Intramolecular Chain Hydrosilylation of Alkynylphenylsilanes Using a Silyl Cation as a Chain Carrier
Abstract
:1. Introduction



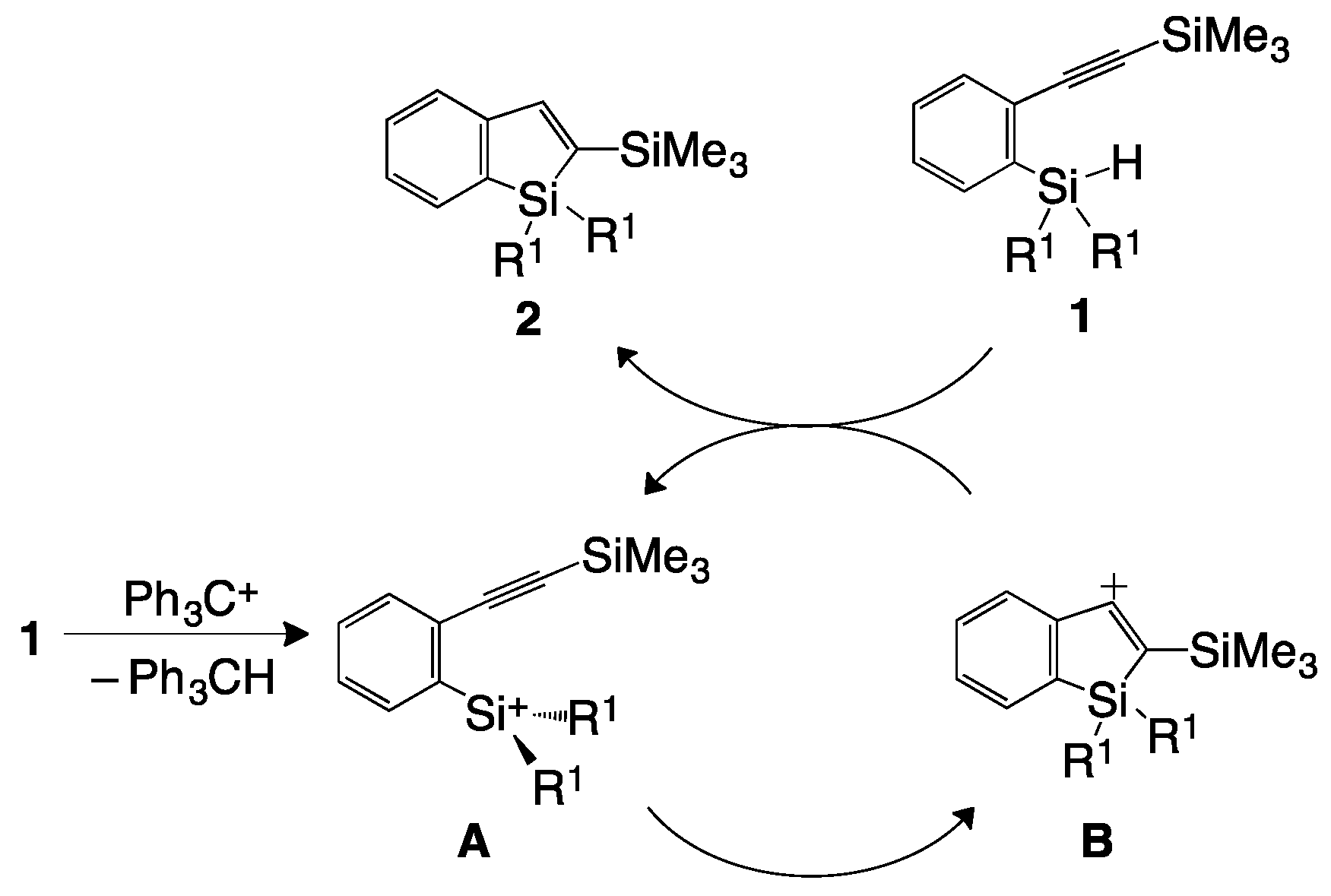
2. Results and Discussion
3. Experimental Section
Preparation of Compounds
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Yamaguchi, S.; Tamao, K. Silole-containing σ- and π-conjugated compounds. J. Chem. Soc. Dalton Trans 1998, 27, 3693–3702. [Google Scholar] [CrossRef]
- Wong, W.W.H.; Holms, A.B. Poly(dibenzosilole)s. Adv. Polym. Sci. 2008, 212, 85–98. [Google Scholar]
- Chen, J.; Cao, Y. Development of novel conjugated donor polymers for high-efficiency bulk-heterojunction photovoltaic devices. Acc. Chem. Res. 2009, 42, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, A. π-Conjugated polymers for organic electronics and photovoltaic cell applications. Chem. Mater. 2011, 23, 733–758. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Tamao, K. Theoretical study of the electronic structure of 2,2’-bisilole in comparison with 1,1’-bi-1,3-cyclopentadiene: σ*-π* Conjugation and a low-lying LUMO as the origin of the unusual optical properties of 3,3’4,4’-tetraphenyl-2,2’-bisilole. Bull. Chem. Soc. Jpn. 1996, 69, 2327–2334. [Google Scholar] [CrossRef]
- Shimizu, M.; Mochida, K.; Hiyama, T. Modular approach to silicon-bridged biaryls: Palladium-catalyzed intramolecular coupling of 2-(arylsilyl)aryl triflates. Angew. Chem. Int. Ed. 2008, 47, 9760–9764. [Google Scholar] [CrossRef] [PubMed]
- Tobisu, M.; Onoe, M.; Kita, Y.; Chatani, N. Rhodium-catalyzed coupling of 2-silylphenylboronic acids with alkynes leading to benzosiloles: Catalytic cleavage of the carbon-silicon bond in trialkylsilyl groups. J. Am. Chem. Soc. 2009, 131, 7506–7507. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, K.; Liang, Y.; Xi, Z. Construction of benzosiloles, six- and eight-membered silacyclic skeletons via a Pd-catalyzed intramolecular Mizoroki–Heck reaction of vinylsilanes. Org. Lett. 2012, 14, 4572–4575. [Google Scholar] [CrossRef] [PubMed]
- Onoe, M.; Baba, K.; Kim, Y.; Kita, Y.; Tobisu, M.; Chatani, N. Rhodium-catalyzed carbon-silicon bond activation for synthesis of benzosilole derivatives. J. Am. Chem. Soc. 2012, 134, 19477–19488. [Google Scholar] [CrossRef] [PubMed]
- Shintani, R.; Otomo, H.; Ota, K.; Hayashi, T. Palladium-catalyzed asymmetric synthesis of silicon-stereogenic dibenzosiloles via enantioselective C–H bond functionalization. J. Am. Chem. Soc. 2012, 134, 7305–7308. [Google Scholar] [CrossRef] [PubMed]
- Shintani, R.; Takagi, C.; Ito, T.; Naito, M.; Nozaki, K. Rhodium-catalyzed asymmetric synthesis of silicon-stereogenic dibenzosiloles by enantioselective [2 + 2 + 2] cycloaddition. Angew. Chem. Int. Ed. 2015, 54, 1616–1620. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Kadowaki, S.; Murakami, M. Ruthenium-catalyzed double trans-hydrosilylation of 1,4-diarylbuta-1,3-diynes leading to 2,5-diarylsiloles. Chem. Commun. 2007, 43, 2627–2629. [Google Scholar] [CrossRef] [PubMed]
