Neuroprotective Effects of Methyl 3,4-Dihydroxybenzoate against TBHP-Induced Oxidative Damage in SH-SY5Y Cells
Abstract
:1. Introduction
2. Results
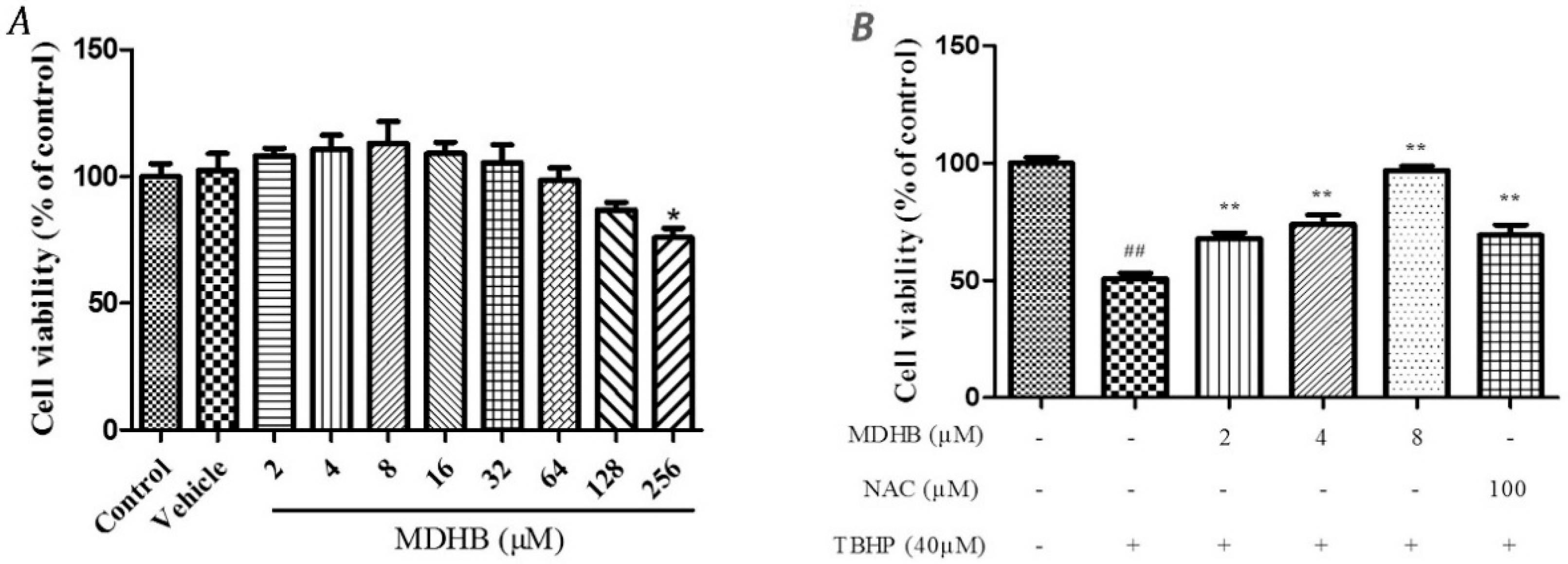
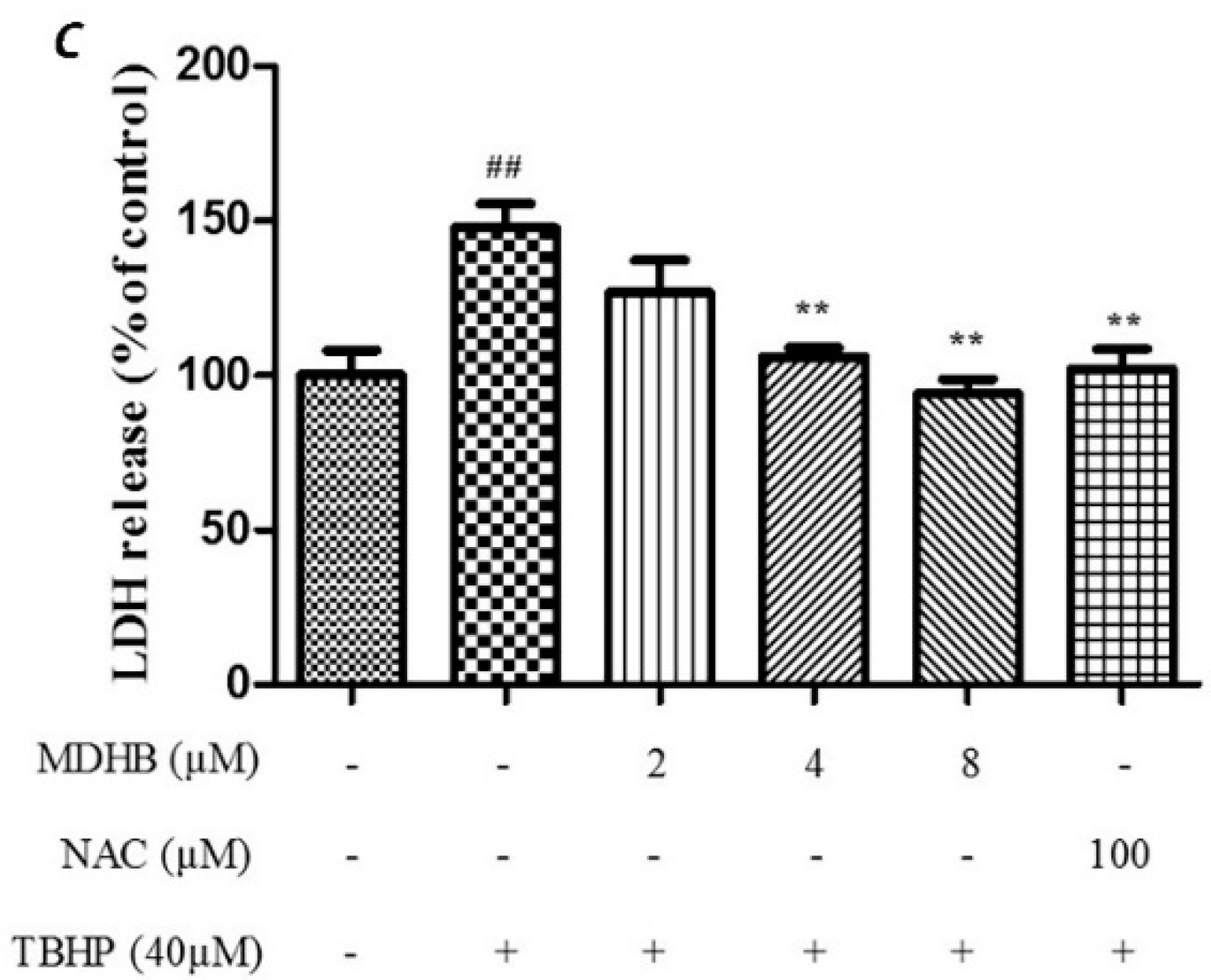
2.1. The Effect of MDHB on SH-SY5Y Cell Viability
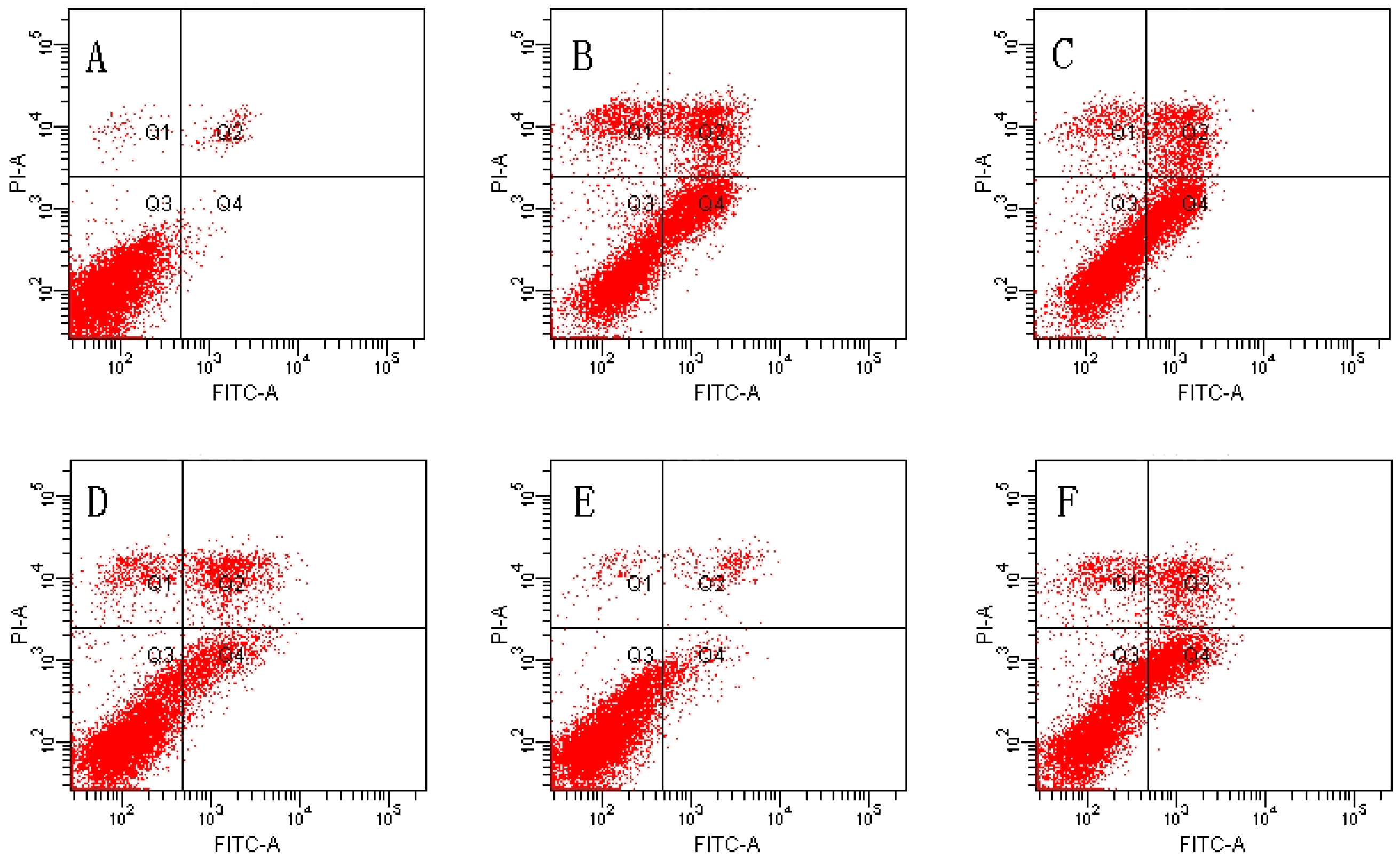
2.2. Effect of MDHB against TBHP-Induced Apoptosis in SH-SY5Y Cells
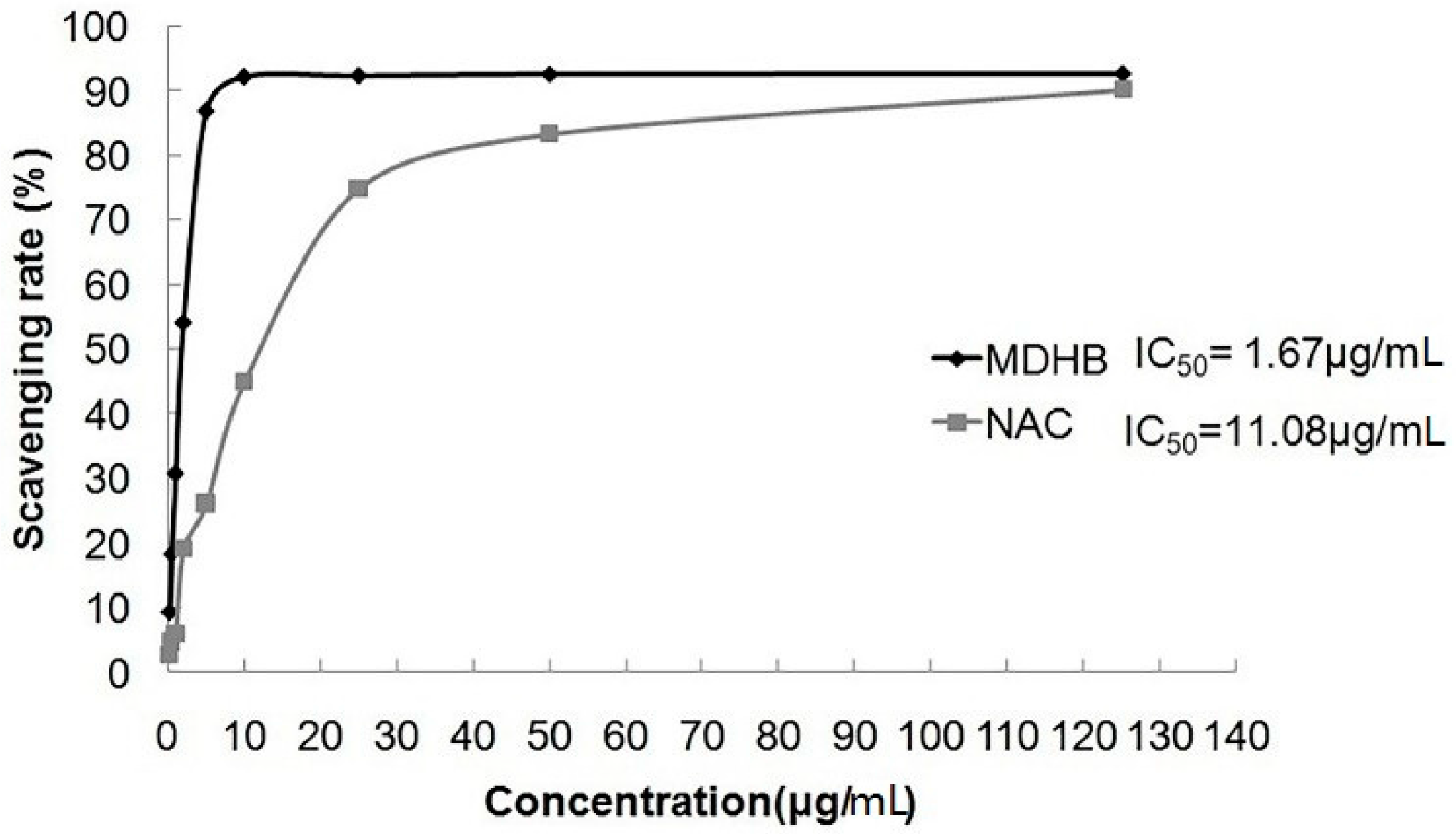
2.3. Effect of MDHB on DPPH Free Radical
2.4. Effect of MDHB on Intracellular ROS, SOD and GSH-Px Activity in SH-SY5Y Cells
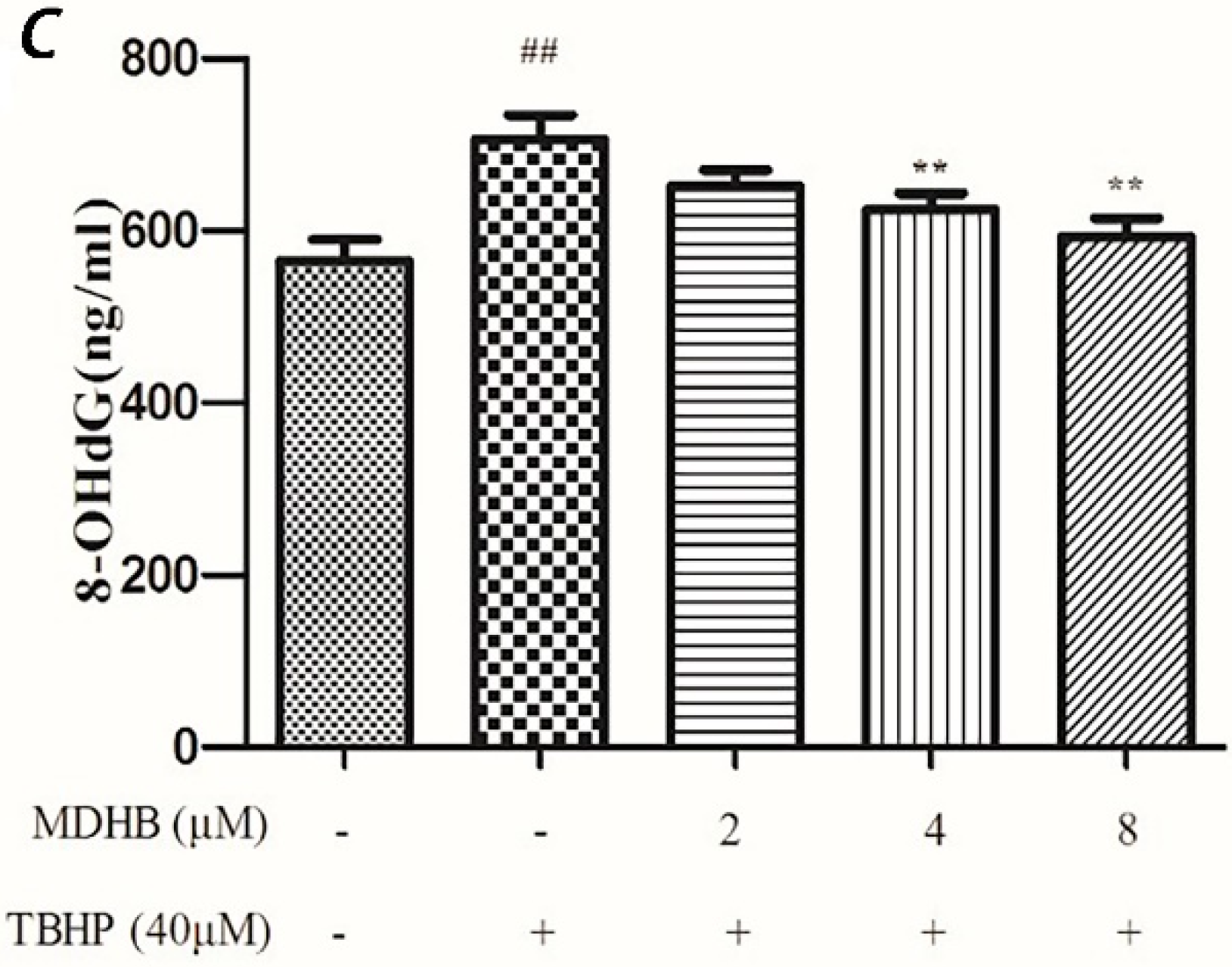
2.5. Effect of MDHB on 8-OHdG Levels in the Cultured Media of SH-SY5Y Cells
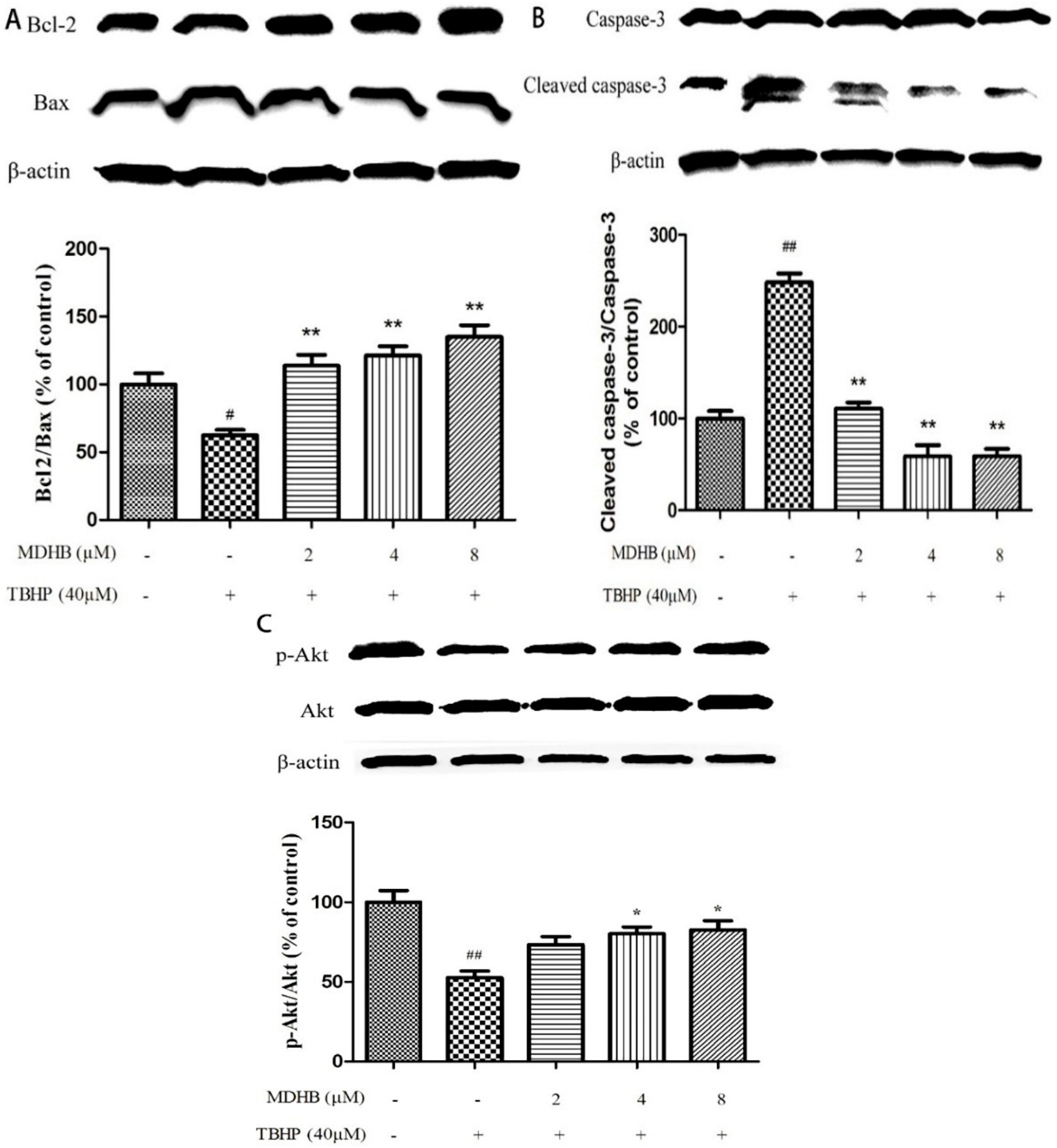
2.6. Effect of MDHB on Protein Expression of the Related Protein
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Culture of SH-SY5Y Cells
4.3. MTT Assay
4.4. LDH Assay
4.5. Detection of Cell Apoptosis by Flow Cytometry
4.6. Chemical Simulation System to Detect the Scavenging Ability of MDHB on DPPH Free Radicals
4.7. Detection of Cell Reactive Oxygen Species Using the DCFH-DA Assay by Flow Cytometry
4.8. Determination of Intracellular SOD Activity
4.9. Detection of 8-Hydroxydeoxyguanosine by ELISA
4.10. Detection of Related Proteins Using Western Blotting
4.11. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MDHB | Methyl 3,4-dihydroxybenzoate |
