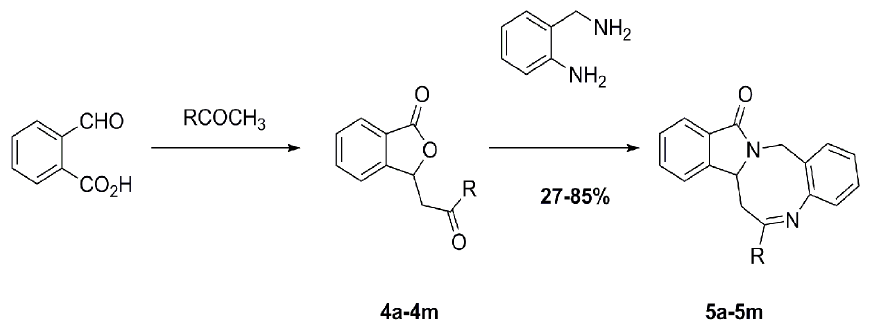
Synthesis and Spectral Characterization of Benzo-[6,7][1,5]diazocino[2,1-a]isoindol-12-(14H)-one Derivatives
Abstract
:1. Introduction
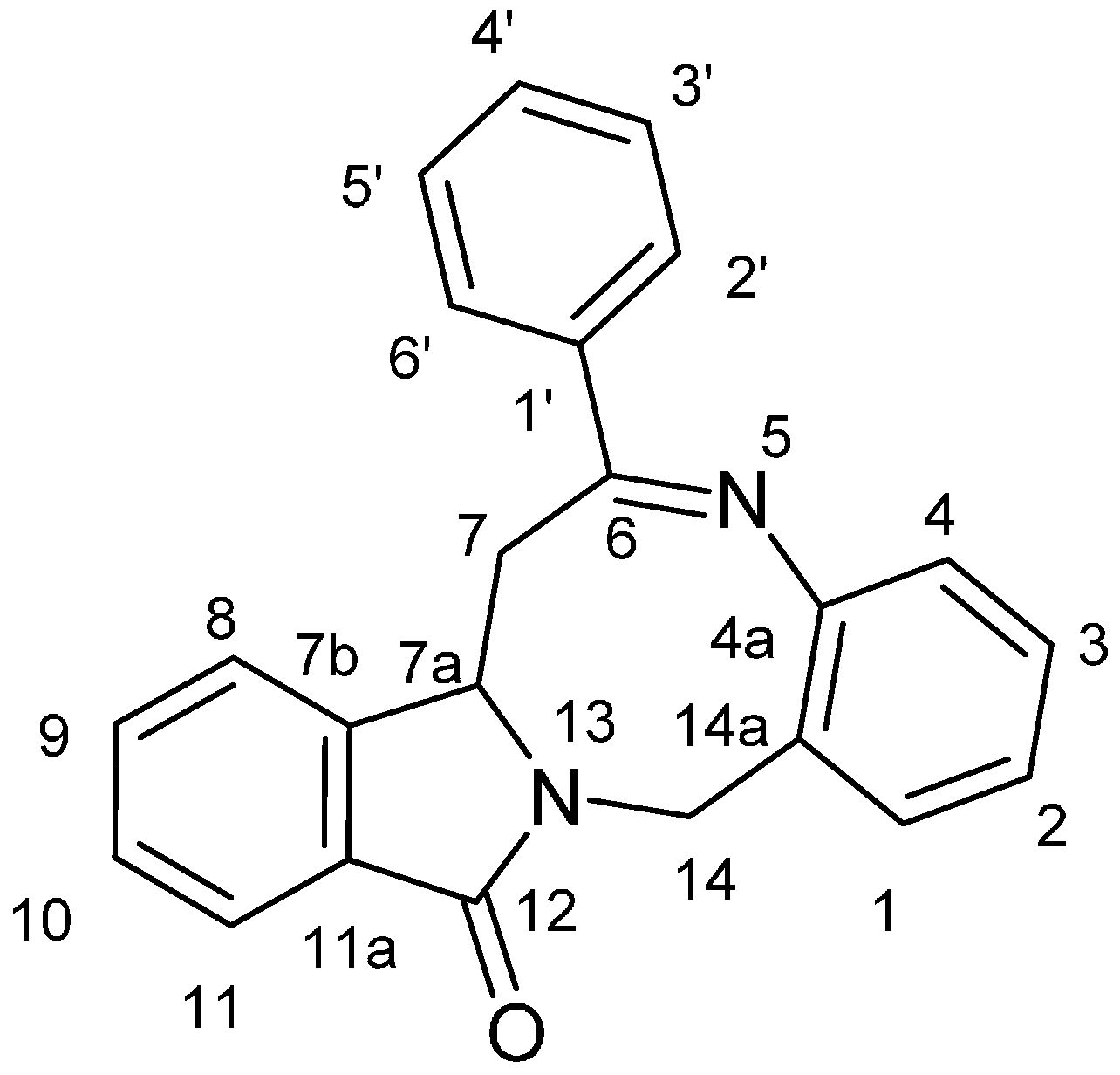
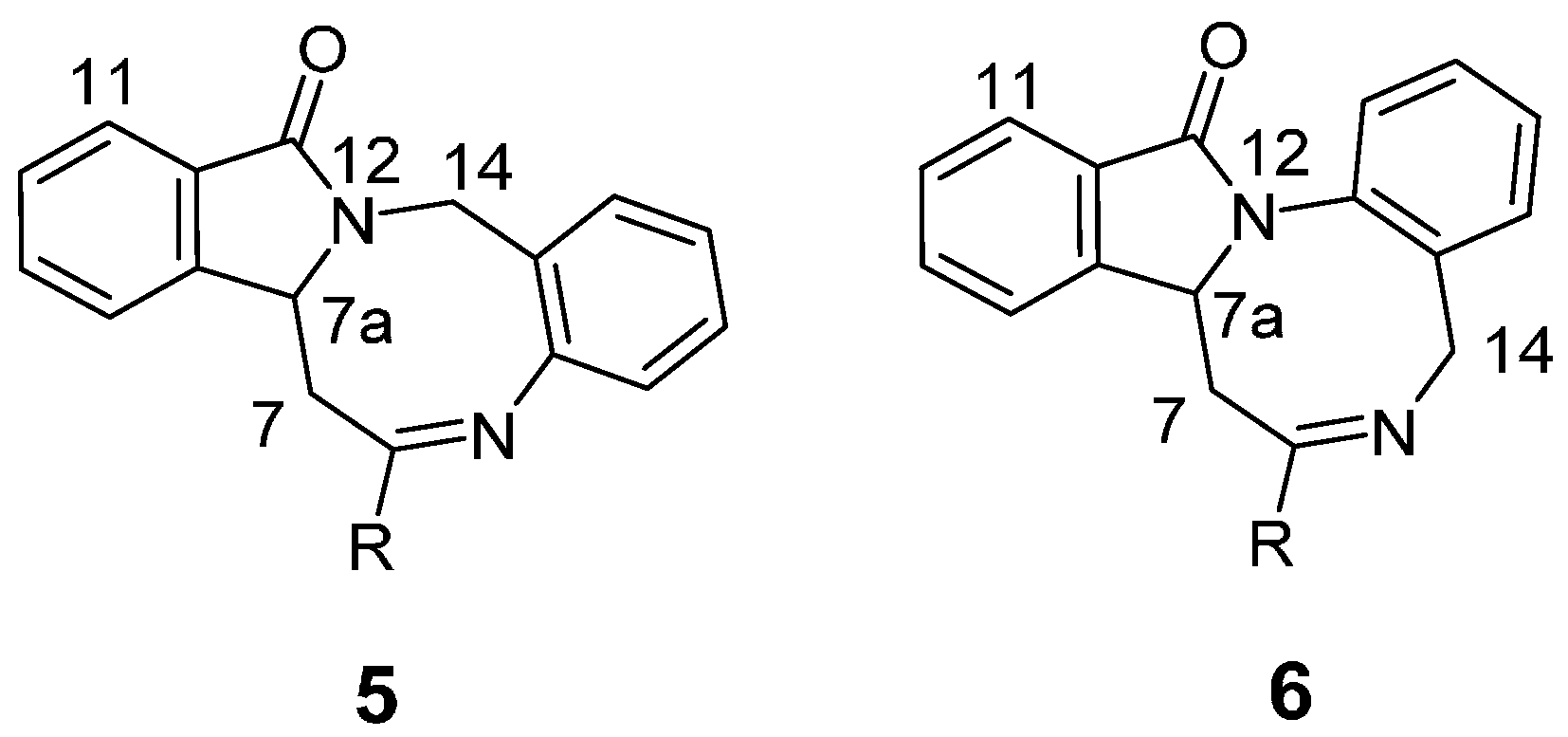
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of 3-(2-Oxo-2-phenylethyl)isobenzofuran-1(3H)-ones 4a–4m
3.3. General Procedure for the Synthesis of Benzo[6,7][1,5]diazocino[2,1-a]isoindol-12(14H)-ones 5a–5m
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, X.; Li, J.; Zhao, N.; Wan, X. A rapid and efficient access to diaryldibenzo[b,f][1,5] diazocines. Org. Lett. 2011, 13, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Zankowskajasinska, W.; Ostrowska, K. The synthesis of some derivatives of the new ring system Pyrrolo [3,4-f][1,5] diazocine. J. Prakt. Chem. 1989, 331, 700–704. [Google Scholar] [CrossRef]
- Fink, B.E.; Gavai, A.V.; Tokarski, J.S.; Goyal, B.; Misra, R.; Xiao, H.-Y.; Kimball, S.D.; Han, W.-C.; Norris, D.; Spires, T.E.; et al. Identification of a novel series of tetrahydrodibenzazocines as inhibitors of 17β-hydroxysteroid dehydrogenase type 3. Bioorg. Med. Chem. Lett. 2006, 16, 1532–1536. [Google Scholar] [CrossRef] [PubMed]
- Mazurov, A.A.; Andronati, S.A.; Korotenko, T.I.; Sokolenko, N.I.; Dyadenko, A.I.; Shapiro, Y.E.; Gorbatyuk, V.Y.; Voronina, T.A. Design of a novel cognitive enhancer (8S,10aS)-8-carbamoyl-1,2,3,6,7,8,9,10a-octahydro-5H,10H-pyrrolo[1,2-a][1,4]diazocin-5,10-dione. Bioorg. Med. Chem. Lett. 1996, 6, 2595–2600. [Google Scholar] [CrossRef]
- Kulsi, G.; Ghorai, A.; Chattopadhyay, P. Tandem one pot synthesis of 1,5-benzodiazocine-2-one by isocyanide based Ugi multicomponent reaction. Tetrahedron Lett. 2012, 53, 3619–3622. [Google Scholar] [CrossRef]
- Mishra, J.K.; Panda, G. Diversity-oriented synthetic approach to naturally abundant S-amino acid based benzannulated enantiomerically pure medium ring heterocyclic scaffolds employing inter- and intramolecular Mitsunobu reactions. J. Comb. Chem. 2007, 9, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Boruah, R.C.; Sandhu, J.S. A facile synthesis of 2,4,8,10-tetrahalo-6,12-diaryldibenzo[b,f][1,5]diazocines. J. Heterocycl. Chem. 1988, 25, 459–462. [Google Scholar] [CrossRef]
