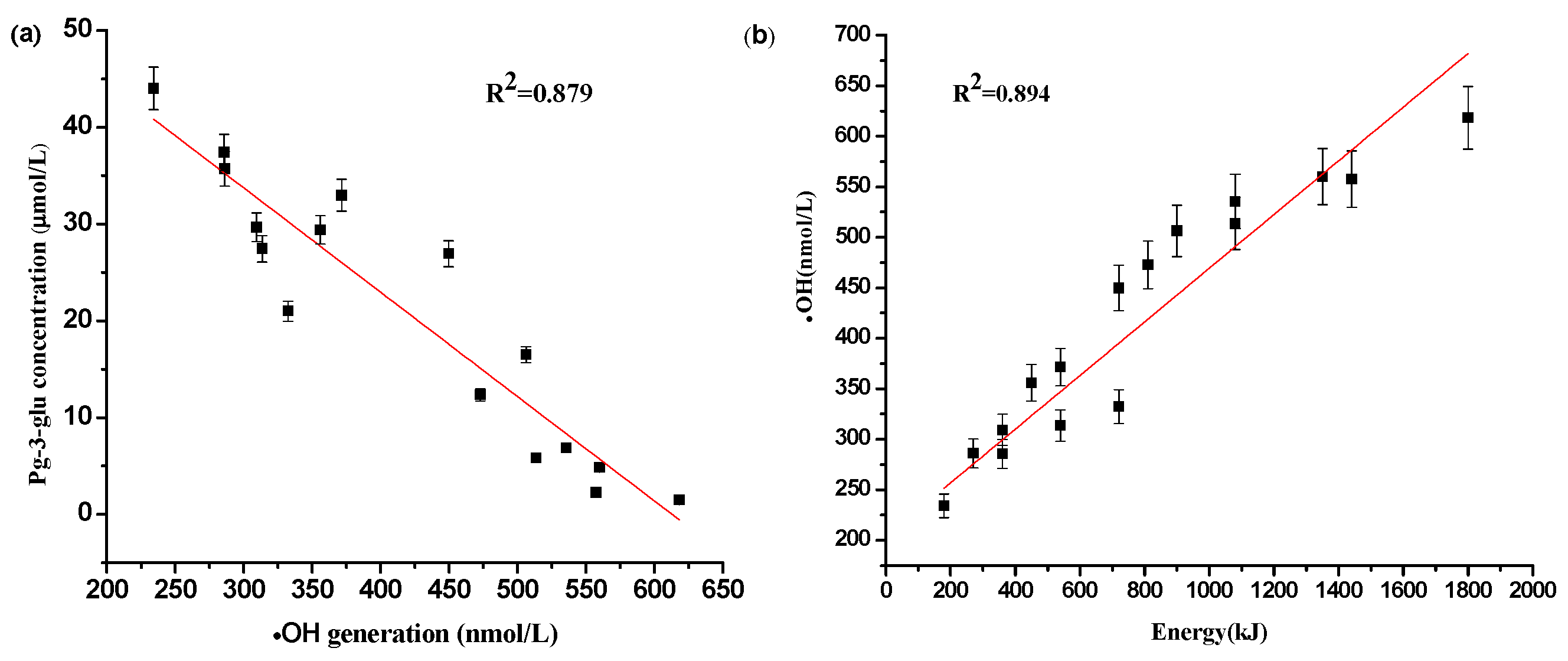
2.4. The Relationship between Reactive Oxygen Specie •OH Generation and Degradation of Pg-3-glu
3. Materials and Methods
3.1. Chemicals
3.2. Extraction and Isolation of Pg-3-glu
3.3. Identification and Purity Analysises of Pg-3-glu
3.4. Ultrasound Treatment
3.5. Evaluation of •OH Formation
3.6. Degradation Kinetics Analysis
3.7. Determination of Ultrasonic Energy
3.8. Methods of Determination
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gabriel, A.A. Microbial inactivation in cloudy apple juice by multi-frequency dynashock power ultrasound. Ultrason. Sonochem. 2012, 19, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, M.; Alzamora, S.M.; Guerrero, S. Study of the inactivation of spoilage microorganisms in apple juice by pulsed light and ultrasound. Food Microbiol. 2015, 46, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Hu, Z.; Pang, B.; Wang, H.; Xie, H.; Wu, F. Effect of ultrasound treatment on fruit decay and quality maintenance in strawberry after harvest. Food Control 2010, 21, 529–532. [Google Scholar] [CrossRef]
- Cheng, X.-F.; Zhang, M.; Adhikari, B. Changes in quality attributes of strawberry purees processed by power ultrasound or thermal treatments. Food Sci. Technol. Res. 2014, 20, 1033–1041. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, Y.; Wu, J.; Xiao, G.; Fu, M.; Zhang, Y. Effect of ultra-high pressure homogenisation processing on phenolic compounds, antioxidant capacity and anti-glucosidase of mulberry juice. Food Chem. 2014, 153, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Anese, M.; Mirolo, G.; Beraldo, P.; Lippe, G. Effect of ultrasound treatments of tomato pulp on microstructure and lycopene in vitro bioaccessibility. Food Chem. 2013, 136, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Valdramidis, V.P.; Cullen, P.J.; Tiwari, B.K.; O’Donnell, C.P. Quantitative modelling approaches for ascorbic acid degradation and non-enzymatic browning of orange juice during ultrasound processing. J. Food Eng. 2010, 96, 449–454. [Google Scholar] [CrossRef]
- Piyasena, P.; Mohareb, E.; McKellar, R.C. Inactivation of microbes using ultrasound: A review. Int. J. Food Microbiol. 2003, 87, 207–216. [Google Scholar] [CrossRef]
- Zenker, M.; Heinz, V.; Knorr, D. Application of ultrasound-assisted thermal processing for preservation and quality retention of liquid foods. J. Food Prot. 2003, 66, 1642–1649. [Google Scholar] [PubMed]
- Pingret, D.; Fabiano-Tixier, A.S.; Chemat, F. Degradation during application of ultrasound in food processing: A review. Food Control 2013, 31, 593–606. [Google Scholar] [CrossRef]
