Indole Alkaloids from the Leaves of Nauclea officinalis
Abstract
:1. Introduction
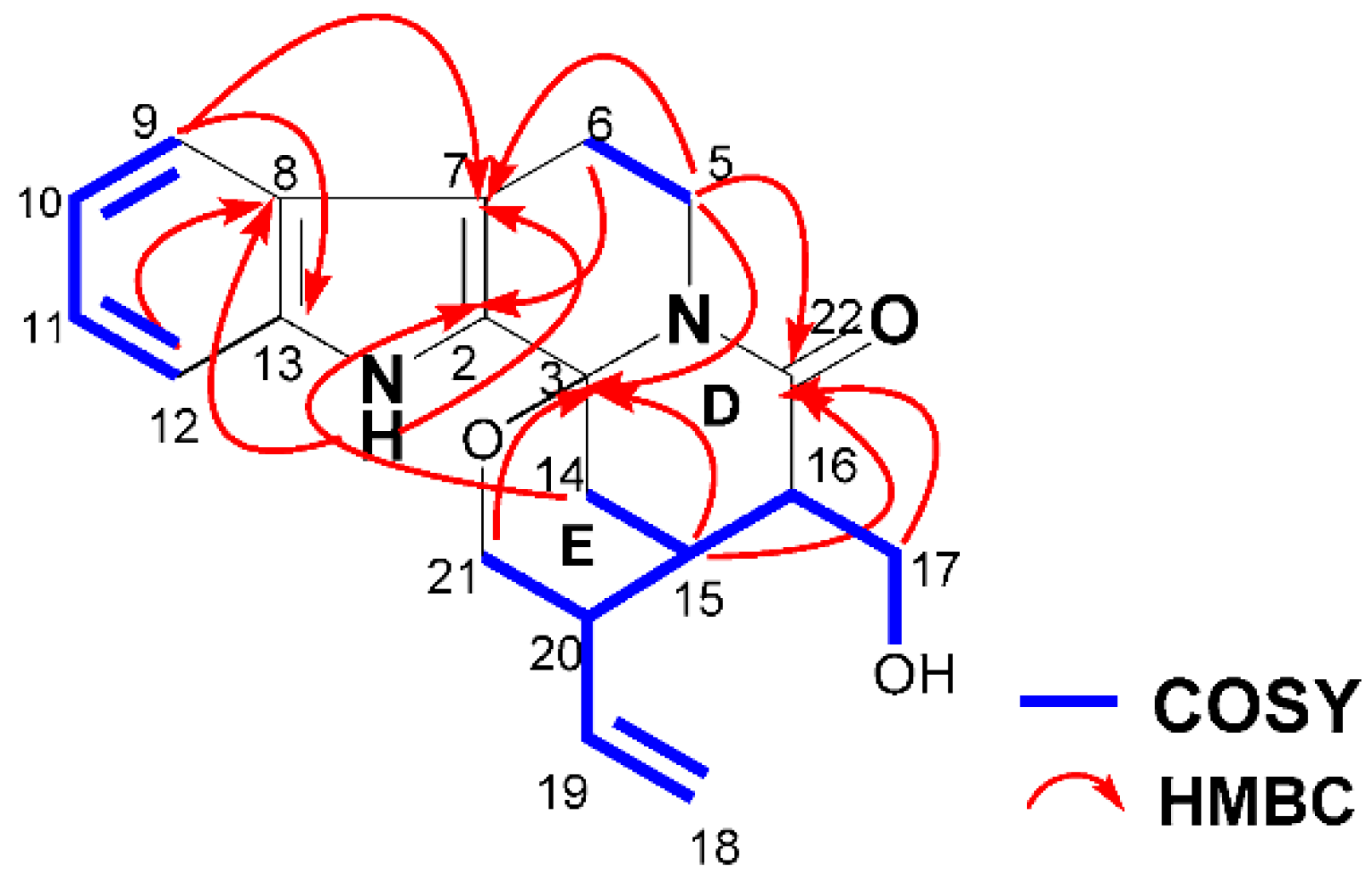
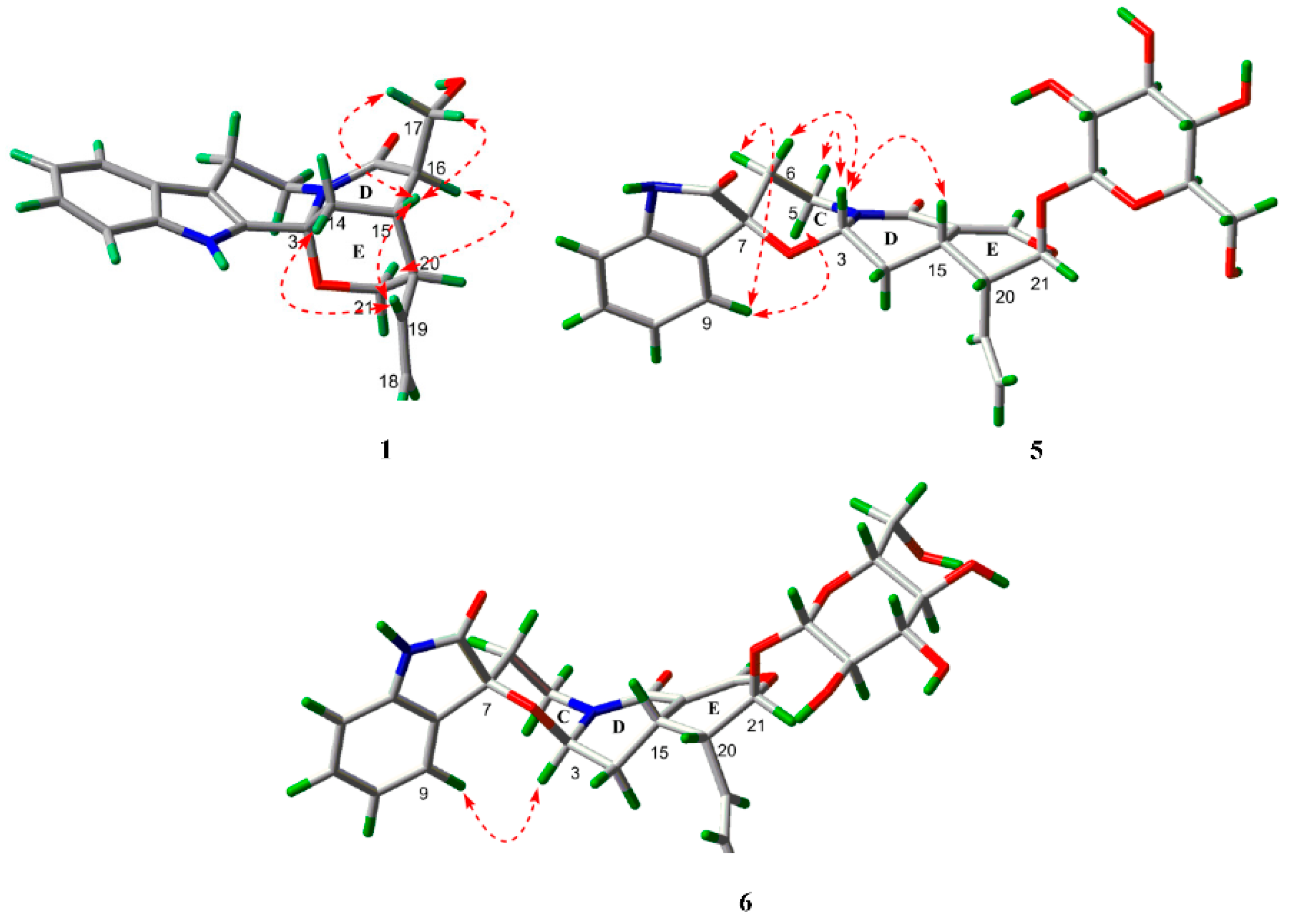
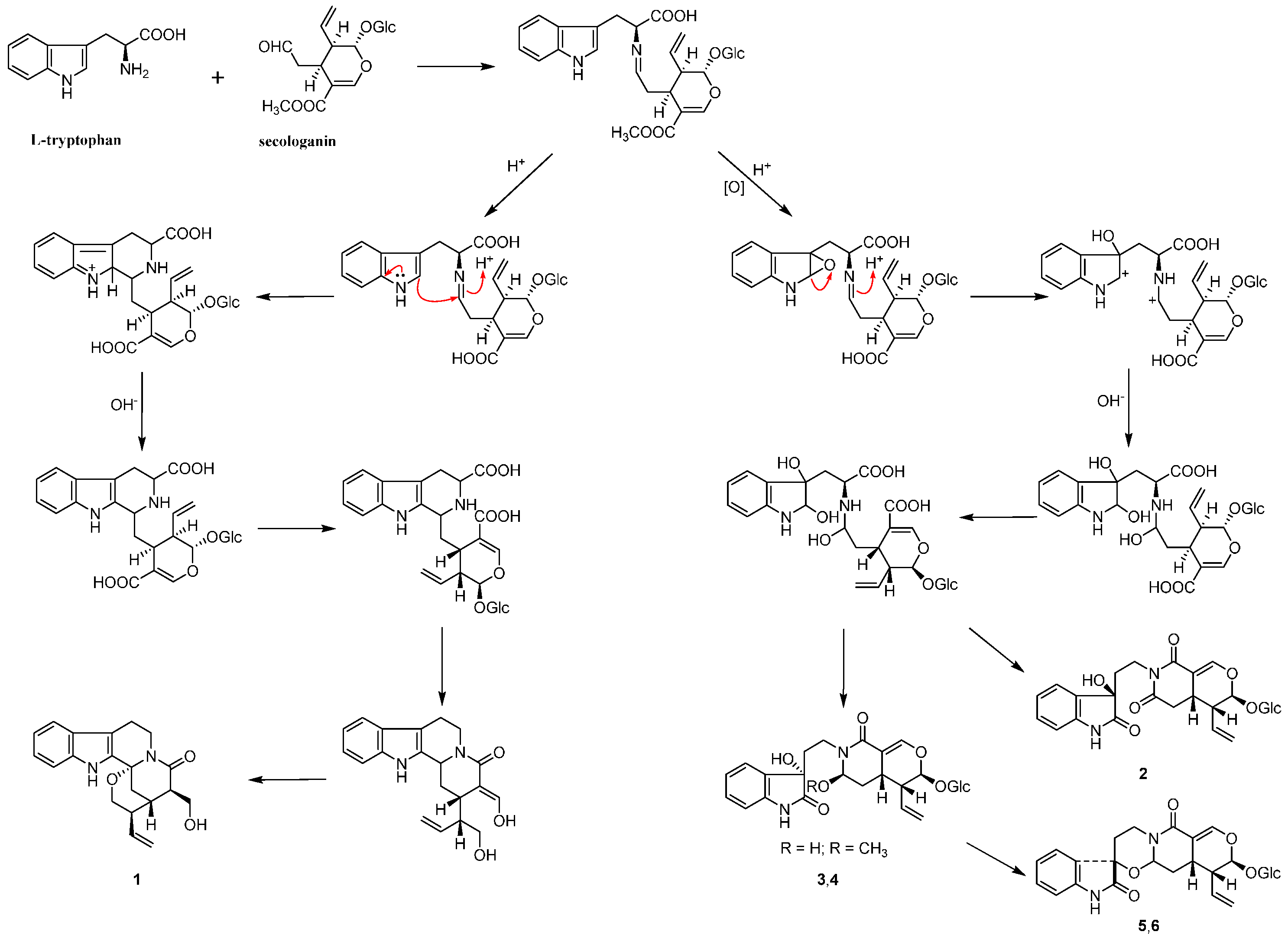
2. Results and Discussion
3. Materials and Methods
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Compound Characterization
3.5. Acid Hydrolysis and Sugar Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Editorial Committee of Chinese Herbs. Chinese Herbs; Shanghai Science and Technology Press: Shanghai, China, 1999; Volume 6, p. 456. [Google Scholar]
- Sun, J.Y.; Lou, H.X.; Xu, H.; Dai, S.J.; Liu, K. Two new indole alkaloids from Nauclea officinalis. Chin. Chem. Lett. 2007, 18, 1084–1086. [Google Scholar] [CrossRef]
- Xuan, W.D.; Chen, H.S.; Du, J.L.; Liang, S.; Li, T.Z.; Cai, D.G. Two new indole alkaloids from Nauclea officinalis. J. Asian Nat. Prod. Res. 2006, 8, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.D.; Bian, J.; Chen, H.S. Alkaloidal constituents of Nauclea officinalis. Chin. Tradit. Herb. Drugs 2007, 38, 170–173. [Google Scholar]