- Csende, F.; Miklos, F.; Stajer, G. Recent Developments in the Synthesis of Heterocycle-Fused Isoindoles. Curr. Org. Chem. 2012, 16, 1005–1050. [Google Scholar] [CrossRef]
- Zhuang, Z.P.; Kung, M.P.; Mu, M.; Kung, H.F. Isoindol-1-one analogues of 4-(2′-methoxyphenyl)-1-2′-N-(2″-pyridyl)-p-iodobenzamido ethyl piperazine (p-MPPI) as 5-HT1A receptor ligands. J. Med. Chem. 1998, 41, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Link, J.T.; Raghavan, S.; Danishefsky, S.J. Total synthesis of staurosporine and ent- staurosporine. J. Am. Chem. Soc. 1995, 117, 552–553. [Google Scholar] [CrossRef]
- Comins, D.L.; Schilling, S.; Zhang, Y.C. Asymmetric synthesis of 3-substituted isoindolinones: Application to the total synthesis of (+)-lennoxamine. Org. Lett. 2005, 7, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Padwa, A.; Beall, L.S.; Eidell, C.K.; Worsencroft, K.J. An approach toward isoindolobenzazepines using the ammonium ylide/Stevens 1,2-rearrangement sequence. J. Org. Chem. 2001, 66, 2414–2421. [Google Scholar] [CrossRef] [PubMed]
- Neumann, H.; Strubing, D.; Lalk, M.; Klaus, S.; Hubner, S.; Spannenberg, A.; Lindequist, U.; Beller, M. Synthesis and antimicrobial activity of N-analogous corollosporines. Org. Biomol. Chem. 2006, 4, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-F.; Neumann, H.; Neumann, S.; Beller, M. Palladium-catalyzed synthesis of phthalazinones: Efficient carbonylative coupling of 2-bromobenzaldehydes and hydrazines. Chem. Eur. J. 2012, 18, 8596–8599. [Google Scholar] [CrossRef] [PubMed]
- Dufort, I.; Rheault, P.; Huang, X.F.; Soucy, P.; Luu-The, V. Characteristics of a highly labile human type 5 17β-hydroxysteroid dehydrogenase. Endocrinology 1999, 140, 568–574. [Google Scholar] [PubMed]
