Neuroprotection by Combined Administration with Maslinic Acid, a Natural Product from Olea europaea, and MK-801 in the Cerebral Ischemia Model
Abstract
:1. Introduction
2. Results
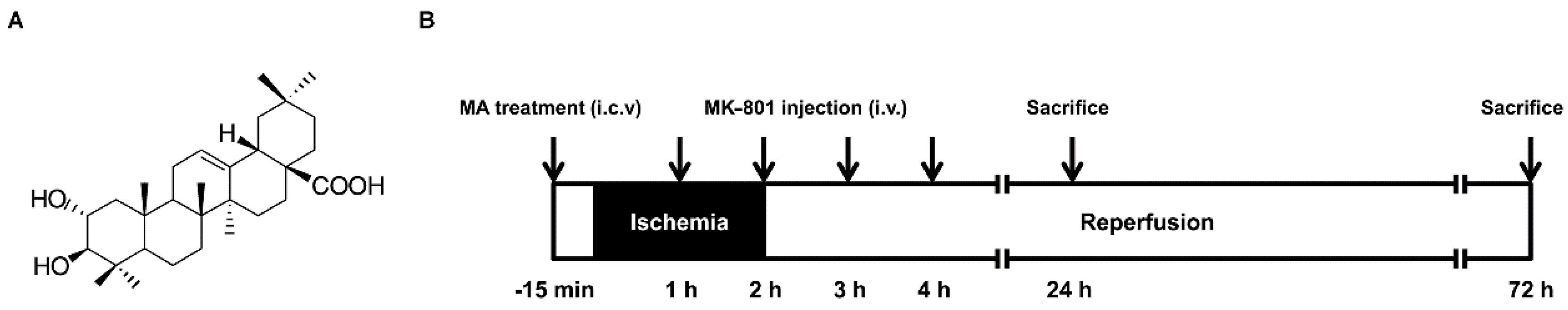
2.1. Physiological Variables
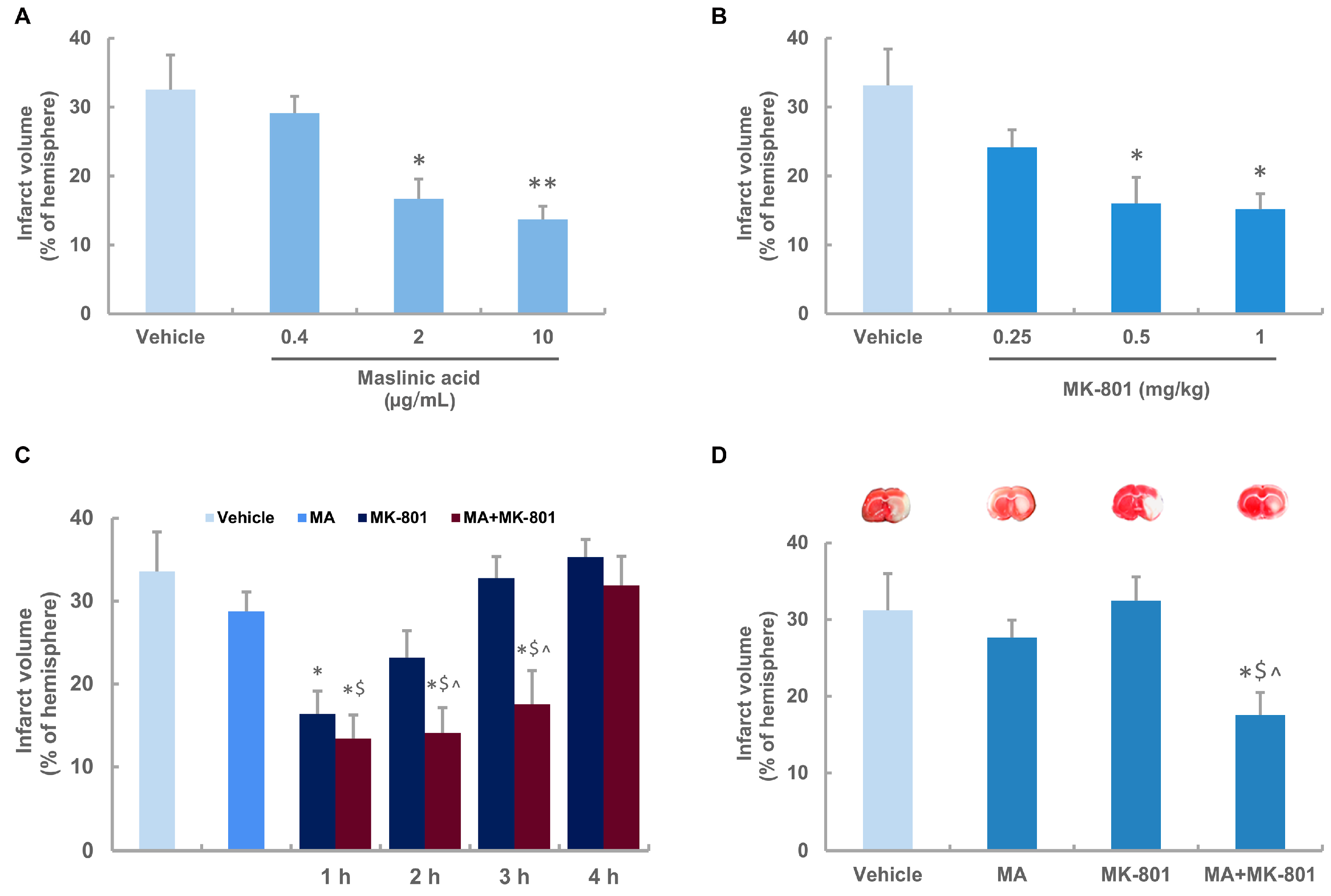
2.2. Neuroprotective Effects of Maslinic Acid and MK-801 on Focal Cerebral Ischemia in Rats
2.3. Maslinic Acid Extends the Time Window and Decreases the Dosage of MK-801 in Ischemic Rats
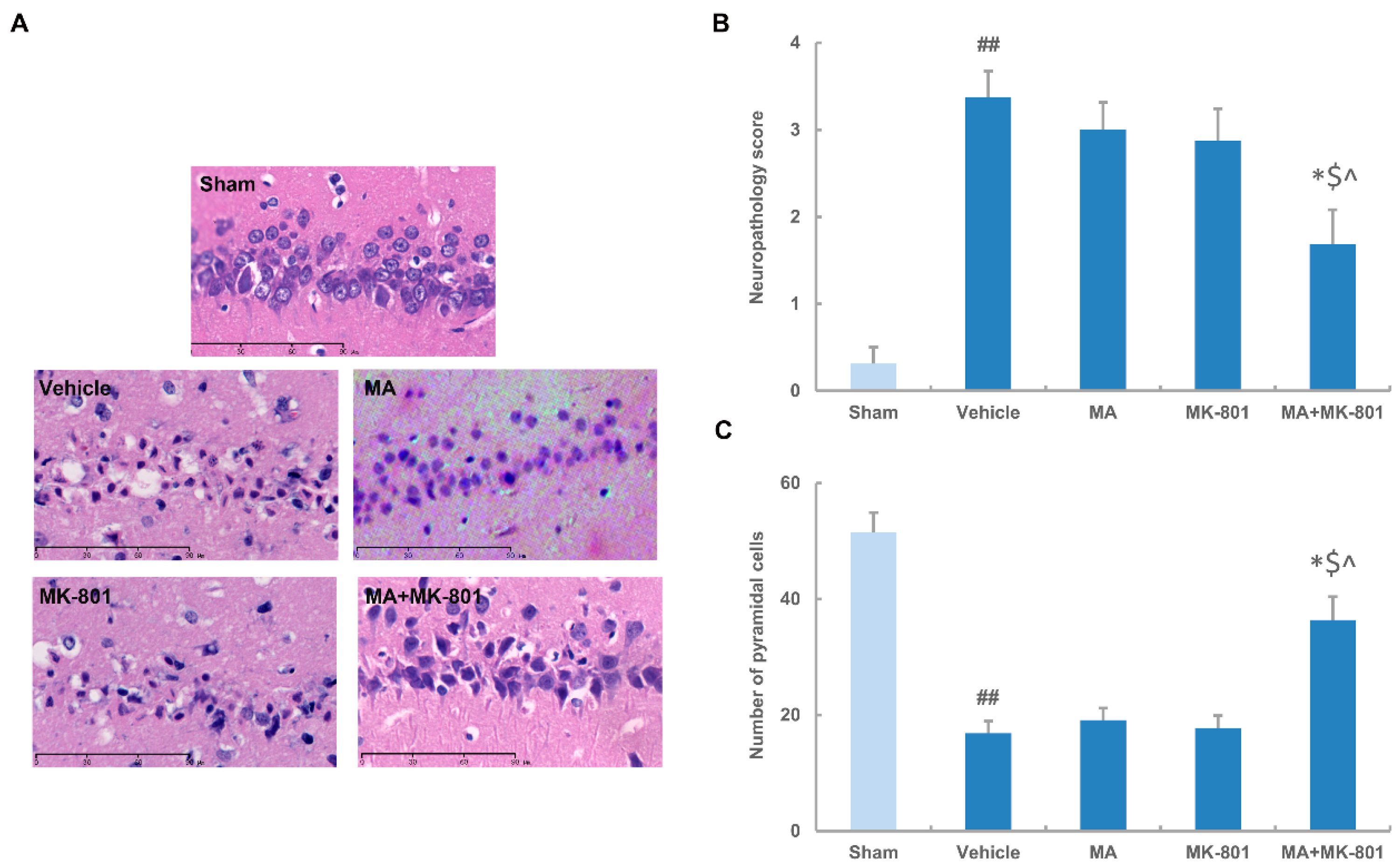
2.4. MK-801 Combined with Maslinic Acid Improves the Outcome in Rats Subjected to Cerebral Ischemia
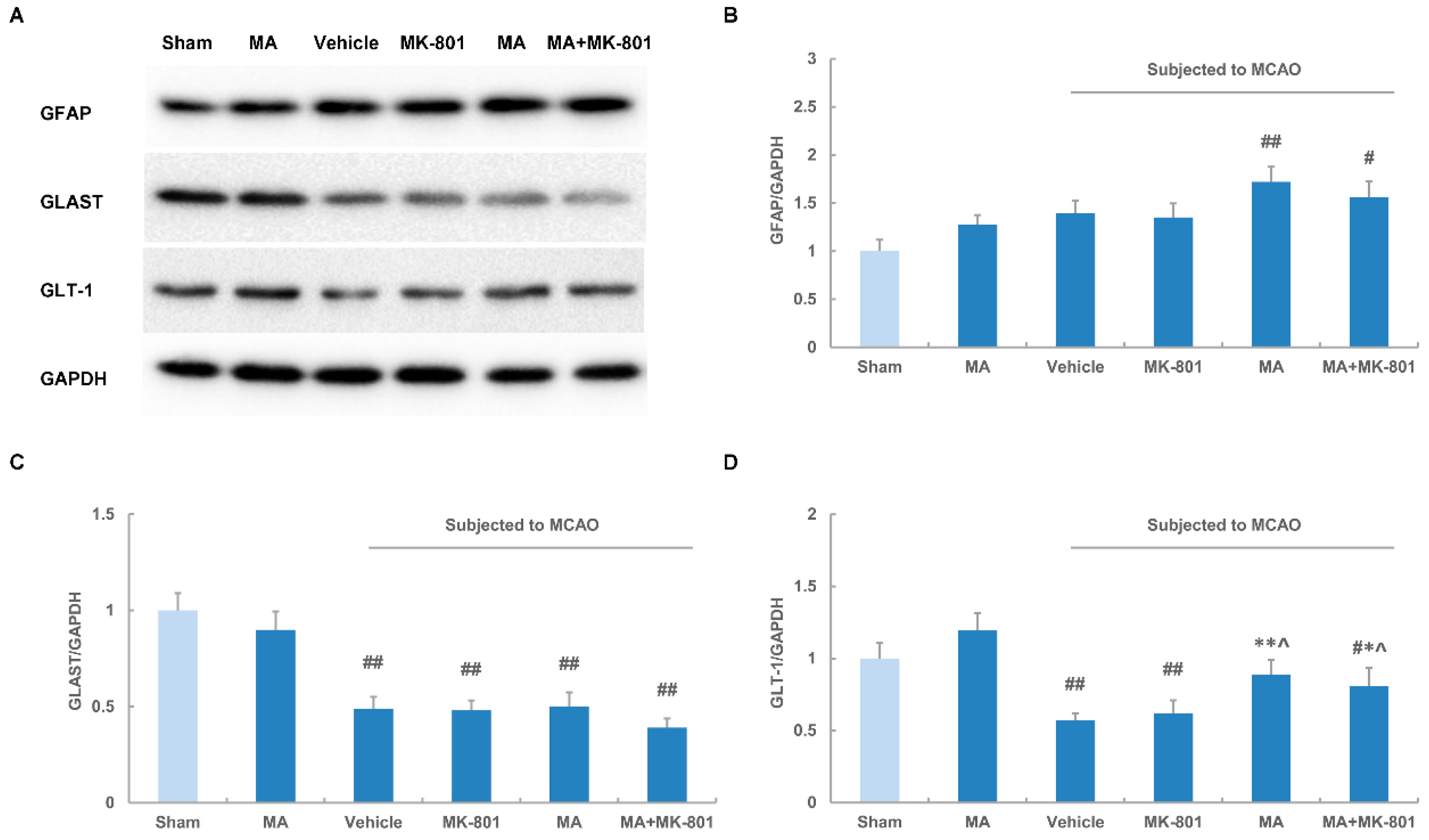
2.5. Maslinic Acid Up-Regulates the Expression of GLT-1 after Cerebral Ischemia
3. Discussion
4. Material and Methods
4.1. Materials
4.2. Animal Model
4.3. Drug Administration
4.4. Measurement of Infarct Volume
4.5. Histology
4.6. Immunohistochemistry
4.7. Western Blot
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lopez-Atalaya, J.P.; Roussel, B.D.; Levrat, D.; Parcq, J.; Nicole, O.; Hommet, Y.; Benchenane, K.; Castel, H.; Leprince, J.; To Van, D.; et al. Toward safer thrombolytic agents in stroke: Molecular requirements for NMDA receptor-mediated neurotoxicity. J. Cereb. Blood Flow Metab. 2008, 28, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- O’Collins, V.E.; Macleod, M.R.; Donnan, G.A.; Horky, L.L.; van der Worp, B.H.; Howells, D.W. 1026 experimental treatments in acute stroke. Ann. Neurol. 2006, 59, 467–477. [Google Scholar] [PubMed]
- Chamorro, A.; Dirnagl, U.; Urra, X.; Planas, A.M. Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016, 15, 869–881. [Google Scholar] [CrossRef]
- Tapia, R. Release and uptake of glutamate as related to excitotoxicity. Rev. Brasil. Biol. 1996, 56 Pt 1 (Suppl. 1), 165–174. [Google Scholar] [PubMed]