- Nikkhah, E.; Khayamy, M.; Heidari, R.; Jamee, R. Effect of sugar treatment on stability of anthocyanin pigments in berries. J. Biol. Sci. 2007, 7, 1412–1417. [Google Scholar]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Stability of anthocyanin-based aqueous extracts of andean purple corn and red-fleshed sweet potato compared to synthetic and natural colorants. Food Chem. 2004, 86, 69–77. [Google Scholar] [CrossRef]
- Tiwari, B.K.; O’Donnell, C.P.; Patras, A.; Cullen, P.J. Anthocyanin and ascorbic acid degradation in sonicated strawberry juice. J. Agric. Food Chem. 2008, 56, 10071–10077. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Sun, Y.; Zhao, G.; Liao, X.; Hu, X.; Wu, J.; Wang, Z. Optimization of ultrasound-assisted extraction of anthocyanins in red raspberries and identification of anthocyanins in extract using high-performance liquid chromatography-mass spectrometry. Ultrason. Sonochem. 2007, 14, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Fossen, T.; Rayyan, S.; Andersen, O.M. Dimeric anthocyanins from strawberry (Fragaria ananassa) consisting of pelargonidin 3-glucoside covalently linked to four flavan-3-ols. Phytochemistry 2004, 65, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, L.; Fossen, T.; Andersen, Ø.M. Colour and stability of the six common anthocyanidin 3-glucosides in aqueous solutions. Food Chem. 2000, 68, 101–107. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Hu, X.; Liao, X. Spectral alteration and degradation of cyanidin-3-glucoside exposed to pulsed electric field. J. Agric. Food Chem. 2010, 58, 3524–3531. [Google Scholar] [CrossRef] [PubMed]
- Furtado, P.; Figueiredo, P.; Neves, H.C.D.; Pina, F. Photochemical and thermal degradation of anthocyanidins. J. Photochem. Photobiol. A Chem. 1993, 75, 113–118. [Google Scholar] [CrossRef]
- Kırca, A.; Cemeroğlu, B. Degradation kinetics of anthocyanins in blood orange juice and concentrate. Food Chem. 2003, 81, 583–587. [Google Scholar] [CrossRef]
- Vercet, A.; Lopez, P.; Burgos, J. Free radical production by manothermosonication. Ultrasonics 1998, 36, 615–618. [Google Scholar] [CrossRef]
- Nayak, B.; Berrios de, J.; Powers, J.R.; Tang, J. Thermal degradation of anthocyanins from purple potato (cv. Purple majesty) and impact on antioxidant capacity. J. Agric. Food Chem. 2011, 59, 11040–11049. [Google Scholar] [CrossRef] [PubMed]