- Bilbao, J.R.; Loridan, L.; Audi, L.; Gonzalo, E.; Castano, L. A novel missense (R80W) mutation in 17-β-hydroxysteroid dehydrogenase type 3 gene associated with male pseudohermaphroditism. Eur. J. Endocrinol. 1998, 139, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Pessoa-Mahana, H.; Aranguiz, K.G.M.; Araya-Maturana, R.; Pessoa-Mahana, C.D. A facile approach for new dibenzo[b,f][1,5]diazocinones. Synth. Commun. 2005, 35, 1493–1500. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Ray, K.; Ganai, S. Intramolecular Azide-Alkyne [3+2] Cycloaddition: A Versatile Route for the Synthesis of 1,2,3-Triazole Fused Dibenzo 1,5 diazocine Derivatives. Synthesis 2010, 12, 2101–2105. [Google Scholar] [CrossRef]
- Das Adhikary, N.; Chattopadhyay, P. Palladium-Catalyzed Intramolecular Aryl Amination Reaction: An Expeditious Approach to the Synthesis of Chiral Benzodiazocine Derivatives. Eur. J. Org. Chem. 2010, 9, 1754–1762. [Google Scholar] [CrossRef]
- Gruit, M.; Michalik, D.; Krueger, K.; Spannenberg, A.; Tillack, A.; Pews-Davtyan, A.; Beller, M. Synthesis of pyrroloazepinones: Platinum- and gold-catalyzed cyclization reactions of alkynes. Tetrahedron 2010, 66, 3341–3352. [Google Scholar] [CrossRef]
- Gruit, M.; Michalik, D.; Tillack, A.; Beller, M. Platinum-Catalyzed Intramolecular Cyclizations of Alkynes: Efficient Synthesis of Pyrroloazepinone Derivatives. Angew. Chem. Int. Ed. 2009, 48, 7212–7216. [Google Scholar] [CrossRef] [PubMed]
- Volland, S.; Gruit, M.; Regnier, T.; Viau, L.; Lavastre, O.; Vioux, A. Encapsulation of Pd(OAc)2 catalyst in an ionic liquid phase confined in silica gels. Application to Heck-Mizoroki reaction. New J. Chem. 2009, 33, 2015–2021. [Google Scholar] [CrossRef]
- Potapov, V.V.; Fetisova, N.A.; Nikitin, A.V.; Ivachtchenko, A.V. One-step assembly of novel carbamoyl substituted 6-oxo-4,5,6,11-tetrahydropyrrolo [1,2-b][2,5] benzodiazocine. Tetrahedron Lett. 2009, 50, 2790–2792. [Google Scholar] [CrossRef]
- Basavaiah, D.; Rao, K.V.; Reddy, R.J. The Baylis-Hillman reaction: A novel source of attraction, opportunities, and challenges in synthetic chemistry. Chem. Soc. Rev. 2007, 36, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Basavaiah, D.; Reddy, B.S.; Badsara, S.S. Recent Contributions from the Baylis-Hillman Reaction to Organic Chemistry. Chem. Rev. 2010, 110, 5447–5674. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.-I.; Song, J.-H.; Lee, E.-J.; Kim, Y.-Y.; Lee, D.-H.; Lee, Y.-G.; Hahn, J.-T. Simple synthesis of quinolines and dibenzo [b,f][1,5] diazocines under microwave irradiation. Tetrahedron Lett. 2009, 50, 5805–5807. [Google Scholar] [CrossRef]
