Plant Resources, 13C-NMR Spectral Characteristic and Pharmacological Activities of Dammarane-Type Triterpenoids
Abstract
:1. Introduction
2. Plant Resources of DTT
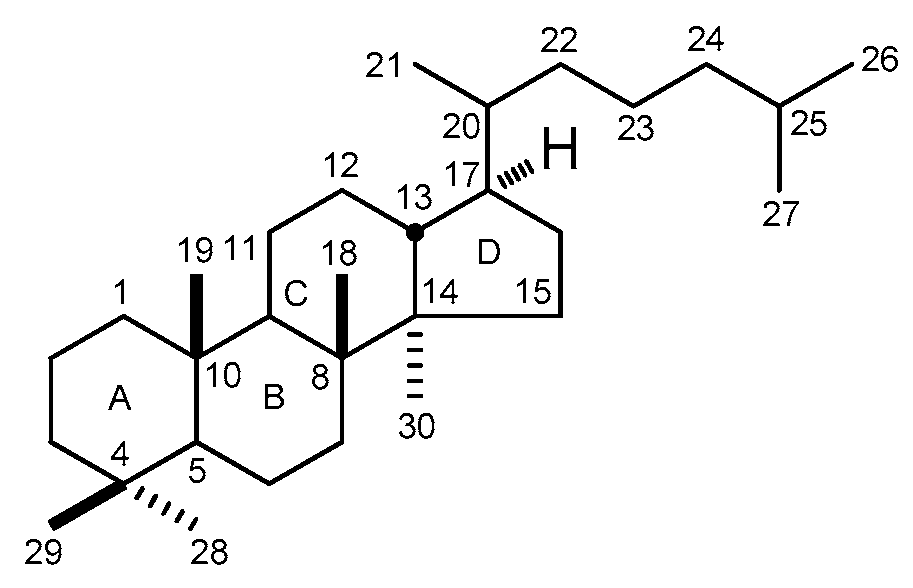
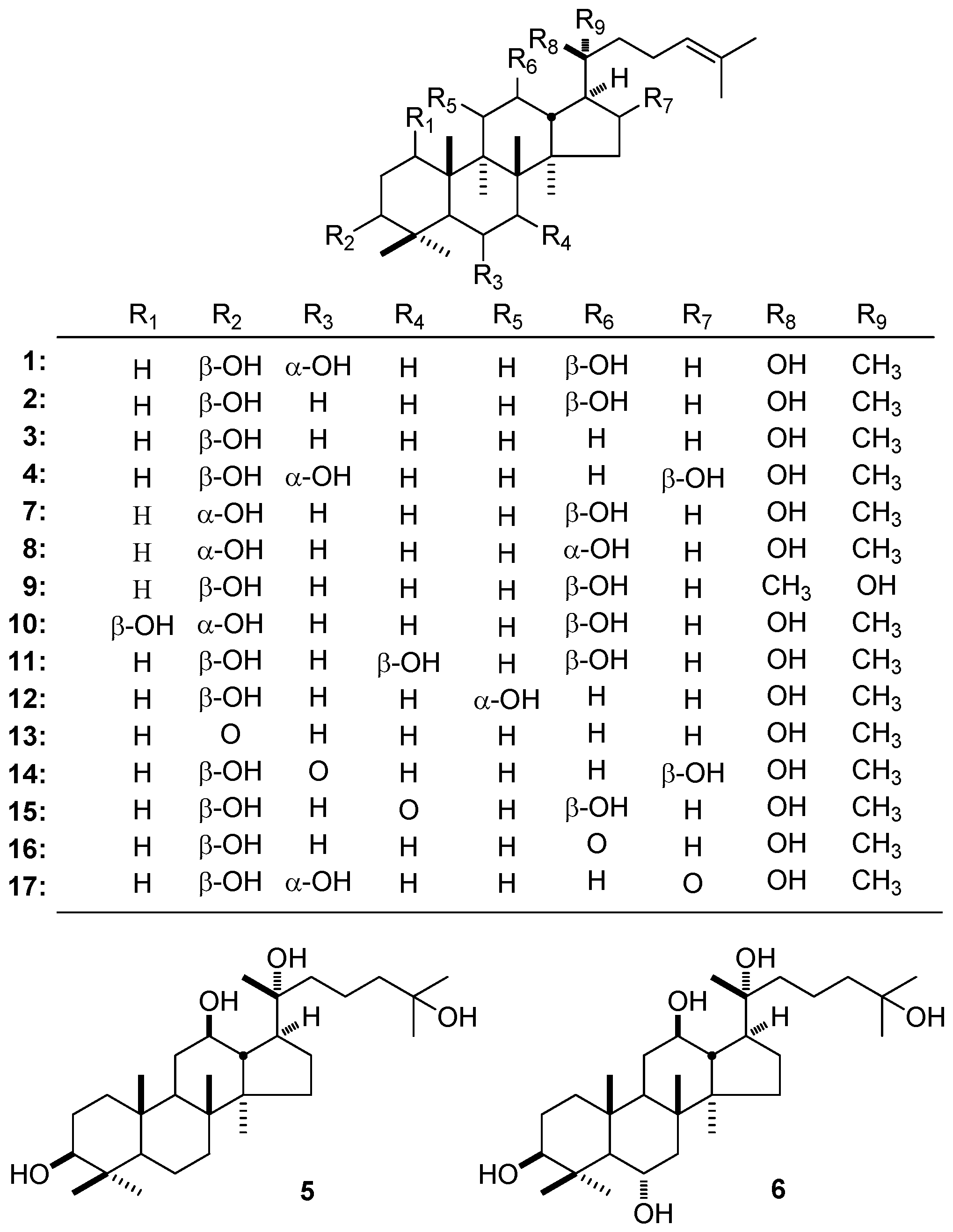
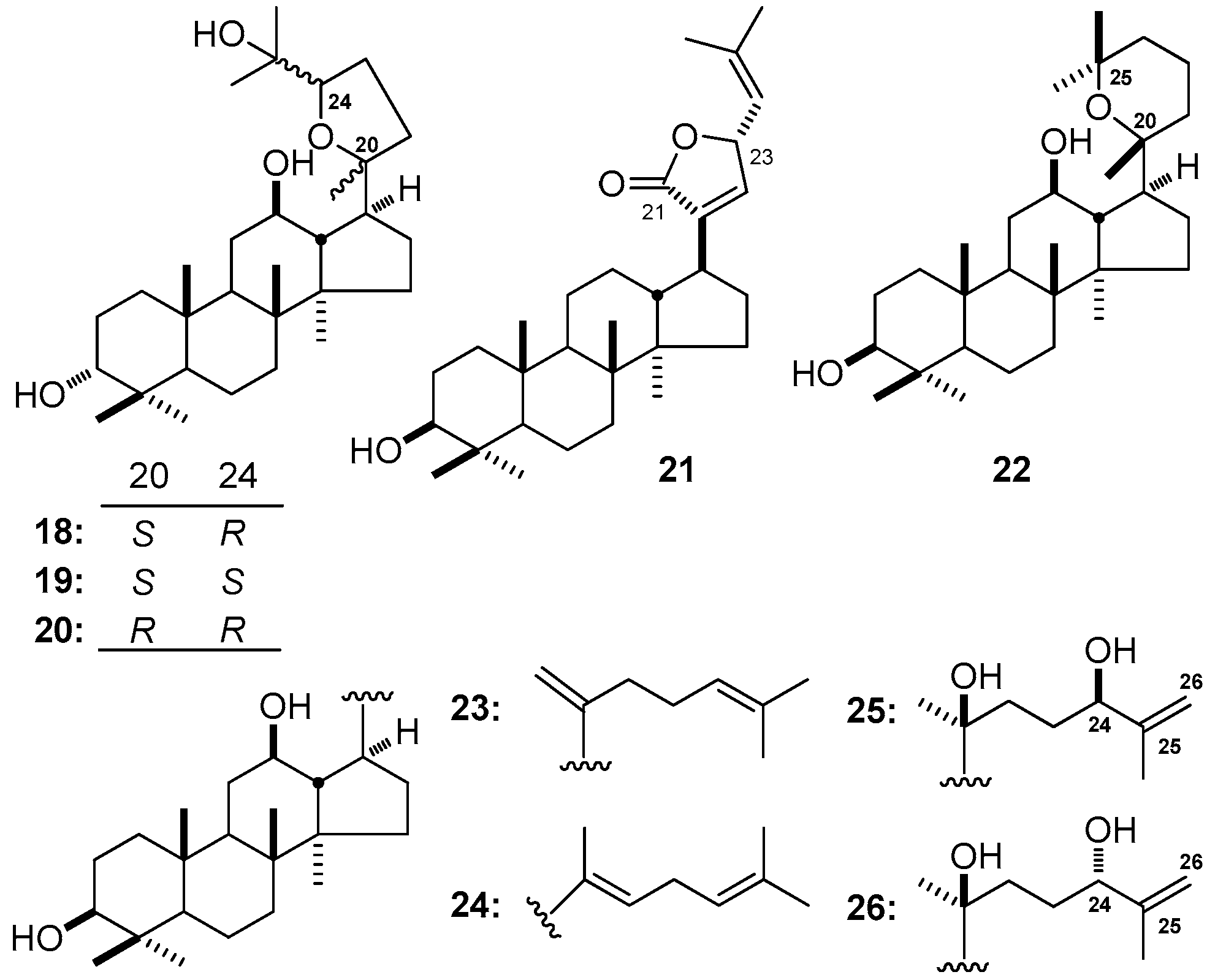
3. NMR Spectral Characteristic of DTT
3.1. Hydroxyl
3.2. Carbonyl
3.3. Cyclization
3.4. Olefinic Bond
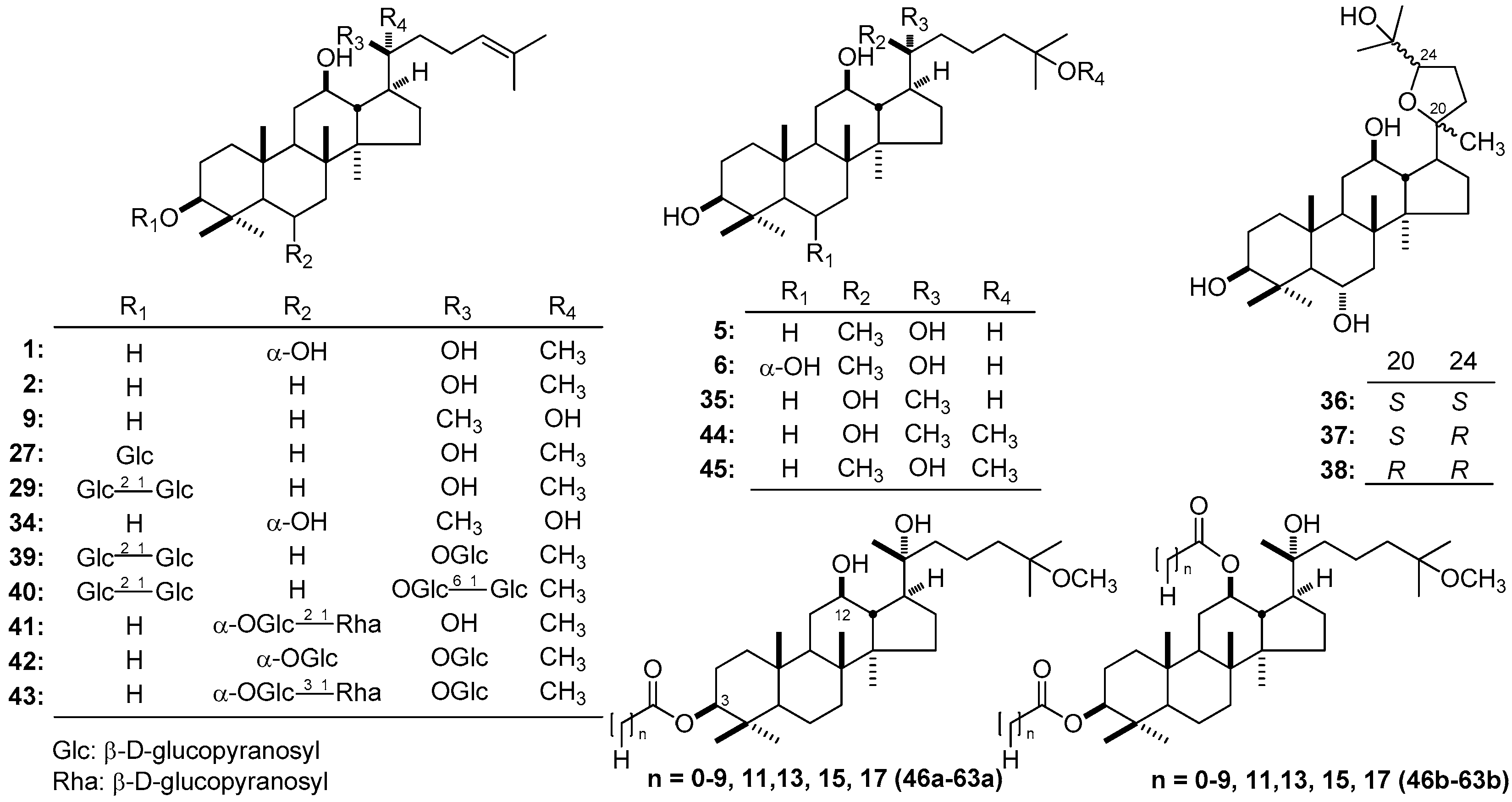
3.5. Glycosyl
4. Pharmacological Effects of DTT
4.1. Anti-Tumor Activity
4.2. Anti-Inflammatory Activity
4.3. Immunomodulatory Activity
4.4. Anti-Diabetic Activity
4.5. Other Biological Activities
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2015, 32, 273–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.W.; Yao, Y.; Shi, Z.H. Observation of curative effect of combined 20(R)-ginsenosided Rg3 with chemotherapy on nonsmall-cell lung cancers. Mil. Med. J. South China 2005, 19, 4–6. [Google Scholar]
- Jin, C.L.; Kou, X.G.; Miao, Z.H. Observation of ginsenosided Rg3 combined with chemotherapy as adjuvant treatment for elder nonsmall-cell lung patients. J. Xinxiang Med. Coll. 2011, 28, 229–232. [Google Scholar]
- Anjaneyulu, V.; Babu, J.S.; Krishna, M.M.; Connolly, J.D. 3-Oxo-20S,24R-epoxy-dammarane-25ξ,26-diol from Mangifera indica. Phytochemistry 1993, 32, 469–471. [Google Scholar] [CrossRef]
- Monaco, P.; Caputo, R.; Palumbo, G.; Mangoni, L. Neutral triterpenes from the galls of Pistacia terebinthus. Phytochemistry 1973, 12, 939–942. [Google Scholar] [CrossRef]
- Kim, G.S.; Jeong, T.S.; Kim, Y.O.; Baek, N.I.; Cha, S.W.; Lee, J.W.; Song, K.S. Human acyl-CoA: Cholesterol acyltransferase-inhibiting dammarane triterpenes from Rhus chinensis. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 417–421. [Google Scholar] [CrossRef]
- Zhao, M.; Fu, L.W.; Li, N.; Tang, W.X.; Li, M.J.; Hasegawa, T.; Ogura, H.; Kataoka, T.; Hirose, K.; Ando, M. The ursane-, oleanane-, dammarane-, lupane-, and taraxasterane-type triterpenes isolated from Nerium oleander and their biological activities. J. Recent Progr. Med. Plants 2007, 16, 83–107. [Google Scholar]
- Siddiqui, B.S.; Ilyas, F.; Rasheed, M.; Begum, S. Chemical constituents of leaves and stem bark of Plumeria obtuse. Phytochemistry 2004, 65, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Choi, Y.H.; Lee, I.R.; Kwon, B.M.; Lee, S.H. A novel nortriterpene from Hedera rhombea. Arch. Pharm. Res. 1997, 20, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, K.; Devkota, H.P.; Fujino, H.; Yahara, S. Saponins composition of rhizomes, taproots, and lateral roots of satsuma-ninjin (Panax japonicus). Chem. Pharm. Bull. 2013, 61, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Y.; Zhou, Q.L.; Yang, X.W. New SIRT1 activator from alkaline hydrolysate of total saponins in the stems-leaves of Panax ginseng. Bioorg. Med. Chem. Lett. 2015, 25, 5321–5325. [Google Scholar] [CrossRef] [PubMed]
- Hamid, K.; Alqahtani, A.; Kim, M.S.; Cho, J.L.; Cui, P.H.; Li, C.G.; Groundwater, P.W.; Li, G.Q. Tetracyclic triterpenoids in herbal medicines and their activities in diabetes and its complications. Curr. Top. Med. Chem. 2015, 15, 2406–2430. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Lu, D.; Li, P.Y. Two new dammarane-type triterpene saponins from red American ginseng. J. Asian Nat. Prod. Res. 2011, 13, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zou, K.; Fushimi, H.; Cai, S.; Komatsu, K. Comparative study on triterpene saponins of Ginseng drugs. Planta Med. 2004, 70, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Bedir, E.; Toyang, N.J.; Khan, I.A.; Walker, L.A.; Clark, A.M. A new dammarane-type triterpene glycoside from Polyscias fulva. J. Nat. Prod. 2001, 64, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Matsunami, K.; Otsuka, H.; Shinzato, T.; Takeda, Y.; Kawahata, M.; Yamaguchi, K. Schefflerins A–G, new triterpene glucosides from the leaves of Schefflera arboricola. Chem. Pharm. Bull. 2010, 58, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Duan, Y.H.; Li, M.M.; Tang, W.; Wu, X.; Wang, G.C.; Ye, W.C.; Zhou, G.X.; Li, Y.L. Triterpenoid saponins from the stem barks of Schefflera heptaphylla. Planta Med. 2013, 79, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.G.; Truong, T.T.C.; Phan, T.S.; Matsunami, K.; Otsuka, H. A new diarylheptanoid and a rare dammarane triterpenoid from Alnus nepalensis. Chem. Nat. Compd. 2011, 47, 735–737. [Google Scholar] [CrossRef]