- Ureshino, T.; Yoshida, T.; Kuninobu, Y.; Takai, K. Rhodium-catalyzed synthesis of silafluorene derivatives via cleavage of silicon–hydrogen and carbon–hydrogen bonds. J. Am. Chem. Soc. 2010, 132, 14324–14326. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Yamaguchi, Y.; Shigeno, M.; Sato, S.; Murakami, M. Gold-catalysed alkenyl- and arylsilylaton reactions forming 1-silaindenes. Chem. Commun. 2011, 47, 8697–8699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-W.; An, K.; He, W. Rhodium-catalyzed tandem cyclization/Si–C activation reaction for the synthesis of siloles. Angew. Chem. Int. Ed. 2014, 53, 5667–5671. [Google Scholar] [CrossRef] [PubMed]
- Omann, L.; Oestreich, M. A catalytic SEAr approach to dibenzosiloles functionalized at both benzene cores. Angew. Chem. Int. Ed. 2015, 54, 10276–10279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-W.; An, K.; Liu, L.-C.; Guo, S.; Jiang, C.; Guo, H.; He, W. Rhodium-catalyzed intramolecular C–H silylation by silacyclobutanes. Angew. Chem. Int. Ed. 2016, 55, 6319–6323. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wakamiya, A.; Yamaguchi, S. General silaindene synthesis based on intramolecular reductive cyclization toward new fluorescent silicon-containing π-electron materials. Org. Lett. 2004, 6, 3707–3710. [Google Scholar] [CrossRef] [PubMed]
- Sudo, T.; Asao, N.; Yamamoto, Y. Synthesis of various silacycles via the Lewis acid-catalyzed intramolecular trans-hydrosilylation of unactivated alkynes. J. Org. Chem. 2000, 65, 8919–8923. [Google Scholar] [CrossRef] [PubMed]
- Curless, L.D.; Ingleson, M.J. B(C6F5)3-catalyzed synthesis of benzofused-siloles. Organometallics 2014, 33, 7241–7246. [Google Scholar] [CrossRef]
- Ilies, L.; Tsuji, H.; Nakamura, E. Synthesis of benzo[b]siloles via KH-promoted cyclization of (2-alkynylphenyl)silanes. Org. Lett. 2009, 11, 3966–3968. [Google Scholar] [CrossRef] [PubMed]
- Leifert, D.; Studer, A. 9-Silafluorenes via base-promoted homolytic aromatic substitution (BHAS)—The electron as catalyst. Org. Lett. 2015, 17, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Kobayashi, J.; Kawashima, T. Development of a sila-Friedel-Crafts reaction and its application to the synthesis of dibenzosilole derivatives. J. Am. Chem. Soc. 2009, 131, 14192–14193. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Kobayashi, J.; Kawashima, T. Application of sila-Friedel-Crafts reaction to the synthesis of π-extended silole derivatives and their properties. Dalton Trans. 2010, 39, 9329–9336. [Google Scholar] [CrossRef] [PubMed]
- Arii, H.; Kurihara, T.; Mochida, K.; Kawashima, T. Silylium ion-promoted dehydrogenative cyclization: Synthesis of silicon-containing compounds derived from alkynes. Chem. Commun. 2014, 50, 6649–6652. [Google Scholar] [CrossRef] [PubMed]
- Synthesis of silacycles using alkenes instead of alkynes: see, Arii, H.; Yano, Y.; Nakabayashi, K.; Yamaguchi, S.; Yamamura, M.; Mochida, K.; Kawashima, T. Regioselective and stereospecific dehydrogenative annulation utilizing silylium ion-activated alkenes. J. Org. Chem. 2016. [Google Scholar] [CrossRef]
- Lambert, J.B.; Zhao, Y.; Wu, H. β-Silyl and β-germyl carbocations stable at room temperature. J. Org. Chem. 1999, 64, 2729–2736. [Google Scholar] See similar self-regeneration of silyl cations in [28,29].