- Volterra, A.; Bezzi, P.; Rizzini, B.L.; Trotti, D.; Ullensvang, K.; Danbolt, N.C.; Racagni, G. The competitive transport inhibitor l-trans-pyrrolidine-2,4-dicarboxylate triggers excitotoxicity in rat cortical neuron-astrocyte co-cultures via glutamate release rather than uptake inhibition. Eur. J. Neurosci. 1996, 8, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Faillace, M.P.; Sarmiento, M.I.; Rosenstein, R.E. Melatonin effect on [3H] glutamate uptake and release in the golden hamster retina. J. Neurochem. 1996, 67, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Gerasimov, V.D.; Artemenko, D.P.; Krishtal, O.A. Therapeutic time window for the neuroprotective action of MK-801 after decapitation ischemia: Hippocampal slice data. Brain Res. 2004, 1017, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Nito, C.; Kamiya, T.; Ueda, M.; Arii, T.; Katayama, Y. Mild hypothermia enhances the neuroprotective effects of FK506 and expands its therapeutic window following transient focal ischemia in rats. Brain Res. 2004, 1008, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Yam, P.S.; Dunn, L.T.; Graham, D.I.; Dewar, D.; McCulloch, J. NMDA receptor blockade fails to alter axonal injury in focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2000, 20, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Pignataro, G.; Simon, R.P.; Xiong, Z.G. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain 2007, 130 Pt 1, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Vlahov, G.; Rinaldi, G.; del Re, P.; Giuliani, A.A. 13C nuclear magnetic resonance spectroscopy for determining the different components of epicuticular waxes of olive fruit (Olea europaea) Dritta cultivar. Anal. Chim. Acta 2008, 624, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhang, P.; Liu, J.; Zhang, L.; Wu, X.; Ni, P.; Sun, H. Pentacyclic triterpenes. Part 2: Synthesis and biological evaluation of maslinic acid derivatives as glycogen phosphorylase inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Li, Y.; Sun, H.; Tang, X.; Qian, Y. Effects of maslinic acid, a natural triterpene, on glycogen metabolism in cultured cortical astrocytes. Planta Med. 2009, 75, 1141–1143. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Chen, Y.P.; Mao, L.F.; Shang, J.; Sun, H.B.; Zhang, L.Y. Maslinic acid modulates glycogen metabolism by enhancing the insulin signaling pathway and inhibiting glycogen phosphorylase. Chin. J. Nat. Med. 2014, 12, 259–265. [Google Scholar] [CrossRef]
- Guan, T.; Qian, Y.; Tang, X.; Huang, M.; Huang, L.; Li, Y.; Sun, H. Maslinic acid, a natural inhibitor of glycogen phosphorylase, reduces cerebral ischemic injury in hyperglycemic rats by GLT-1 up-regulation. J. Neurosci. Res. 2011, 89, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Guan, T.; Tang, X.; Huang, L.; Huang, M.; Li, Y.; Sun, H.; Yu, R.; Zhang, F. Astrocytic glutamate transporter-dependent neuroprotection against glutamate toxicity: An in vitro study of maslinic acid. Eur. J. Pharmacol. 2011, 651, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Danbolt, N.C. Glutamate uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [CrossRef]
- Sattler, R.; Tymianski, M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol. Neurobiol. 2001, 24, 107–129. [Google Scholar] [CrossRef]