- Hirata, T.; Ideo, R.; Aoki, T.; Suga, T. The structure of alnuserrutriol, a new C31 dammarane-type triterpenoid from the male flowers of Alnus serrulatoides. Bull. Chem. Soc. Jpn. 1982, 55, 639–640. [Google Scholar] [CrossRef]
- Fuchino, H.; Satoh, T.; Tanaka, N. Chemical evaluation of Betula species in Japan. III. Constituents of Betula maximowicziana. Chem. Pharm. Bull. 1996, 44, 1748–1753. [Google Scholar] [CrossRef]
- Hilpisch, U.; Hartmann, R.; Glombitza, K.W. New dammaranes, esterified with malonic acid, from leaves of Betula pendula. Planta Med. 1997, 63, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Taniguchi, M.; Kashiwada, Y.; Yamagishi, T.; Takaishi, Y. Seven new dammarane triterpenes from the floral spikes of Betula platyphylla var. japonica. J. Nat. Med. 2011, 65, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Fuchino, H.; Satoh, T.; Yokochi, M.; Tanaka, N. Chemical evaluation of Betula species in Japan. V. Constituents of Betula ovalifolia. Chem. Pharm. Bull. 1998, 46, 169–170. [Google Scholar] [CrossRef]
- Kuroyanagi, M.; Kawahara, N.; Sekita, S.; Satake, M.; Hayashi, T.; Takase, Y.; Masuda, K. Dammarane-type triterpenes from the Brazilian medicinal plant Cordia multispicata. J. Nat. Prod. 2003, 66, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Kojima, S.; Lim, Y.A.; Meselhy, M.R.; Hattori, M.; Gupta, M.P.; Correa, M. Dammarane-type triterpenes from Cordia spinescens. Phytochemistry 1997, 46, 1139–1141. [Google Scholar] [CrossRef]
- Vande Velde, V.; Lavie, D.; Zelnik, R.; Matida, A.K.; Panizza, S. Cordialin A and B, two new triterpenes from Cordia verbenacea DC. J. Chem. Soc. Perkin 1 1982, 11, 2697–2700. [Google Scholar] [CrossRef]
- Fattorusso, E.; Santacroce, C.; Xaasan, C.F. Dammarane triterpenes from the resin of Boswellia freerana. Phytochemistry 1985, 24, 1035–1036. [Google Scholar] [CrossRef]
- Manguro, L.O.; Ugi, I.; Lemmen, P. Dammarane triterpenes of Commiphora confusa resin. Chem. Pharm. Bull. (Tokyo) 2003, 51, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Waterman, P.G.; Ampofo, S. Chemistry of the Burseraceae. Part 2. Dammarane triterpenes from the stem bark of Commiphora dalzielii. Phytochemistry 1985, 24, 2925–2928. [Google Scholar]
- Provan, G.J.; Waterman, P.G. Chemistry of the Burseraceae. Part 3. The mansumbinanes: Octanordammaranes from the resin of Commiphora incisa. Phytochemistry 1986, 25, 917–922. [Google Scholar] [CrossRef]
- Manguro, L.O.; Ugi, I.; Lemmen, P. Further bisabolenes and dammarane triterpenes of Commiphora kua resin. Chem. Pharm. Bull. (Tokyo) 2003, 51, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Wan, W.Z.; Wang, X.N.; Yuan, H.Q.; Ji, M.; Lou, H.X. A triterpenoid and sesquiterpenoids from the resinous exudates of Commiphora myrrha. Helv. Chim. Acta 2009, 92, 645–652. [Google Scholar] [CrossRef]
- Nagaya, H.; Tobita, Y.; Nagae, T.; Itokawa, H.; Takeya, K.; Halim, A.F.; Abdel Halim, O.B. Cytotoxic triterpenes from Cleome Africana. Phytochemistry 1997, 44, 1115–1119. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Kattab, A.M.; Bodige, S.G.; Mao, Y.; Minter, D.E.; Reinecke, M.G.; Watson, W.H.; Mabry, T.J. 15α-Acetoxycleomblynol A from Cleome amblyocarpa. J. Nat. Prod. 2001, 64, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, V.U.; Qazi, S.; Zia, N.B.; Xu, C.F.; Clardy, J. Cleocarpone, a triterpenoid from Cleome brachycarpa. Phytochemistry 1990, 29, 670–672. [Google Scholar] [CrossRef]
- Das, P.C.; Patra, A.; Mandal, S.; Mallick, B.; Das, A.; Chatterjee, A. Cleogynol, a novel dammarane triterpenoid from Cleome gynandra. J. Nat. Prod. 1999, 62, 616–618. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Zhao, Y.; Yu, Z.Y.; Cong, Y.W.; Xu, G.; Peng, L.Y.; Zhang, P.T.; Cheng, X.; Zhao, Q.S. Six new dammarane triterpenoids from Viburnum cylindricum. Helv. Chim. Acta 2008, 91, 1578–1587. [Google Scholar] [CrossRef]
- Machida, K.; Kikuchi, M. Studies on the constituents of Viburnum species. XIX. Six new triterpenoids from Viburnum dilatatum Thunb. Chem. Pharm. Bull. 1999, 47, 692–694. [Google Scholar] [CrossRef]
- Wang, K.W.; Sun, C.R.; Wu, X.D.; Pan, Y.J. Novel bioactive dammarane caffeoyl esters from Celastrus rosthornianus. Planta Med. 2006, 72, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Fukuhara, K. Elabunin, a new cytotoxic triterpene from an East African medicinal plant, Elaeodendron buchananii. J. Nat. Prod. 1999, 53, 968–971. [Google Scholar] [CrossRef]