- BouzBouz, S.; Sanselme, M. A rapid and efficient synthesis of a new pyrrolobenzodiazocines via an intramolecular Friedel-Crafts reaction. Tetrahedron Lett. 2009, 50, 5884–5887. [Google Scholar] [CrossRef]
- Cho, H.I.; Lee, S.W.; Lee, K.J. A new synthesis of 1,2,3,5-tetrahydroimidazo [2,3-b][1,3] benzodiazocines. J. Heterocycl. Chem. 2004, 41, 799–802. [Google Scholar] [CrossRef]
- Cliffe, I.A.; Heatherington, K.; White, A.C. Rearrangements of pyrimido [1,2-a] indoles and diazepino [1,2-a] indoles-synthesis of 1,5-benzodiazonines. J. Chem. Soc.-Perkin Trans. 1 1991, 1975–1979. [Google Scholar] [CrossRef]
- Korakas, D.; Varvounis, G. Synthesis of 5,6-dihydro-4H-pyrrolo [1,2-a][1,4] benzodiazepine and 10,11-dihydro-5H,12H-pyrrolo [2,1-c][1,4] benzodiazocine derivatives via cyclization of 2-aminomethylpyrroles. J. Heterocycl. Chem. 1994, 31, 1317–1320. [Google Scholar] [CrossRef]
- Mishra, A.; Batra, S. Expeditious Synthesis of Imidazole- and Pyrrole-Fused Benzodiazocines. Eur. J. Org. Chem. 2010, 25, 4832–4840. [Google Scholar] [CrossRef]
- Othman, M.; Pigeon, P.; Netchitailo, P.; Daich, A.; Decroix, B. Quinoxalines, benzodiazepines and benzodiazocines fused to pyrrole and isoindole via N-acyliminium ion aromatic cyclization. Heterocycles 2000, 52, 273–281. [Google Scholar]
- Sharp, J.T.; Wilson, P.; Parsons, S.; Gould, R.O. Reactions of triene-conjugated diazo-compounds: Reaction paths from o-(1,3-dienyl)aryldiazomethanes to 3,8-methano-1,2-diazocines and to pyrrolo [2,1-a] phthalazines via intramolecular [3 + 2] and 1,1-cycloaddition reactions. J. Chem. Soc.-Perkin Trans. 1 2000, 7, 1139–1148. [Google Scholar] [CrossRef]