- Mercali, G.D.; Jaeschke, D.P.; Tessaro, I.C.; Marczak, L.D. Degradation kinetics of anthocyanins in acerola pulp: Comparison between ohmic and conventional heat treatment. Food Chem. 2013, 136, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Dong, X.; Zhou, W. Combined effect of ph and high temperature on the stability and antioxidant capacity of two anthocyanins in aqueous solution. Food Chem. 2014, 163, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Xu, Z. Changes of anthocyanins, anthocyanidins, and antioxidant activity in bilberry extract during dry heating. J. Food Sci. 2008, 73, C494–C499. [Google Scholar] [CrossRef] [PubMed]
- Andersen, Ø.M. Anthocyanins. In Encyclopedia of Life Sciences; MacMillan Publishers Ltd.: London, UK, 2002; pp. 1–8. [Google Scholar]
- Brouillard, R.; Markakis, P. Anthocyanins as Food Colors; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Sun, J.; Bai, W.; Zhang, Y.; Liao, X.; Hu, X. Identification of degradation pathways and products of cyanidin-3-sophoroside exposed to pulsed electric field. Food Chem. 2011, 126, 1203–1210. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Li, W.; Pickard, M.D.; Beta, T. Effect of thermal processing on antioxidant properties of purple wheat bran. Food Chem. 2007, 104, 1080–1086. [Google Scholar] [CrossRef]
- Zhang, Y. Extraction of Raspberry Anthocyanins Assisted by Pulsed Electric Field. Ph.D. Thesis, China Agricultural University, Beijing, China, 2007. [Google Scholar]
- Lopes, P.; Richard, T.; Saucier, C.; Teissedre, P.L.; Monti, J.P.; Glories, Y. Anthocyanone a: A quinone methide derivative resulting from malvidin 3-O-glucoside degradation. J. Agric. Food Chem. 2007, 55, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Floros, J.D.; Liang, H. Acoustically assisted diffusion through membranes and biomaterials. Food Toconol. 1994, 48, 79–84. [Google Scholar]
- Riesz, P.; Berdahl, D.; Christman, C.L. Free radical generation by ultrasound in aqueous and nonaqueous solutions. Environ. Health Perspect. 1986, 64, 233–252. [Google Scholar] [CrossRef]
- Kanthale, P.; Ashokkumar, M.; Grieser, F. Sonoluminescence, sonochemistry (H2O2 yield) and bubble dynamics: Frequency and power effects. Ultrason. Sonochem. 2008, 15, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Eren, Z. Degradation of an azo dye with homogeneous and heterogeneous catalysts by sonophotolysis. CLEAN—Soil Air Water 2012, 40, 1284–1289. [Google Scholar] [CrossRef]
- Ruenroengklin, N.; Yang, B.; Lin, H.; Chen, F.; Jiang, Y. Degradation of anthocyanin from litchi fruit pericarp by H2O2 and hydroxyl radical. Food Chem. 2009, 116, 995–998. [Google Scholar] [CrossRef]
- K De, A.; Chaudhuri, B.; Bhattacharjee, S. A kinetic study of the oxidation of phenol, o-chlorophenol and catechol by hydrogen peroxide between 298 K and 333 K: The effect of ph, temperature and ratio of oxidant to substrate. J. Chem. Technol. Biotechnol. 1999, 74, 162–168. [Google Scholar] [CrossRef]