- Müther, K.; Oestreich, M. Self-regeneration of a silylium ion catalyst in carbonyl reduction. Chem. Commun. 2011, 47, 334–336. [Google Scholar] See similar self-regeneration of silyl cations in [27,29].
- Müther, K.; Mohr, J.; Oestreich, M. Silylium ion promoted reduction of imines with hydrosilanes. Organometallics 2013, 32, 6643–6646. [Google Scholar] See similar self-regeneration of silyl cations in [27,28].
- Ihara, E.; Young, V.G., Jr.; Jordan, R.F. Cationic aluminium alkyl complexes incorporating aminotroponiminate ligands. J. Am. Chem. Soc. 1998, 120, 8277–8278. [Google Scholar] [CrossRef]
- Matsuda, T.; Kato, K.; Goya, T.; Shimada, S.; Murakami, M. Ruthenium-catalyzed cycloisomerization of 2,2’-diethynylbiphenyls involving cleavage of a carbon–carbon triple bond. Chem. Eur. J. 2016, 22, 1941–1943. [Google Scholar] [CrossRef] [PubMed]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nuldelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR chemical shifts of trace impurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1 and 2 are available from the authors.
| Entry | R1 | R2 | R3 | Solvent | TPFPB/mol % | Time/min | Yield of 2/% a | |
|---|---|---|---|---|---|---|---|---|
| 1 | 1a: | Me | TMS | H | benzene | 4 | 5 | 37 (2a) |
| 2 | 1b: | i-Pr | TMS | H | benzene | 4 | 5 | 61 (2b) |
| 3 | 1c: | Ph | TMS | H | benzene | 4 | 5 | 21 (2c) |
| 4 | 1b: | i-Pr | TMS | H | benzene | 3 | 15 | 70 (2b) |
| 5 | 1b: | i-Pr | TMS | H | benzene | 1 | 30 | 75 (2b) |
| 6 | 1b: | i-Pr | TMS | H | toluene | 1 | 30 | 72 (2b) |
| 7 | 1b: | i-Pr | TMS | H | mesitylene | 1 | 30 | 70 (2b) |
| 8 | 1b: | i-Pr | TMS | H | CH2Cl2 | 1 | 30 | 60 (2b) |
| 9 | 1a: | Me | TMS | H | benzene | 1 | 30 | 34 (2a) |
| 10 | 1c: | Ph | TMS | H | benzene | 1 | 30 | 55 (2c) |
| 11 | 1d: | i-Pr | TMS | 4-Me | benzene | 1 | 30 | 72 (2d) |
| 12 | 1e: | i-Pr | TMS | 5-Me | benzene | 1 | 50 | 81 (2e) |
| 13 | 1f: | i-Pr | n-Bu | H | benzene | 1 | 720 | 0 (2f) b |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arii, H.; Nakabayashi, K.; Mochida, K.; Kawashima, T. Intramolecular Chain Hydrosilylation of Alkynylphenylsilanes Using a Silyl Cation as a Chain Carrier. Molecules 2016, 21, 999. https://doi.org/10.3390/molecules21080999
Arii H, Nakabayashi K, Mochida K, Kawashima T. Intramolecular Chain Hydrosilylation of Alkynylphenylsilanes Using a Silyl Cation as a Chain Carrier. Molecules. 2016; 21(8):999. https://doi.org/10.3390/molecules21080999
Chicago/Turabian StyleArii, Hidekazu, Kenichi Nakabayashi, Kunio Mochida, and Takayuki Kawashima. 2016. "Intramolecular Chain Hydrosilylation of Alkynylphenylsilanes Using a Silyl Cation as a Chain Carrier" Molecules 21, no. 8: 999. https://doi.org/10.3390/molecules21080999