- King, F.D.; Aliev, A.E.; Caddick, S.; Tocher, D.A. A novel synthesis of (di)-benzazocinones via an endocyclic N-acyliminium ion cyclisation. Org. Biomol. Chem. 2011, 9, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Sun, H.; Peng, Y.; Lu, J.; Nikolovska-Coleska, Z.; McEachern, D.; Liu, L.; Qiu, S.; Yang, C.-Y.; Miller, R.; et al. A Potent and Orally Active Antagonist (SM-406/AT-406) of Multiple Inhibitor of Apoptosis Proteins (IAPs) in Clinical Development for Cancer Treatment. J. Med. Chem. 2011, 54, 2714–2726. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Wick, W.; Weller, M.; Debatin, K.M. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat. Med. 2002, 8, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Arnt, C.R.; Chiorean, M.V.; Heldebrant, M.V.; Gores, G.J.; Kaufmann, S.H. Synthetic Smac/DIABLO peptides enhance the effects of chemotherapeutic agents by binding XIAP and cIAP1 in situ. J. Biol. Chem. 2002, 277, 44236–44243. [Google Scholar] [CrossRef] [PubMed]
- Bassin, J.P.; Shah, V.P.; Martin, L.; Horton, P.N. Racemic 9,10-dimethoxy-3-methyl-6-phenyl-7,7a-dihydrobenzo[b]benzo 4,5 isothiazo lo [2,3-d][1,4] diazepine 12,12-dioxide. Acta Cryst. 2011, E67, o684–o685. [Google Scholar]
- Bassin, J.P.; Frearson, M.J.; Al-Nawwar, K. Synthesis of benzo d benzo[2,3][1,4] diazepino 1,7-(b)under-bar isothiazole, a new heterocyclic ring system. Synth. Commun. 2000, 30, 2961–2965. [Google Scholar] [CrossRef]
- Nandi, D.; Ghosh, D.; Chen, S.-J.; Kuo, B.-C.; Wang, N.M.; Lee, H.M. One-Step Synthesis of Isocoumarins and 3-Benzylidenephthalides via Ligandless Pd-Catalyzed Oxidative Coupling of Benzoic Acids and Vinylarenes. J. Org. Chem. 2013, 78, 3445–3451. [Google Scholar] [CrossRef] [PubMed]
- Landge, S.M.; Berryman, M.; Torok, B. Microwave-assisted solid acid-catalyzed one-pot synthesis of isobenzofuran-1(3H)-ones. Tetrahedron Lett. 2008, 49, 4505–4508. [Google Scholar] [CrossRef]
- Goncalves, C.J.; Lenoir, A.S.; Padaratz, P.; Correa, R.; Niero, R.; Cechinel-Filho, V.; Buzzi, F.D. Benzofuranones as potential antinociceptive agents: Structure-activity relationships. Eur. J. Med. Chem. 2012, 56, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Paradkar, M.V.; Gadre, S.Y.; Pujari, T.A.; Khandekar, P.P.; Kumbhar, V.B. One-pot synthesis of 3-phenacylphthalides. Synth. Commun. 2005, 35, 471–474. [Google Scholar] [CrossRef]