- Fan, L.; Fan, C.L.; Wang, Y.; Zhang, X.Q.; Zhang, Q.W.; Zhang, J.Q.; Ye, W.C. Alkaloids from the leaves of Nauclea officinalis. Acta Pharm. Sin. 2010, 45, 747–751. [Google Scholar]
- Lin, M.; Liu, X.; Yu, D.Q.; Dou, S.Q.; Zhang, Y.J.; Li, Q.M. The structure determination of a new alkaloid-nauclefiline in Nauclea officinalis. Acta Pharm. Sin. 1985, 20, 902–905. [Google Scholar]
- Liew, S.Y.; Mukhtar, M.R.; Hadi, A.H.; Awang, K.; Mustafa, M.R.; Zaima, K.; Morita, H.; Litaudon, M. Naucline, a new indole alkaloid from the bark of Nauclea officinalis. Molecules 2012, 17, 4028–4036. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Lou, H.X.; Dai, S.J.; Xu, H.; Zhao, F.; Liu, K. Indole alkaloids from Nauclea officinalis with weak antimalarial activity. Phytochemistry 2008, 69, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Liu, X.; Yu, D.Q. Alkaloids of Nauclea officinalis. Planta Med. 1984, 50, 459–461. [Google Scholar]
- Lin, M.; Li, S.Z.; Liu, X.; Yu, D.Q. Studies on the structures of two new alkaloidal glucosides of Nauclea officinalis Pierre ex pitard. Acta Pharm. Sin. 1989, 24, 32–36. [Google Scholar]
- Xuan, W.D.; Chen, H.S.; Bian, J. A new indole alkaloid glycoside from stems of Nauclea officinalis. Acta Pharm. Sin. 2006, 41, 1064–1067. [Google Scholar]
- Xuan, W.D.; Chen, H.S.; Yuan, Z.X.; Zhu, P. Chemical constituents of Nauclea officinalis. Chin. J. Nat. Med. 2005, 3, 181–183. [Google Scholar]
- Fan, L.; Huang, X.J.; Fan, C.L.; Li, G.Q.; Wu, Z.L.; Li, S.G.; He, Z.D.; Wang, Y.; Ye, W.C. Two new oxindole alkaloid glycosides from the leaves of Nauclea officinalis. Nat. Prod. Commun. 2015, 10, 2087–2090. [Google Scholar] [PubMed]
- Shigemori, H.; Kagata, T.; Ishiyama, H.; Morah, F.; Ohsaki, A.; Kobayashi, J. Naucleamides A–E, new monoterpene indole alkaloids from Nauclea latifolia. Chem. Pharm. Bull. 2003, 51, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Hecht, S.M. Javaniside, a novel DNA cleavage agent from Alangium javanicum having an unusual oxindole skeleton. Chem. Commun. 2004, 40, 1190–1191. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.C.; Ma, J.; Thomas, S.J.; Xu, Z.D.; Hecht, S.M. Alkaloids from Alangium javanicum and Alangium grisolleoides that mediate Cu2+-dependent DNA strand scission. J. Nat. Prod. 2005, 68, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wu, P.; Xie, H.H.; Wu, G.J.; Wei, X.Y. Monoterpenoid indole alkaloids mediating DNA strand scission from Turpinia arguta. Planta Med. 2011, 77, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Kagata, T.; Saito, S.; Shigemori, H.; Ohsaki, A.; Ishiyama, H.; Kubota, T.; Kobayashi, J. Paratunamides A–D, oxindole alkaloids from Cinnamodendron axillare. J. Nat. Prod. 2006, 69, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.