- Chen, Y.; Swanson, R.A. Astrocytes and brain injury. J. Cereb. Blood Flow Metab. 2003, 23, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Hazell, A.S. Excitotoxic mechanisms in stroke: An update of concepts and treatment strategies. Neurochem. Int. 2007, 50, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra Rao, V.L.; Rao, A.M.; Dogan, A.; Bowen, K.K.; Hatcher, J.; Rothstein, J.D.; Dempsey, R.J. Glial glutamate transporter GLT-1 down-regulation precedes delayed neuronal death in gerbil hippocampus following transient global cerebral ischemia. Neurochem. Int. 2000, 36, 531–537. [Google Scholar] [CrossRef]
- Barreto, G.; White, R.E.; Ouyang, Y.; Xu, L.; Giffard, R.G. Astrocytes: Targets for neuroprotection in stroke. Cent. Nervous Syst. Agents Med. Chem. 2011, 11, 164–173. [Google Scholar] [CrossRef]
- Sidoryk-Wegrzynowicz, M.; Wegrzynowicz, M.; Lee, E.; Bowman, A.B.; Aschner, M. Role of astrocytes in brain function and disease. Toxicologic pathology 2011, 39, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Nawashiro, H.; Brenner, M.; Fukui, S.; Shima, K.; Hallenbeck, J.M. High susceptibility to cerebral ischemia in GFAP-null mice. J. Cereb. Blood Flow Metab. 2000, 20, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, A.E.; Berlinger, E.; Langer, J.; Kafitz, K.W.; Rose, C.R. Lesion-induced alterations in astrocyte glutamate transporter expression and function in the hippocampus. ISRN Neurol. 2013, 2013, 893605. [Google Scholar] [CrossRef] [PubMed]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Sobrado, M.; Lopez, M.G.; Carceller, F.; Garcia, A.G.; Roda, J.M. Combined nimodipine and citicoline reduce infarct size, attenuate apoptosis and increase bcl-2 expression after focal cerebral ischemia. Neuroscience 2003, 118, 107–113. [Google Scholar] [CrossRef]
- Yang, Y.; Shuaib, A.; Li, Q. Quantification of infarct size on focal cerebral ischemia model of rats using a simple and economical method. J. Neurosci. Methods 1998, 84, 9–16. [Google Scholar] [CrossRef]