- Torpocco, V.; Chavez, H.; Estevez-Braun, A.; Ravelo, A.G. New dammarane triterpenes from Maytenus macrocarpa. Chem. Pharm. Bull. 2007, 55, 812–814. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.B.; Norman, V.L.; Goering, M.G.; O’Neil-Johnson, M.; Eldridge, G.R.; Starks, C.M. Acetylated dammarane-type bisdesmosides from Combretum inflatum. J. Nat. Prod. 2013, 76, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.; Dewelle, J.; Nacoulma, O.; Guissou, P.; Kiss, R.; Daloze, D.; Braekman, J.C. Cytotoxic pentacyclic triterpenes from Combretum nigricans. Fitoterapia 2003, 74, 339–344. [Google Scholar] [CrossRef]
- Sharma, S.C.; Tandon, J.S. A dammarane triterpene from Commelina undulate. Phytochemistry 1982, 21, 2420–2421. [Google Scholar] [CrossRef]
- Schmidt, T.J.; von Raison, J.; Willuhn, G. New triterpene esters from flowerheads of Arnica lonchophylla. Planta Med. 2004, 70, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, G.; Wang, G.; Yu, B.; Wang, F.; Liu, J. Study on Kalimeris indica triterpenes. Zhongchengyao 2010, 32, 462–465. [Google Scholar]
- Li, X.H.; Qi, H.Y.; Shi, Y.P. Dammarane- and taraxastane-type triterpenoids from Saussurea oligantha Franch. J. Asian Nat. Prod. Res. 2008, 10, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zeng, F.L.; Xu, L.X.; Chen, Y.Y.; Wang, Y.F.; Wei, X.Y. Bioactive dammarane-type saponins from Operculina turpethum. J. Nat. Prod. 2011, 74, 1868–1874. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, T.; Iwase, Y.; Okabe, H.; Mihashi, K.; Yamauchi, T. Studies on the constituents of Actinostemma lobatum Maxim. II. Structures of actinostemmosides G and H, new dammarane triterpene glycosides isolated from the herb. Chem. Pharm. Bull. 1987, 35, 3870–3873. [Google Scholar] [CrossRef]
- Yin, F.; Hu, L.H.; Lou, F.C.; Pan, R.X. Chemical studies on Gynostemma pentaphyllum Makino. In Proceedings of the 227th ACS National Meeting, Anaheim, CA, USA, 28 March–1 April 2004.
- Yin, F.; Zhang, Y.N.; Yang, Z.Y.; Cheng, Q.Q.; Hu, L.H. Triterpene saponins from Gynostemma cardiospermum. J. Nat. Prod. 2006, 69, 1394–1398. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.L.; Zhu, Z.Y. Gycomoside I: A new dammarane saponin from Gynostemma compressum. Planta Med. 1993, 59, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.J.; Guo, C.Y.; Ma, L.; Hu, L.H. Dammarane-type glycosides and long chain sesquiterpene glycosides from Gynostemma yixingense. Fitoterapia 2010, 81, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, K.; Nagao, T.; Okabe, H.; Yamauchi, T. Studies on the constituents of Luffa operculata Cogn. I. Isolation and structures of luperosides A-H, dammarane-type triterpene glycosides in the herb. Chem. Pharm. Bull. 1989, 37, 18–22. [Google Scholar] [CrossRef]
- Fujita, S.J.; Kasai, R.; Ohtani, K.; Yamasaki, K.; Chiu, M.H.; Nie, R.L.; Tanaka, O. Dammarane glycosides from aerial part of Neoalsomitra integrifoliola. Phytochemistry 1995, 39, 591–602. [Google Scholar] [CrossRef]
- Yang, J.L.; Shi, Y.P. Structurally diverse terpenoids from the rhizomes of Cyperus rotundus L. Planta Med. 2012, 78, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.H.; Luo, W.; Guo, Z.Y.; Deng, Z.S.; Zou, K. Chemical constituents of petroleum ether part of endophytic fungus Lophiostoma sp. (X1-2) from Davidia involucrata Baill. Sanxia Daxue Xuebao Ziran Kexueban 2012, 34, 92–94. [Google Scholar]
- Zhang, Z.; Koike, K.; Guo, D.; Li, C.; Zheng, J.; Jia, Z.; Nikaido, T. Chemical constituents of Yunnan wintergreen root (Gaultheria yunnanensis) (III). Zhongcaoyao 1999, 30, 247–250. [Google Scholar]
- Yu, J.H.; Shen, Y.; Liu, H.B.; Leng, Y.; Zhang, H.; Yue, J.M. Dammarane-type triterpenoids as 11β-HSD1 inhibitors from Homonoia riparia. Org. Biomol. Chem. 2014, 12, 4716–4722. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.Y.; Wu, D.; Lu, C.; Xu, X.M.; Huang, J.; Sun, B.H.; Wu, L.J. Constituents from the testas of Castanea mollissima Blume with α-glucosidase inhibitory activity. J. Asian Nat. Prod. Res. 2010, 12, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Garo, E.; Hung, C.S.; Williams, R.B.; Olson, K.M.; Hu, J.F.; Rice, S.M.; Hough, G.W.; Goering, M.G.; O’Neil-Johnson, M.; Eldridge, G.R.; et al. Dammarane-type triterpene glycosides from Oncoba manii active against methicillin-resistant Staphylococcus aureus. Planta Med. 2009, 75, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, D.; Zhang, Y.J.; Yang, C.R. Dammarane triterpenoids from the roots of Gentiana rigescens. J. Nat. Prod. 2007, 70, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Zhang, Y.; Wang, T.; Nakamura, S.; Matsuda, H. New triterpene constituents, foliasalacins A1-A4, B1-B3, and C, from the leaves of Salacia chinensis. Chem. Pharm. Bull. 2008, 56, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, M.Q.; Li, X.L.; Xu, T.H.; Xie, S.X.; Xu, Y.J.; Xu, D.M. Study on chemical constituents of Cyclocarya paliurus. J. Asian Nat. Prod. Res. 2014, 16, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, X.; Zhu, H.; Yao, Y.; Hao, X.; Song, B. Triterpenoids of Glechoma longituba (Nakai) Kupr. Zhongcaoyao 2006, 37, 1780–1781. [Google Scholar]