| TBHP | t-Butylhydroperoxide |
| NAC | N-Acetyl-l-cysteine |
| ROS | Reactive oxygen species |
| LDH | Lactic dehydrogenase |
| SOD | Superoxide dismutase |
| GSH-Px | Glutathione peroxidase |
| 8-OhdG | 8-Hydroxy-2-deoxyguanosine |
| DPPH | α,α-Diphenyl-β-picrylhydrazyl |
| Nrf2 | Nuclear factor E2-related factor 2 |
References
- Jomova, K.; Vondrakova, D.; Lawson, M.; Valko, M. Metals, oxidative stress and neurodegenerative disorders. Mol. Cell. Biochem. 2010, 345, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Barone, E.; Cenini, G.; di Domenico, F.; Martin, S.; Sultana, R.; Mancuso, C.; Murphy, M.P.; Head, E.; Butterfield, D.A. Long-term high-dose atorvastatin decreases brain oxidative and nitrosative stress in a preclinical model of Alzheimer disease: A novel mechanism of action. Pharmacol. Res. 2011, 63, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell. Physiol. 2002, 192, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Von Arnim, C.A.; Gola, U.; Biesalski, H.K. More than the sum of its parts? Nutrition in Alzheimer’s disease. Nutrition 2010, 26, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.; Grunblatt, E.; Riederer, P.; Gerlach, M.; Levites, Y.; Youdim, M.B. Neuroprotective strategies in Parkinson’s disease: An update on progress. CNS Drugs 2003, 17, 729–762. [Google Scholar] [CrossRef] [PubMed]