- Lee, S.R.; Tsuji, K.; Lo, E.H. Role of matrix metalloproteinases in delayed neuronal damage after transient global cerebral ischemia. J. Neurosci. 2004, 24, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Bowerman, M.; Beauvais, A.; Anderson, C.L.; Kothary, R. Rho-kinase inactivation prolongs survival of an intermediate SMA mouse model. Hum. Mol. Genet. 2010, 19, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Zhang, H.L.; Han, R.; Gu, Z.L.; Qin, Z.H. Enhancement of neuroprotection and heat shock protein induction by combined prostaglandin A1 and lithium in rodent models of focal ischemia. Brain Res. 2006, 1102, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Muranyi, M.; Ding, C.; He, Q.; Lin, Y.; Li, P.A. Streptozotocin-induced diabetes causes astrocyte death after ischemia and reperfusion injury. Diabetes 2006, 55, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds MK-801 are available from the authors.

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, Y.; Tang, X.; Guan, T.; Li, Y.; Sun, H. Neuroprotection by Combined Administration with Maslinic Acid, a Natural Product from Olea europaea, and MK-801 in the Cerebral Ischemia Model. Molecules 2016, 21, 1093. https://doi.org/10.3390/molecules21081093
Qian Y, Tang X, Guan T, Li Y, Sun H. Neuroprotection by Combined Administration with Maslinic Acid, a Natural Product from Olea europaea, and MK-801 in the Cerebral Ischemia Model. Molecules. 2016; 21(8):1093. https://doi.org/10.3390/molecules21081093
Chicago/Turabian StyleQian, Yisong, Xuzhen Tang, Teng Guan, Yunman Li, and Hongbin Sun. 2016. "Neuroprotection by Combined Administration with Maslinic Acid, a Natural Product from Olea europaea, and MK-801 in the Cerebral Ischemia Model" Molecules 21, no. 8: 1093. https://doi.org/10.3390/molecules21081093