- Esquivel, B.; Guerrero, F.; Toscano, R.A. Tri-nordammarane triterpenoids and neoclerodane diterpenoids from Salvia aspera (Labiatae). Nat. Prod. Lett. 2002, 16, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Kolak, U.; Kabouche, A.; Ozturk, M.; Kabouche, Z.; Topcu, G.; Ulubelen, A. Antioxidant diterpenoids from the roots of Salvia barrelieri. Phytochem. Anal. 2009, 20, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Pedreros, S.; Rodriguez, B.; De la Torre, M.C.; Bruno, M.; Savona, G.; Perales, A.; Torres, M.R. Dammarane triterpenes of Salvia hierosolymitana. Phytochemistry 1990, 29, 919–922. [Google Scholar] [CrossRef]
- Ding, M.M.; Yan, F.L.; Tan, J.; Bai, Y.X.; Wang, X.; Yang, Y.X. Two new dammarane-type glycosides from Phlomis umbrosa. Nat. Prod. Res. 2014, 28, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.L.; Liu, M.T.; Gan, L.S.; Lin, S.; Liu, B.; Zhang, Y.L.; Zi, J.C.; Song, W.X.; Shi, J.G. Dammarane glycosides from the root of Machilus yaoshansis. J. Nat. Prod. 2012, 75, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yu, F.; Yu, S. Triterpenoids from Erythrophleum fordii. Acta Bot. Sin. 2004, 46, 371–374. [Google Scholar]
- Asai, T.; Hara, N.; Fujimoto, Y. Fatty acid derivatives and dammarane triterpenes from the glandular trichome exudates of Ibicella lutea and Proboscidea louisiana. Phytochemistry 2010, 71, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.L.; Chai, H.; Santisuk, T.; Reutrakul, V.; Farnsworth, N.R.; Cordell, G.A.; Pezzuto, J.M.; Kinghorn, A.D. Novel cytotoxic 1H-cyclopenta[b]benzofuran lignans from Aglaia elliptica. Tetrahedron 1997, 53, 17625–17632. [Google Scholar] [CrossRef]
- Phongmaykin, J.; Kumamoto, T.; Ishikawa, T.; Saifah, E.; Suttisri, R. Biologically active constituents of Aglaia erythrosperma. Nat. Prod. Res. 2011, 25, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Chantrapromma, S.; Supriadin, A.; Harneti, D.; Supratman, U. 3-epi-Dammarenediol II 1.075 hydrate. A dammarane triterpene from the bark of Aglaia eximia. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, o3089–o3090. [Google Scholar] [CrossRef] [PubMed]
- Joycharat, N.; Plodpai, P.; Panthong, K.; Yingyongnarongkul, B.; Voravuthikunchai, S.P. Terpenoid constituents and antifungal activity of Aglaia forbesii seed against phytopathogens. Can. J. Chem. 2010, 88, 937–944. [Google Scholar] [CrossRef]
- Roux, D.; Martin, M.T.; Adeline, M.T.; Hevenet, T.; Hadi, A.H.A.; Pais, M. Foveolins A and B, dammarane triterpenes from Aglaia foveolata. Phytochemistry 1998, 49, 1745–1748. [Google Scholar] [CrossRef]
- Mohamad, K.; Sevenet, T.; Dumontet, V.; Pais, M.; Van, T.M.; Hadi, H.; Awang, K.; Martin, M.T. Dammarane triterpenes and pregnane steroids from Aglaia lawii and A. tomentosa. Phytochemistry 1999, 51, 1031–1037. [Google Scholar] [CrossRef]
- Wang, D.X.; Yang, S.M. Chemical constituents from the leaves of Aglaia odorata. Z. Naturforsch. C 2013, 68, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Hofer, O.; Pointinger, S.; Brecker, L.; Peter, K.; Greger, H. Silvaglenamin-a novel dimeric triterpene alkaloid from Aglaia silvestris. Tetrahedron Lett. 2009, 50, 467–468. [Google Scholar] [CrossRef]
- Harneti, D.; Tjokronegoro, R.; Safari, A.; Supratman, U.; Loong, X.M.; Mukhtar, M.R.; Mohamad, K.; Awang, K.; Hayashi, H. Cytotoxic triterpenoids from the bark of Aglaia smithii (Meliaceae). Phytochem. Lett. 2012, 5, 496–499. [Google Scholar] [CrossRef]
- Luo, X.D.; Wu, S.H.; Ma, Y.B.; Wu, D.G. Dammarane triterpenoids from Amoora yunnanensis. Heterocycles 2000, 53, 2795–2802. [Google Scholar] [CrossRef]
- Huang, S.S.; Jian, K.L.; Li, R.J.; Kong, L.Y.; Yang, M.H. Phytosteroids and triterpenoids with potent cytotoxicities from the leaves of Chisocheton cumingianus. RSC Adv. 2016, 6, 6320–6328. [Google Scholar] [CrossRef]