| Position | δC | δH |
|---|---|---|
| 1 | - | 11.19 s |
| 2 | 133.8 | - |
| 3 | 80.9 | - |
| 5 | 34.6 | a 4.80 dd (12.7, 4.7) |
| b 2.82 m | ||
| 6 | 20.7 | a 2.74 m |
| b 2.61 m | ||
| 7 | 108.7 | - |
| 8 | 125.3 | - |
| 9 | 118.6 | 7.46 d (8.0) |
| 10 | 118.7 | 7.00 t (8.0) |
| 11 | 121.9 | 7.12 t (8.0) |
| 12 | 111.5 | 7.37 d (8.0) |
| 13 | 136.2 | - |
| 14 | 31.5 | a 2.89 dd (13.0, 3.3) |
| b 1.72 dd (13.0, 2.0) | ||
| 15 | 30.9 | 2.33 br s |
| 16 | 46.8 | 2.79 m |
| 17 | 58.9 | a 4.08 m |
| b 3.65 m | ||
| 17-OH | - | 4.69 dd (6.0, 4.3) |
| 18 | 115.5 | a 5.28 d (17.3) |
| b 5.23 d (10.4) | ||
| 19 | 140.4 | 6.39 ddd (17.3, 10.4, 7.3) |
| 20 | 35.8 | 2.51 m |
| 21 | 61.7 | a 3.66 m |
| b 3.59 dd (12.7, 3.1) | ||
| 22 | 170.6 | - |
| Position | Nauclealomide A | 5 | 6 | |||
|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | |
| 2 | 180.1 | - | 179.5 | - | 177.8 | - |
| 3 | 80.0 | 6.28 t (2.8) | 80.4 | 6.04 dd (9.5, 4.6) | 81.2 | 5.72 dd (3.6, 2.1) |
| 5 | 39.3 | a 4.60 ddd (13.0, 4.9, 1.8) | 36.1 | a 4.43 dt (13.3, 4.7) | 39.7 | a 4.73 ddd (13.6, 5.4, 2.8) |
| b 3.75 dd (13.0, 3.0) | b 3.73 ddd (13.3, 11.0, 3.7) | b 3.55 td (13.6, 3.3) | ||||
| 6 | 32.4 | a 2.11 td (14.1, 5.4) | 31.7 | a 2.16 ddd (14.2, 11.0, 4.9) | 31.3 | a 2.17 td (13.3, 5.6) |
| b 1.77 m | b 2.03 dt (14.2, 4.0) | b 1.67 m | ||||
| 7 | 77.6 | - | 76.3 | - | 79.4 | - |
| 8 | 131.4 | - | 131.5 | - | 131.6 | - |
| 9 | 125.4 | 7.25 br d (7.5) | 125.1 | 7.27 br d (7.7) | 126.6 | 7.82 br d (7.6) |
| 10 | 123.9 | 7.02 br t (7.5) | 123.9 | 7.00 td (7.7, 1.1) | 123.7 | 7.07 td (7.6, 0.9) |
| 11 | 131.1 | 7.25 br t (7.5) | 131.2 | 7.24 td (7.7, 1.1) | 131.1 | 7.31 td (7.6, 0.9) |
| 12 | 111.0 | 6.84 br d (7.5) | 111.2 | 6.85 br d (7.7) | 111.9 | 6.94 br d (7.6) |
| 13 | 142.3 | - | 142.4 | - | 142.6 | - |
| 14 | 29.7 | 1.76 m | 32.2 | a 1.45 dt (13.9, 9.5) | 29.9 | a 1.78 td (14.0, 3.6) |
| b 1.95 dt (12.9, 4.6) | b 1.71 m | |||||
| 15 | 23.4 | 3.27 m | 24.4 | 3.02 m | 23.5 | 3.34 m |