- Seet, R.C.; Lee, C.Y.; Lim, E.C.; Tan, J.J.; Quek, A.M.; Chong, W.L.; Looi, W.F.; Huang, S.H.; Wang, H.; Chan, Y.H.; et al. Oxidative damage in Parkinson disease: Measurement using accurate biomarkers. Free Radic. Biol. Med. 2010, 48, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Budihardjo, I.; Oliver, H.; Lutter, M.; Luo, X.; Wang, X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell. Dev. Biol. 1999, 15, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Kubli, D.A.; Gustafsson, A.B. Mitochondria and mitophagy: The yin and yang of cell death control. Circ. Res. 2012, 111, 1208–1221. [Google Scholar] [CrossRef] [PubMed]
- Caroppi, P.; Sinibaldi, F.; Fiorucci, L.; Santucci, R. Apoptosis and human diseases: mitochondrion damage and lethal role of released cytochrome C as proapoptotic protein. Curr. Med. Chem. 2009, 16, 4058–4065. [Google Scholar] [CrossRef] [PubMed]
- Garcimartin, A.; Merino, J.J.; Gonzalez, M.P.; Sanchez-Reus, M.I.; Sanchez-Muniz, F.J.; Bastida, S.; Benedi, J. Organic silicon protects human neuroblastoma SH-SY5Y cells against hydrogen peroxide effects. BMC Complement. Altern. Med. 2014, 14, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, S.; Plesca, D.; Almasan, A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol. Biol. 2008, 414, 13–21. [Google Scholar] [PubMed]