- Phongmaykin, J.; Kumamoto, T.; Ishikawa, T.; Suttisri, R.; Saifah, E. A new sesquiterpene and other terpenoid constituents of Chisocheton penduliflorus. Arch. Pharm. Res. 2008, 31, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; Mohamad, K.O.; Audrey, J.A.; Imiyabir, Z.; Chung, L.Y. Bioactivity-guided fractionation of the lipoxygenase and cyclooxygenase inhibiting constituents from Chisocheton polyandrus Merr. Fitoterapia 2012, 83, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.J.; Wang, J.S.; Kong, L.Y. Cytotoxic dammarane-type triterpenoids from the stem bark of Dysoxylum binectariferum. J. Nat. Prod. 2014, 77, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Harrison, L.J.; Sim, K.Y. A triterpenoid with a novel abeo-dammarane skeleton from Dysoxylum cauliflorum. Tetrahedron Lett. 1999, 40, 1607–1610. [Google Scholar] [CrossRef]
- Nugroho, A.E.; Momota, T.; Sugiura, R.; Hanzawa, M.; Yajima, E.; Nagakura, Y.; Yasuda, N.; Hirasawa, Y.; Wong, C.P.; Kaneda, T.; et al. Dysotriflorins A-M, triterpenoids from Dysoxylum densiflorum. Tetrahedron 2014, 70, 9661–9667. [Google Scholar] [CrossRef]
- Cao, P.; Liang, G.B.; Gao, X.; Wang, X.G.; Li, Z.Q. Three new nor-dammarane triterpenoids from Dysoxylum hainanense with particular cytotoxicity against glioma cell line. Arch. Pharm. Res. 2013, 36, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Luo, S.; Wang, H. Dammarane triterpenoid from Dysoxylum hongkongense. Yunnan Zhiwu Yanjiu 1998, 20, 362–368. [Google Scholar]
- Hisham, A.; Bai, M.D.A.; Fumimoto, Y.; Hara, N.; Shimada, H. Complete 1H and 13C-NMR spectra assignment of cabraleadiol, a dammarane triterpene from Dysoxylum malabaricum Bedd. Magn. Reson. Chem. 1996, 34, 146–150. [Google Scholar] [CrossRef]
- Tantapakul, C.; Maneerat, W.; Sripisut, T.; Ritthiwigrom, T. Dammarane terpenoids from the fruits of Dysoxylum mollissimum. Nat. Prod. Commun. 2014, 9, 1553–1556. [Google Scholar] [PubMed]
- Mulholland, D.A.; Naidoo, N. Dammarane triterpenoids from Dysoxylum muellerii. Biochem. Syst. Ecol. 2000, 28, 295–297. [Google Scholar] [CrossRef]
- Singh, Y.; Aalbersberg, W. Dammarane triterpenoids from a Fijian medicinal plant, Dysoxylum richii. Part 2. Dammarane triterpenoids from Dysoxylum richii. Phytochemistry 1992, 31, 4033–4035. [Google Scholar] [CrossRef]
- Mahmod, I.I.; Kwong, H.C.; Mohamed, T.; Mohamed, I.; Ismail, I.S. (20S*,24S*)-25-Hydroxy-20,24-epoxy-A-homo-4-oxadammaran-3-one (Chrysura) isolated from the leaves of Walsura chrysogyne. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, o3296. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, J.; Kimizuka, K.; Tanaka, Y. New dammarane-type acetylated triterpenoids and their related compounds of Ficus pumila fruit. Chem. Pharm. Bull. 1999, 47, 1138–1140. [Google Scholar] [CrossRef]
- Suga, A.; Takaishi, Y.; Nakagawa, H.; Iwasa, T.; Sato, M.; Okamoto, M. Chemical constituents from fruits and seeds of Myrica rubra (Myricaceae). Nat. Med. 2005, 59, 70–75. [Google Scholar]
- Januario, A.H.; Da Silva, M.F.D.G.F.; Vieira, P.C.; Fernandes, J.B. Dammarane and cycloartane triterpenoids from three Rapanea species. Phytochemistry 1992, 31, 1251–1253. [Google Scholar] [CrossRef]
- Xue, J.; Xie, L.; Liu, B.R.; Yu, L.X. Triterpenoids from the fruits of Forsythia suspense. Zhongguo Tianran Yaowu 2010, 8, 414–418. [Google Scholar]
- Hawas, U.W.; Gamal-Eldeen, A.M.; El-Desouky, S.K.; Kim, Y.K.; Huefner, A.; Saf, R. Induction of caspase-8 and death receptors by a new dammarane skeleton from the dried fruits of Forsythia koreana. Z. Naturforsch. C 2013, 68, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Yang, N.Y.; Qian, S.H.; Xie, N.; Duan, J.A. Dammarane triterpenes from Ligustrum lucidum. J. Asian Nat. Prod. Res. 2008, 10, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Revesz, L.; Hiestand, P.; La Vecchia, L.; Naef, R.; Naegeli, H.U.; Oberer, L.; Roth, H.J. Isolation and synthesis of a novel immunosuppressive 17α-substituted dammarane from the flour of the Palmyrah palm (Borassus flabellifer). Bioorg. Med. Chem. Lett. 1999, 9, 1521–1526. [Google Scholar] [CrossRef]
- Yamashita, H.; Masuda, K.; Kobayashi, T.; Ageta, H.; Shiojima, K. Dammarane triterpenoids from rhizomes of Pyrrosia lingua. Phytochemistry 1998, 49, 2461–2466. [Google Scholar] [CrossRef]
- Arai, Y.; Hirohara, M.; Ageta, H. Fern constituents: Three new skeletal triterpenoid hydrocarbons isolated from Polypodiodes niponica. Tetrahedron Lett. 1989, 30, 7209–7212. [Google Scholar] [CrossRef]