| 16 | 109.0 | - | 109.1 | - | 109.2 | - |
| 17 | 149.7 | 7.44 d (2.4) | 149.1 | 7.40 d (2.3) | 149.5 | 7.42 d (2.4) |
| 18 | 120.7 | a 5.29 dd (12.1, 1.6) | 120.7 | 5.24 dd (14.5, 1.6) | 120.6 | 5.22 m |
| b 5.24 td (5.2, 1.8) | 5.21 dd (7.8, 1.8) | |||||
| 19 | 134.1 | 5.56 dt (17.2, 10.1) | 133.8 | 5.53 dt (17.2, 10.0) | 134.0 | 5.54 dt (17.6, 9.8) |
| 20 | 44.3 | 2.61 ddd (10.1, 5.8, 1.7) | 44.4 | 2.60 ddd (10.0, 5.8, 1.7) | 44.2 | 2.56 ddd (9.8, 5.8, 1.7) |
| 21 | 98.1 | 5.46 d (1.7) | 97.3 | 5.47 d (1.7) | 97.6 | 5.45 d (1.7) |
| 22 | 166.1 | - | 165.7 | - | 166.1 | - |
| 1’ | 100.4 | 4.69 d (7.9) | 99.6 | 4.65 d (7.9) | 100.0 | 4.66 d (7.8) |
| 2’ | 74.7 | 3.20 m | 74.6 | 3.18 dd (8.9, 7.9) | 74.6 | 3.24 dd (9.0, 7.8) |
| 3’ | 78.2 | 3.37 m | 77.8 | 3.36 m | 77.7 | 3.39 m |
| 4’ | 71.5 | 3.30 m | 71.5 | 3.30 m | 71.5 | 3.31 m |
| 5’ | 78.3 | 3.31 m | 78.2 | 3.31 m | 78.3 | 3.31 m |
| 6’ | 62.6 | a 3.87 dd (11.8, 1.7) | 62.6 | a 3.87 dd (11.9, 1.6) | 62.7 | a 3.88 dd (11.9, 1.6) |
| b 3.66 dd (11.8, 5.3) | b 3.66 dd (11.9, 5.4) | b 3.67 dd (11.9, 5.3) | ||||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, L.; Liao, C.-H.; Kang, Q.-R.; Zheng, K.; Jiang, Y.-C.; He, Z.-D. Indole Alkaloids from the Leaves of Nauclea officinalis. Molecules 2016, 21, 968. https://doi.org/10.3390/molecules21080968
Fan L, Liao C-H, Kang Q-R, Zheng K, Jiang Y-C, He Z-D. Indole Alkaloids from the Leaves of Nauclea officinalis. Molecules. 2016; 21(8):968. https://doi.org/10.3390/molecules21080968
Chicago/Turabian StyleFan, Long, Cheng-Hui Liao, Qiang-Rong Kang, Kai Zheng, Ying-Chun Jiang, and Zhen-Dan He. 2016. "Indole Alkaloids from the Leaves of Nauclea officinalis" Molecules 21, no. 8: 968. https://doi.org/10.3390/molecules21080968
APA StyleFan, L., Liao, C.-H., Kang, Q.-R., Zheng, K., Jiang, Y.-C., & He, Z.-D. (2016). Indole Alkaloids from the Leaves of Nauclea officinalis. Molecules, 21(8), 968. https://doi.org/10.3390/molecules21080968