- Wang, N.; Feng, Y.; Zhu, M.; Tsang, C.M.; Man, K.; Tong, Y.; Tsao, S.W. Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: The cellular mechanism. J. Cell. Biochem. 2010, 111, 1426–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Gong, X.; Zhou, X.; Zhao, C.; Chen, H. Chemical constituents and bioactivity of Kalimeris indica. Zhongguo Zhong Yao Za Zhi 2010, 35, 3172–3174. [Google Scholar] [PubMed]
- Zhang, Z.; Zhou, X.; Zhou, X.; Xu, X.; Liao, M.; Yan, L.; Lv, R.; Luo, H. Methyl 3,4-dihydroxybenzoate promotes neurite outgrowth of cortical neurons cultured in vitro. Neural Regen. Res. 2012, 7, 971–977. [Google Scholar] [PubMed]
- Zhou, X.W.; Zhang, Z.; Su, C.F.; Lv, R.H.; Zhou, X.; Cai, L.; Wang, C.Y.; Yan, L.; Zhang, W.; Luo, H.M. Methyl 3,4-dihydroxybenzoate protects primary cortical neurons against Aβ25–35-induced neurotoxicity through mitochondria pathway. J. Neurosci. Res. 2013, 91, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Su, C.F.; Zhang, Z.; Wang, C.Y.; Luo, J.Q.; Zhou, X.W.; Cai, L.; Yan, L.; Zhang, W.; Luo, H.M. Neuroprotective effects of methyl 3,4-dihydroxybenzoate against H2O2-induced apoptosis in RGC-5 cells. J. Pharmacol. Sci. 2014, 125, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cai, L.; Geng, H.J.; Su, C.F.; Yan, L.; Wang, J.H.; Gao, Q.; Luo, H.M. Methyl 3,4-Dihydroxybenzoate Extends the Lifespan of Caenorhabditis elegans, Partly via W06A7.4 Gene. Exp. Gerontol. 