- Mata, R.; Macias, M.L.; Rojas, I.S.; Lotina-Hennsen, B.; Toscano, R.A.; Anaya, A.L. Phytotoxic compounds from Esenbeckia yaxhoob. Phytochemistry 1998, 49, 441–449. [Google Scholar] [CrossRef]
- Oulad-Ali, A.; Guillaume, D.; Jiang, Y.L.; Weniger, B.; Anton, R. Mabioside B, a novel saponin from Colubrina elliptica. Nat. Prod. Lett. 1993, 2, 203–207. [Google Scholar] [CrossRef]
- Kennelly, E.J.; Lewis, W.H.; Winter, R.E.K.; Johnson, S.; Elvin-Lewis, M.; Gossling, J. Triterpenoid saponins from Gouania lupuloides. J. Nat. Prod. 1993, 56, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.F.; Zhang, X.Q.; Zhang, J.; Liu, B.; Jiang, J.; Wang, W.J.; Gao, M.H.; Jiang, R.W.; Ye, W.C. Two methyl-migrated 16,17-seco-dammarane triterpenoid saponins from the seeds of Hovenia acerba. J. Asian Nat. Prod. Res. 2012, 14, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Murakami, T.; Ueda, T.; Matsuda, H.; Yamahara, J.; Murakami, N. Bioactive saponins and glycosides. IV. Four methyl-migrated 16,17-seco-dammarane triterpene glycosides from Chinese natural medicine, Hoveniae Semen Seu Fructus, the seeds and fruit of Hovenia dulcis Thunb.: Absolute stereostructures and inhibitory activity on histamine release of hovenidulciosides A1, A2, B1, and B2. Chem. Pharm. Bull. 1996, 44, 1736–1743. [Google Scholar] [PubMed]
- Rambabu, P.; Ramana, K.V.; Ganapaty, S. Isolation and characterization of triterpenes from Zizyphus glabrata. Int. J. Chem. Sci. 2011, 9, 1014–1024. [Google Scholar]
- Schuhly, W.; Heilmann, J.; Calis, I.; Sticher, O. Novel triterpene saponins from Zizyphus joazeiro. Helv. Chim. Acta 2000, 83, 1509–1516. [Google Scholar] [CrossRef]
- Inoue, O.; Ogihara, Y.; Yamasaki, K. Application of carbon-13 nuclear magnetic resonance spectroscopy to the elucidation of the structure of a minor dammarane saponin from the seeds of Zizyphus jujuba. J. Chem. Res. 1978, 4, 144–145. [Google Scholar]
- Maciuk, A.; Lavaud, C.; Thepenier, P.; Jacquier, M.J.; Ghedira, K.; Zeches-Hanrot, M. Four new dammarane saponins from Zizyphus lotus. J. Nat. Prod. 2004, 67, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Ma, G.J.; Xie, J.B. Tissue distribution of Jujuboside A in sprague-dawley rats determined by an efficient HPLC-ESI-MS/MS method. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 215–221. [Google Scholar] [CrossRef]
- Rambabu, P.; Ramana, K.V.; Ganapaty, S. Dammarane and ceanothane triterpenes from Zizyphus xylopyra. Int. J. Chem. Sci. 2010, 8, 1231–1239. [Google Scholar]
- Homhual, S.; Bunyapraphatsara, N.; Kondratyuk, T.; Herunsalee, A.; Chaukul, W.; Pezzuto, J.M.; Fong, H.H.S.; Zhang, H.J. Bioactive dammarane triterpenes from the Mangrove plant Bruguiera gymnorrhiza. J. Nat. Prod. 2006, 69, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Pakhathirathien, C.; Karalai, C.; Ponglimanont, C.; Subhadhirasakul, S.; Chantrapromma, K. Dammarane triterpenes from the hypocotyls and fruits of Ceriops tagal. J. Nat. Prod. 2005, 68, 1787–1789. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.H.; Fukuoka, R.; Aoki, F.; Tanaka, T.; Kouno, I. Dammarane-type triterpene glycosides from the leaves of Rhoiptelea chiliantha. Chem. Pharm. Bull. 1999, 47, 257–262. [Google Scholar] [CrossRef]
- Asai, T.; Fujimoto, Y. 2-Acety-1-(3-glycosyloxyoctade- canoyl)glycerol and dammarane triterpenes in the exudates from glandular trichome-like secretory organs on the stipules and leaves of Cerasus yedoensis. Phytochem. Lett. 2011, 4, 38–42. [Google Scholar] [CrossRef]
- Grougnet, R.; Magiatis, P.; Mitaku, S.; Skaltsounis, A.L.; Cabalion, P.; Tillequin, F.; Michel, S. Dammarane triterpenes from Gardenia aubryi Vieill. Helv. Chim. Acta 2011, 94, 656–661. [Google Scholar] [CrossRef]
- Nuanyai, T.; Sappapan, R.; Vilaivan, T.; Pudhom, K. Dammarane triterpenes from the apical buds of Gardenia collinsae. Phytochem. Lett. 2011, 4, 183–186. [Google Scholar] [CrossRef]
- Mai, H.L.; Grellier, P.; Prost, E.; Lemoine, P.; Poullain, C.; Dumontet, V.; Deguin, B.; Vo, T.B.H.; Michel, S.; Grougnet, R. Triterpenes from the exudate of Gardenia urvillei. Phytochemistry 2016, 122, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Shen, L.M.; Zhang, M.; Li, N.; Li, X.; Ma, Z.J.; Qu, H.B. Eleven new triterpenes from Eurycorymbus cavaleriei. Helv. Chim. Acta 2010, 93, 2263–2275. [Google Scholar] [CrossRef]
- Suhagia, B.N.; Rathod, I.S.; Sindhu, S. Sapindus mukorossi (areetha): An overview. Int. J. Pharm. Sci. Res. 2011, 2, 1905–1913. [Google Scholar]