- Rendy, R.; Zhang, Y.; McElrea, A.; Gomez, A.; Klumpp, D.A. Superacid-catalyzed reactions of cinnamic acids and the role of superelectrophiles. J. Org. Chem. 2004, 69, 2340–2347. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Silva, A.M.S.; Cavaleiro, J.A.S.; Elguero, J. New bis(chalcones) and their transformation into bis(pyrazoline) and bis(pyrazole) derivatives. Eur. J. Org. Chem. 2003, 4, 747–755. [Google Scholar] [CrossRef]
- Lee, D.Y.; Cho, C.S.; Jiang, L.H.; Wu, X.; Shim, S.C.; Oh, D.H. Palladium-catalyzed synthesis of 3-alkylphthalides via carbonylative cyclization of o-bromobenzaldehyde with 1,3-dicarbonyl compounds. Synth. Commun. 1997, 27, 3449–3455. [Google Scholar] [CrossRef]
- Houbion, J.A. Synthesis of 3-phenactlidenephthalides, 3-phenacylidenephtalimidines and 3-phenacylidenethiophthalides. Am. Chem. Soc. 1981, 181, 229. [Google Scholar]
- Sangshetti, J.N.; Ansari, S.A.M.K.; Shinde, D.B. ZrOCl2 center dot H2O catalyzed solvent-free synthesis of isobenzofuran-1(3H)-ones. Chin. Chem. Lett. 2011, 22, 163–166. [Google Scholar] [CrossRef]
- Limaye, R.A.; Kumbhar, V.B.; Natu, A.D.; Paradkar, M.V.; Honmore, V.S.; Chauhan, R.R.; Gample, S.P.; Sarkar, D. One pot solvent free synthesis and in vitro antitubercular screening of 3-Aracylphthalides against Mycobacterium tuberculosis. Bioorg. Med. Chem. Lett. 2013, 23, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Houbion, J.A.; Schafer, D.E. Treating Rice Seeds and Plants to Prevent Herbicidal Damage by Application of 3-Acyl-methyl-phthalide. U.S. Patent US4185989-A, 28 July 1978. [Google Scholar]
- Rahmani, F.; Mohammadpoor-Baltork, I.; Khosropour, A.R.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V. Propylphosphonium hydrogen carbonate ionic liquid supported on nano-silica as a reusable catalyst for the efficient multicomponent synthesis of fully substituted pyridines and bis-pyridines. RSC Adv. 2015, 5, 39978–39991. [Google Scholar] [CrossRef]
- Bousquet, E.W.; Moran, M.D.; Harmon, J.; Johnson, A.L.; Summers, J.C. Synthesis of 3,3a-dihydro-8H-pyrazolo [5,1-a] isoindolo-8-ones and 8H-pyrazolo[5,1-a]isoindol-8-ones. J. Org. Chem. 1975, 40, 2208–2211. [Google Scholar] [CrossRef]