2014, 60, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.O.; Pant, A.B.; Farombi, E.O. 4-Hydroxynonenal induces mitochondrial-mediated apoptosis and oxidative stress in SH-SY5Y human neuronal cells. Basic Clin. Pharmacol. Toxicol. 2012, 110, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- Tezel, G. Oxidative stress in glaucomatous neurodegeneration: Mechanisms and consequences. Prog. Retin. Eye Res. 2006, 25, 490–513. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Gachhui, R.; Sil, P.C. Hepatoprotective properties of kombucha tea against TBHP-induced oxidative stress via suppression of mitochondria dependent apoptosis. Pathophysiology 2011, 18, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, S.; D′Alessio, A.; Sirabella, R.; di Renzo, G.; Annunziato, L. Ca2+-independent caspase-3 but not Ca(2+)-dependent caspase-2 activation induced by oxidative stress leads to SH-SY5Y human neuroblastoma cell apoptosis. J. Neurosci. Res. 2002, 68, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Kanupriya; Prasad, D.; Sai Ram, M.; Sawhney, R.C.; Ilavazhagan, G.; Banerjee, P.K. Mechanism of tert-butylhydroperoxide induced cytotoxicity in U-937 macrophages by alteration of mitochondrial function and generation of ROS. Toxicol. In Vitro 2007, 21, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Gurer, H.; Ercal, N. Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic. Biol. Med. 2000, 29, 927–945. [Google Scholar] [CrossRef]
- Da Silva, D.G.; Ricci, O., Jr.; de Almeida, E.A.; Bonini-Domingos, C.R. Potential utility of melatonin as an antioxidant therapy in the management of sickle cell anemia. J. Pineal. Res. 2015, 58, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, G.F.; Megson, I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, L.; Sun, W.; Tang, J.; Lv, Z.; Xu, Z.; Yu, H. Protective effects of a wheat germ peptide (RVF) against H2O2-induced oxidative stress in human neuroblastoma cells. Biotechnol. Lett. 2014, 36, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Rockenstein, E.; Crews, L.; Masliah, E. Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer′s and Parkinson′s diseases. Neuromol. Med. 2003, 4, 21–36. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Q.; Wu, Y.; Chen, G.; Yue, W.; Liang, Q. Response surface optimized ultrasonic-assisted extraction of flavonoids from Sparganii rhizoma and evaluation of their in vitro antioxidant activities. Molecules 2012, 17, 6769–6783. [Google Scholar] [CrossRef] [PubMed]
- Akanni, O.O.; Owumi, S.E.; Adaramoye, O.A. In vitro studies to assess the antioxidative, radical scavenging and arginase inhibitory potentials of extracts from Artocarpus altilis, Ficus exasperate and Kigelia africana. Asian Pac. J. Trop. Biomed. 2014, 4, S492–S499. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.S.; Olinski, R.; Evans, M.D. Does measurement of oxidative damage to DNA have clinical significance? Clin. Chim. Acta 2006, 365, 30–49. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Ismail, M.; Imam, M.U.; Azmi, N.H.; Fathy, S.F.; Foo, J.B. Mechanistic basis for protection of differentiated SH-SY5Y cells by oryzanol-rich fraction against hydrogen peroxide-induced neurotoxicity. BMC Complement. Altern. Med. 2014, 14, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Hartley, R.C.; Cocheme, H.M.; Murphy, M.P. Mitochondrial pharmacology. Trends Pharmacol. Sci. 2012, 33, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Borner, C. The Bcl-2 protein family: Sensors and checkpoints for life-or-death decisions. Mol. Immunol. 2003, 39, 615–47. [Google Scholar] [CrossRef]
- Tomek, M.; Akiyama, T.; Dass, C.R. Role of Bcl-2 in tumour cell survival and implications for pharmacotherapy. J. Pharm. Pharmacol. 2012, 64, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Hassa, P.O.; Hottiger, M.O. The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Front. Biosci. 