- Sun, J.; Cao, X.; Bai, w.; Liao, X.; Hu, X. Comparative analyses of copigmentation of cyanidin 3-glucoside and cyanidin 3-sophoroside from red raspberry fruits. Food Chem. 2010, 120, 1131–1137. [Google Scholar] [CrossRef]
- Freinbichler, W.; Colivicchi, M.A.; Fattori, M.; Ballini, C.; Tipton, K.F.; Linert, W.; Della Corte, L. Validation of a robust and sensitive method for detecting hydroxyl radical formation together with evoked neurotransmitter release in brain microdialysis. J. Neurochem. 2008, 105, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.J.; Cheng, J.J.; Liao, X.Y.; Zhang, T.; Leng, X.; Zhao, G. Comparative Study on Iron Release from Soybean (Glycine max) Seed Ferritin Induced by Anthocyanins and Ascorbate. J. Agric. Food Chem. 2010, 58, 635–664. [Google Scholar] [CrossRef] [PubMed]
- Aljadi, A.M.; Kamaruddin, M.Y. Evaluation of the phenolic contents and antioxidant capacities of two malaysian floral honeys. Food Chem. 2004, 85, 513–518. [Google Scholar] [CrossRef]
- Du, G.; Li, M.; Ma, F.; Dong, L. Antioxidant capacity and the relationship with polyphenol and vitamin c in actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds Pg-3-glu are available from the authors.
| Parameters | 200 W | 300 W | 400 W | 500 W |
|---|---|---|---|---|
| k (min−1) | 1.69 × 10−2 | 4.28 × 10−2 | 6.50 × 10−2 | 6.72 × 10−2 |
| t1/2 (min) | 41.0146 | 16.1950 | 11.4570 | 10.3147 |
| R2 | 0.9861 | 0.9211 | 0.9170 | 0.9790 |
| Treatment Conditions | 200 W | 300 W | 400 W | 500 W |
|---|---|---|---|---|
| 15 min | 5.7 | 10.8 | 13 | 17.3 |
| 30 min | 5.5 | 10.5 | 14.6 | 15.9 |
| 45 min | 5 | 8.9 | 14.6 | 16.5 |
| 60 min | 4.8 | 9.3 | 14.6 | 16.1 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Mei, Z.; Tang, Y.; Ding, L.; Jiang, G.; Zhang, C.; Sun, A.; Bai, W. Stability, Antioxidant Capacity and Degradation Kinetics of Pelargonidin-3-glucoside Exposed to Ultrasound Power at Low Temperature. Molecules 2016, 21, 1109. https://doi.org/10.3390/molecules21091109
Sun J, Mei Z, Tang Y, Ding L, Jiang G, Zhang C, Sun A, Bai W. Stability, Antioxidant Capacity and Degradation Kinetics of Pelargonidin-3-glucoside Exposed to Ultrasound Power at Low Temperature. Molecules. 2016; 21(9):1109. https://doi.org/10.3390/molecules21091109
Chicago/Turabian StyleSun, Jianxia, Zhouxiong Mei, Yajuan Tang, Lijun Ding, Guichuan Jiang, Chi Zhang, Aidong Sun, and Weibin Bai. 2016. "Stability, Antioxidant Capacity and Degradation Kinetics of Pelargonidin-3-glucoside Exposed to Ultrasound Power at Low Temperature" Molecules 21, no. 9: 1109. https://doi.org/10.3390/molecules21091109