- Yaremenko, A.G.; Shelyakin, V.V.; Volochnyuk, D.M.; Rusanov, E.B.; Grygorenko, O.O. An approach to dihydroisoindolobenzodiazepinones-three-dimensional molecular frameworks. Tetrahedron Lett. 2013, 54, 1195–1197. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 4a–4m and 5a–5m are available from the authors.


| Compound | R | Yield (%) | Melting Points (°C) | |
|---|---|---|---|---|
| Found | Literature [Reference] | |||
| 4a | C6H5 | 74 | 142–44 | 146–147 [42] |
| 4b | 4-ClC6H4 | 65 | 139–41 | 146 [48] |
| 4c | 3-ClC6H4 | 76 | 136–37 | 142–144 [51] |
| 4d | 2-ClC6H4 | 79 | 95–96 | 91–92 [47] |
| 4e | 4-BrC6H4 | 45 | 144–45 | 147–149 [42] |
| 4f | 3-BrC6H4 | 75 | 127–29 | 124–127 [52] |
| 4g | 2-BrC6H4 | 67 | 107–108 | — |
| 4h | 4-FC6H4 | 81 | 135–36 | 130–133 [52] |
| 4i | 2-FC6H4 | 62 | 116–117 | 114 [47] |
| 4j | 3-CH3-C6H4 | 56 | 108–109 | 104–105 [47] |
| 4k | 2-CH3C6H4 | 63 | 102–103 | — |
| 4l | 3-CH3OC6H4 | 68 | 110–111 | 134–135 [42] |
| 4m | 2-Thienyl | 54 | 135–137 | 138 [49] |
| R | Compound | Yield (%) |
|---|---|---|
| C6H5 | 5a | 74 |
| 4-ClC6H4 | 5b | 75 |
| 3-ClC6H4 | 5c | 73 |
| 2-ClC6H4 | 5d | 73 |
| 4-BrC6H4 | 5e | 85 |
| 3-BrC6H4 | 5f | 72 |
| 2-BrC6H4 | 5g | 27 |
| 4-FC6H4 | 5h | 72 |
| 3-FC6H4 | 5i | 55 |
| 3-CH3C6H4 | 5j | 68 |
| 2-CH3C6H4 | 5k | 40 |
| 3-CH3OC6H4 | 5l | 62 |
| 2-Thienyl | 5m | 32 |
| Proton [b] | Compound 5a | Compound 5b | Compound 5f | Compound 5h |
|---|---|---|---|---|
| 7<‘> | 2.30 | 2.30 | 2.30 | 2.31 |
| 7<‘‘> | 3.66 | 3.59 | 3.58 | 3.60 |
| 14<‘> | 3.71 | 3.66 | 3.67 | 3.68 |
| 7a | 4.55 | 4.52 | 4.53 | 4.53 |
| 14<‘‘> | 5.31 | 5.31 | 5.32 | 5.31 |
| 4 | 7.03 | 7.02 | 7.02 | 7.02 |
| 2 | 7.13 | 7.14 | 7.15 | 7.14 |
| 3 | 7.30 | 7.30 | 7.31 | 7.23–7.33 |
| 10 | 7.47 | 7.49 | 7.43–7.51 | 7.48 |
| 9 | 7.52–7.65 | 7.53–7.63 | 7.55–7.63 | 7.55–7.62 |
| 8 | 7.52–7.65 | 7.53–7.63 | 7.55–7.63 | 7.55–7.62 |
| 4’ | 7.52–7.65 | — | 7.72 | — |
| 3’ | 7.52–7.65 | 7.53–7.63 | — | 7.23–7.33 |
| 5’ | 7.52–7.65 | 7.53–7.63 | 7.43–7.51 | 7.23–7.33 |
| 1 | 7.76 | 7.76 | 7.76 | 7.76 |