2008, 13, 3046–3082. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Fujimura, M.; Noshita, N.; Kim, G.W.; Saito, A.; Hayashi, T.; Narasimhan, P.; Maier, C.M.; Chan, P.H. Neuronal death/survival signaling pathways in cerebral ischemia. NeuroRx 2004, 1, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples are available from the authors.



© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, L.; Wang, L.-F.; Pan, J.-P.; Mi, X.-N.; Zhang, Z.; Geng, H.-J.; Wang, J.-H.; Hu, S.-H.; Zhang, W.; Gao, Q.; et al. Neuroprotective Effects of Methyl 3,4-Dihydroxybenzoate against TBHP-Induced Oxidative Damage in SH-SY5Y Cells. Molecules 2016, 21, 1071. https://doi.org/10.3390/molecules21081071
Cai L, Wang L-F, Pan J-P, Mi X-N, Zhang Z, Geng H-J, Wang J-H, Hu S-H, Zhang W, Gao Q, et al. Neuroprotective Effects of Methyl 3,4-Dihydroxybenzoate against TBHP-Induced Oxidative Damage in SH-SY5Y Cells. Molecules. 2016; 21(8):1071. https://doi.org/10.3390/molecules21081071
Chicago/Turabian StyleCai, Liang, Li-Fang Wang, Jun-Ping Pan, Xiang-Nan Mi, Zheng Zhang, Hai-Ju Geng, Jia-Hui Wang, Song-Hui Hu, Wei Zhang, Qin Gao, and et al. 2016. "Neuroprotective Effects of Methyl 3,4-Dihydroxybenzoate against TBHP-Induced Oxidative Damage in SH-SY5Y Cells" Molecules 21, no. 8: 1071. https://doi.org/10.3390/molecules21081071