- Ramasamy, S.; Chin, S.P.; Sukumaran, S.D.; Buckle, M.J.C.; Kiew, L.V.; Chung, L.Y. In silico and in vitro analysis of bacoside A aglycones and its derivatives as the constituents responsible for the cognitive effects of Bacopa monnieri. PLoS ONE 2015, 10, e0126565/1–e0126565/19. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.L.; Xiong, J.; Wu, S.B.; Zhu, J.J.; Hong, J.L.; Zhao, Y.; Xia, G.; Hu, J.F. Tetracyclic triterpenoids and terpenylated coumarins from the bark of Ailanthus altissima (“Tree of Heaven”). Phytochemistry 2013, 86, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, P.V.; Rao, R.R.; Rao, J.M. Two new tetracyclic triterpenes from the heartwood of Ailanthus excelsa Roxb. Chem. Biodivers. 2006, 3, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Oh, S.R.; Ahn, K.S.; Lee, H.K. Semialactone, isofouquierone peroxide and fouquierone, three new dammarane triterpenes from Rhus javanica. Chem. Pharm. Bull. 2001, 49, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Gariboldi, P.; Wollenweber, E.; Sironi, A.; Molinari, H. Triterpenes from the frond exudate of the fern Notholaena greggii. Phytochemistry 1992, 31, 923–927. [Google Scholar] [CrossRef]
- Arriaga-Giner, J.F.; Rumbero, A.; Wollenweber, E. Notholaena terpenoids: Two new epimeric diterpenes from the frond exudate of the fern, Notholaena rigida. Z. Naturforsch. C 1997, 52, 292–294. [Google Scholar]
- Abdul Quader, M.; Gray, A.I.; Waterman, P.G.; Lavaud, C.; Massiot, G.; Hasan, C.M.; Ahmed, M.D. Capsugenin-25,30-O-β-diglucopyranoside: A new glycoside from the leaves of Corchorus capsularis. J. Nat. Prod. 1990, 53, 527–530. [Google Scholar] [CrossRef]
- Weng, X.X.; Shao, Y.; Chen, Y.Y.; Gao, W.; Cheng, L.; Kong, D.Y. Two new dammarane monodesmosides from Centella asiatica. J. Asian Nat. Prod. Res. 2011, 13, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Y.; Yang, C. A further investigation on the chemical constituents from the leaves of Panax notoginseng. Nat. Prod. Res. Dev. 2006, 18, 549–554. [Google Scholar]
- Asakawa, J.; Kasai, R.; Yamasaki, K.; Tanaka, O. Carbon-13 NMR study of Ginseng sapogenins and their related dammarane type triterpenes. Tetrahedron 1977, 33, 1935–1939. [Google Scholar] [CrossRef]
- Bianchini, J.P.; Gaydou, E.M.; Rafaralahitsimba, G.; Waegell, B.; Zahra, J.P. Dammarane derivatives in the fruit lipids of Olea madagascariensis. Phytochemistry 1988, 27, 2301–2304. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Y.; Rayburn, E.R.; Hill, D.L.; Wang, H.; Zhang, R. In vitro anti-cancer activity and structure-activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemother. Pharmacol. 2007, 59, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.T.; Yang, M.; Nong, S.J.; Yang, X.; Ling, Y.; Wang, D.G.; Wang, X.Y.; Zhang, W. Microbial transformation of 20(S)-protopanaxadiol by Absidia corymbifera. Cytotoxic activity of the metabolites against human prostate cancer cells. Fitoterapia 2013, 84, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Fuchino, H.; Satoh, T.; Tanaka, N. Chemical evaluation of Betula species in Japan. I. Constituents of Betula ermanii. Chem. Pharm. Bull. 1995, 43, 1937–1942. [Google Scholar] [CrossRef]
- Atopkina, L.N.; Denisenko, V.A. Synthesis of 3β,20S-dihydroxydammar-24-en-12-one 3,20-di-O-β-d-glucopyranoside (chikusetsusaponin-LT8), a glycoside from Panax japonicas. Chem. Nat. Compd. 2006, 42, 55–60. [Google Scholar] [CrossRef]
- Tanaka, O.; Yahara, S. Dammarane saponins of leaves of Panax pseudo-ginseng subsp. himalaicus. Phytochemistry 1978, 17, 1353–1358. [Google Scholar] [CrossRef]
- Bai, M.S.; Gao, J.M.; Fan, C.; Yang, S.X.; Zhang, G.; Zheng, C.D. Bioactive dammarane-type triterpenoids derived from the acid hydrolysate of Gynostemma pentaphyllum saponins. Food Chem. 2010, 119, 306–310. [Google Scholar] [CrossRef]
- Atopkina, L.N.; Denisenko, V.A. Glycosylation of panaxadiol. Chem. Nat. Compd. 2011, 46, 892–896. [Google Scholar] [CrossRef]
- Kang, K.S.; Kim, H.Y.; Yoo, H.H.; Piao, X.L.; Ham, J.; Yang, H.O.; Park, J.H. Protective effect of ginseng sapogenins against 2,2′-azobis (1-aminopropane) dihydrochloride (AAPH)-induced LLC-PK1 cell damage. Bioorg. Med. Chem. Lett. 2012, 22, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.T.; Yang, X.; Li, J.L.; Ge, H.J.; Song, Y.; Ren, J. Biotransformation of 20(S)-protopanaxadiol by Aspergillus niger AS 3.1858. Fitoterapia 2013, 91, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Han, L.F.; Kaunda, J.S.; Liu, L.L.; Agyemang, K.; Wang, T.; Zhang, Y. Saponins from roots of Panax notoginseng. Chin. Herb. Med. 2014, 6, 159–163. [Google Scholar] [CrossRef]