| 11 | 7.83 | 7.83 | 7.83 | 7.83 |
| 2’ | 8.18 | 8.13 | 8.36 | 8.19 |
| 6’ | 8.18 | 8.13 | 8.07 | 8.19 |
| Carbon | Compound 5a | Compound 5b | Compound 5f | Compound 5h | |
|---|---|---|---|---|---|
| 7 | 36.28 | 36.19 | 36.30 | 36.24 | |
| 14 | 42.02 | 42.00 | 41.99 | 42.01 | |
| 7a | 56.11 | 56.30 | 56.22 | 56.33 | |
| 4 | 121.21 | 121.14 | 121.11 | 121.19 | |
| 8 | 121.91 | 121.84 | 121.89 | 121.84 | |
| 11 | 123.99 | 124.07 | 124.06 | 124.06 | |
| 2 | 125.09 | 125.28 | 125.40 | 125.19 | |
| 14a | 126.16 | 126.08 | 125.95 | 126.14 | |
| 2’ | 127.74 | 129.04 | 131.02 | 129.92 | 3JC-F 8.7 Hz |
| 6’ | 127.80 | 129.08 | 126.09 | 129.86 | 3JC-F 8.7 Hz |
| 3 | 128.80 | 128.84 | 128.87 | 128.83 | |
| 10 | 128.84 | 128.94 | 128.96 | 128.92 | |
| 3’ | 129.00 | 129.40 | 123.57 | 116.09 | 2JC-F 21.7 Hz |
| 5’ | 129.13 | 129.34 | 130.54 | 116.23 | 2JC-F 21.7 Hz |
| 4’ | 131.42 | 137.76 | 134.29 | 164.80 | 1JC-F 252.9 Hz |
| 1 | 131.64 | 131.68 | 131.69 | 131.66 | |
| 9 | 131.78 | 131.85 | 131.88 | 131.82 | |
| 11a | 132.62 | 132.59 | 132.57 | 132.60 | |
| 1’ | 137.18 | 135.58 | 139.20 | 133.38 | |
| 7b | 143.90 | 143.69 | 143.66 | 143.75 | |
| 4a | 148.78 | 148.50 | 148.35 | 148.58 | |
| 6 | 165.68 | 164.43 | 164.23 | 164.35 | |
| 12 | 167.16 | 167.12 | 167.12 | 167.13 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassin, J.P.; Anagani, B.; Benham, C.; Goyal, M.; Hashemian, M.; Gerhard, U. Synthesis and Spectral Characterization of Benzo-[6,7][1,5]diazocino[2,1-a]isoindol-12-(14H)-one Derivatives. Molecules 2016, 21, 967. https://doi.org/10.3390/molecules21080967
Bassin JP, Anagani B, Benham C, Goyal M, Hashemian M, Gerhard U. Synthesis and Spectral Characterization of Benzo-[6,7][1,5]diazocino[2,1-a]isoindol-12-(14H)-one Derivatives. Molecules. 2016; 21(8):967. https://doi.org/10.3390/molecules21080967
Chicago/Turabian StyleBassin, Jatinder P., Bhavani Anagani, Christopher Benham, Madhu Goyal, Maryam Hashemian, and Ute Gerhard. 2016. "Synthesis and Spectral Characterization of Benzo-[6,7][1,5]diazocino[2,1-a]isoindol-12-(14H)-one Derivatives" Molecules 21, no. 8: 967. https://doi.org/10.3390/molecules21080967