- Yoshizaki, K.; Yahara, S. New triterpenoid saponins from fruits specimens of Panax japonicas collected in Kumamoto and Miyazaki Prefectures (1). Chem. Pharm. Bull. 2012, 60, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Kim, K. Effect of ginseng and ginsenosides on melanogenesis and their mechanism of action. J. Ginseng Res. 2015, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, A.K. Anti-breast cancer activity of Fine Black ginseng (Panax ginseng Meyer) and ginsenoside Rg5. J. Ginseng Res. 2015, 39, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Shareef, M.; Ashraf, M.A.; Sarfraz, M. Natural cures for breast cancer treatment. Saudi. Pharm. J. 2016, 24, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Y.; Yang, X.W. Six new dammarane-type triterpenes from acidic hydrolysate of the stems-leaves of Panax ginseng and their inhibitory-activities against three human cancer cell lines. Phytochem. Lett. 2015, 13, 406–412. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.; Han, L.; Rayburn, E.R.; Hill, D.L.; Wang, H.; Zhang, R. Isolation, structural determination, and evaluation of the biological activity of 20(S)-25-methoxyl-dammarane-3β,12β,20-triol 20(S)-25-OCH3-PPD, a novel natural product from Panax notoginseng. Med. Chem. 2007, 3, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Yuan, H.N.; Bi, X.L.; Piao, H.R.; Cao, J.Q.; Li, W.; Wang, P.; Zhao, Y.Q. 25-Methoxylprotopanaxadiol derivatives and their anti-proliferative activities. Steroids 2013, 78, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhou, Y.; Kong, D.Y.; Zhang, W.D. Antitumor activities of dammarane triterpene saponins from Bacopa monniera. Phytother. Res. 2010, 24, 864–868. [Google Scholar] [PubMed]
- Cheng, Y.; Hua, H.Q. Clinical research progress in anti-tumor effects of ginsenoside Rg3. Yixue Zongshu 2015, 21, 2938–2940. [Google Scholar]
- Lee, S.Y.; Kim, G.T.; Roh, S.H.; Song, J.S.; Kim, H.J.; Hong, S.S.; Kwon, S.W.; Park, J.H. Proteomic analysis of the anti-cancer effect of 20S-ginsenoside Rg3 in human colon cancer cell lines. Biosci. Biotechnol. Biochem. 2009, 73, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xu, H.; Hu, M.N.; Luan, X.J.; Wang, K.Q.; Fu, Y.S.; Zhang, D.; Li, J.Y. Ginsenoside Rg3 bile salt-phosphatidylcholine-based mixed micelles: Design, characterization, and evaluation. Chem. Pharm. Bull. 2015, 63, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.M.; Thu, C.V.; Cuong, T.D.; Hung, N.P.; Kwack, S.J.; Huh, J.I.; Min, B.S.; Choi, J.S.; Lee, H.K.; Bae, K.H. Dammarane-type glycosides from Gynostemma pentaphyllum and their effects on IL-4-induced eotaxin expression in human bronchial epithelial cells. J. Nat. Prod. 2010, 73, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.P.; Yang, X.B.; Yang, X.W.; Liu, J.X.; Xu, W.; Zhang, Y.B.; Zhang, L.X.; Wang, Y.P. Ginsenjilinol, a new protopanaxatriol-type saponin with inhibitory activity on LPS-activated NO production in macrophage RAW 264.7 cells from the roots and rhizomes of Panax ginseng. J. Asian Nat. Prod. Res. 2013, 15, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.L.; Kim, Y.R.; Yang, J.L.; Oh, D.R.; Dao, T.T.; Oh, W.K. Dammarane triterpenes from the leaves of Panax ginseng enhance cellular immunity. Bioorg. Med. Chem. 2014, 22, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Bi, X.L.; Xiao, W.; Cao, J.Q.; Xia, X.C.; Diao, Y.P.; Zhao, Y.Q. Protein tyrosine phosphatase 1B inhibitory effect by dammarane-type triterpenes from hydrolyzate of total Gynostemma pentaphyllum saponins. Bioorg. Med. Chem. Lett. 2013, 23, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.Z.; Lv, J.J.; Zhang, X.X.; Yan, H.; Zhu, H.T.; Luo, H.R.; Wang, D.; Yang, C.R.; Xu, M.; Zhang, Y.J. Minor dehydrogenated and cleavaged dammarane-type saponins from the steamed roots of Panax notoginseng. Fitoterapia 2015, 103, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Ha, T.K.Q.; Dhodary, B.; Kim, K.H.; Park, J.; Lee, C.H.; Kim, Y.C.; Oh, W.K. Dammarane triterpenes as potential SIRT1 activators from the leaves of Panax ginseng. J. Nat. Prod. 2014, 77, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Y.; Zhou, Q.L.; Yang, X.B.; Wang, H.P.; Yang, X.W. Metabolism of 20(S)-ginsenoside Rg2 by rat liver microsomes: Bioactivation to SIRT1-activating metabolites. Molecules 2016, 21, 757. [Google Scholar] [CrossRef] [PubMed]

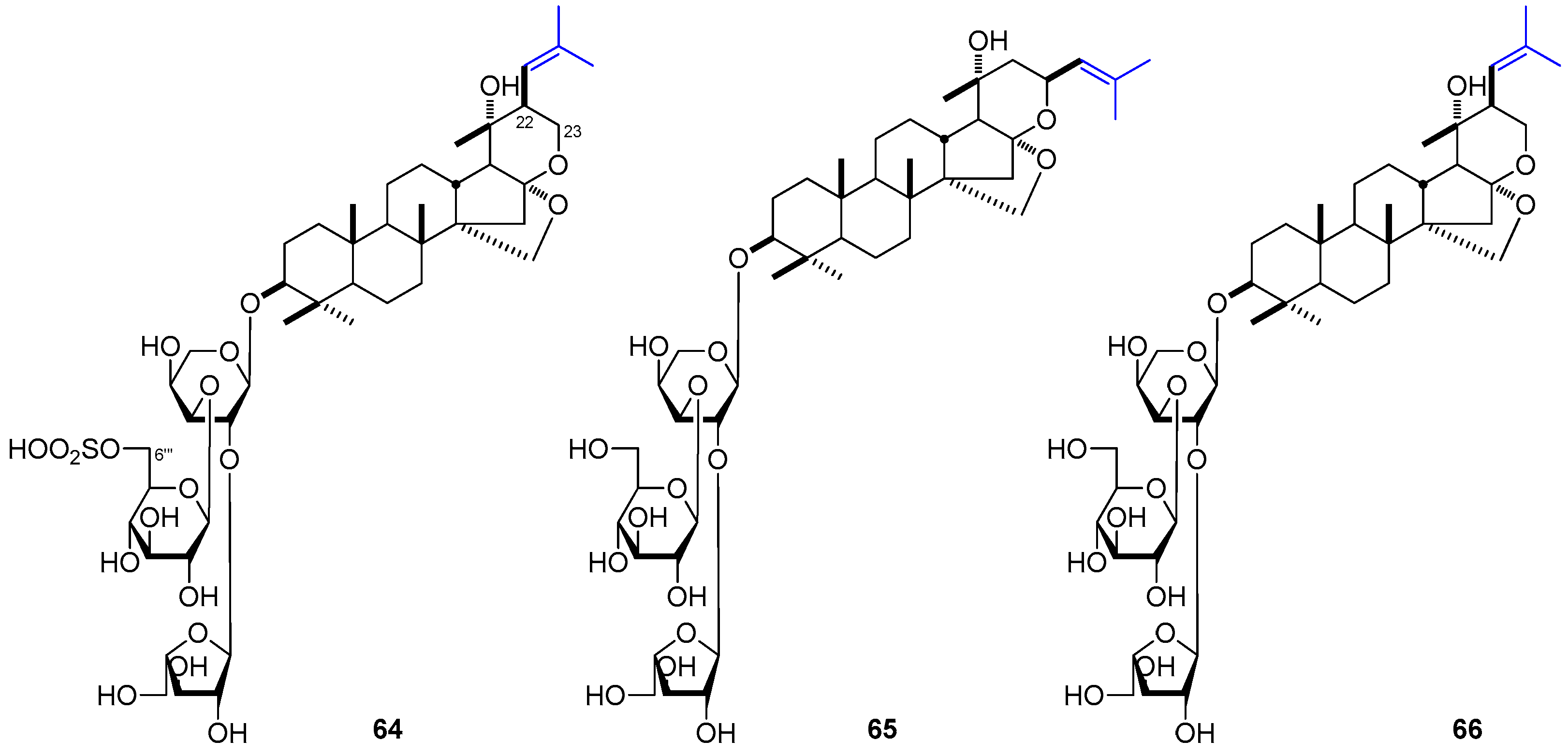
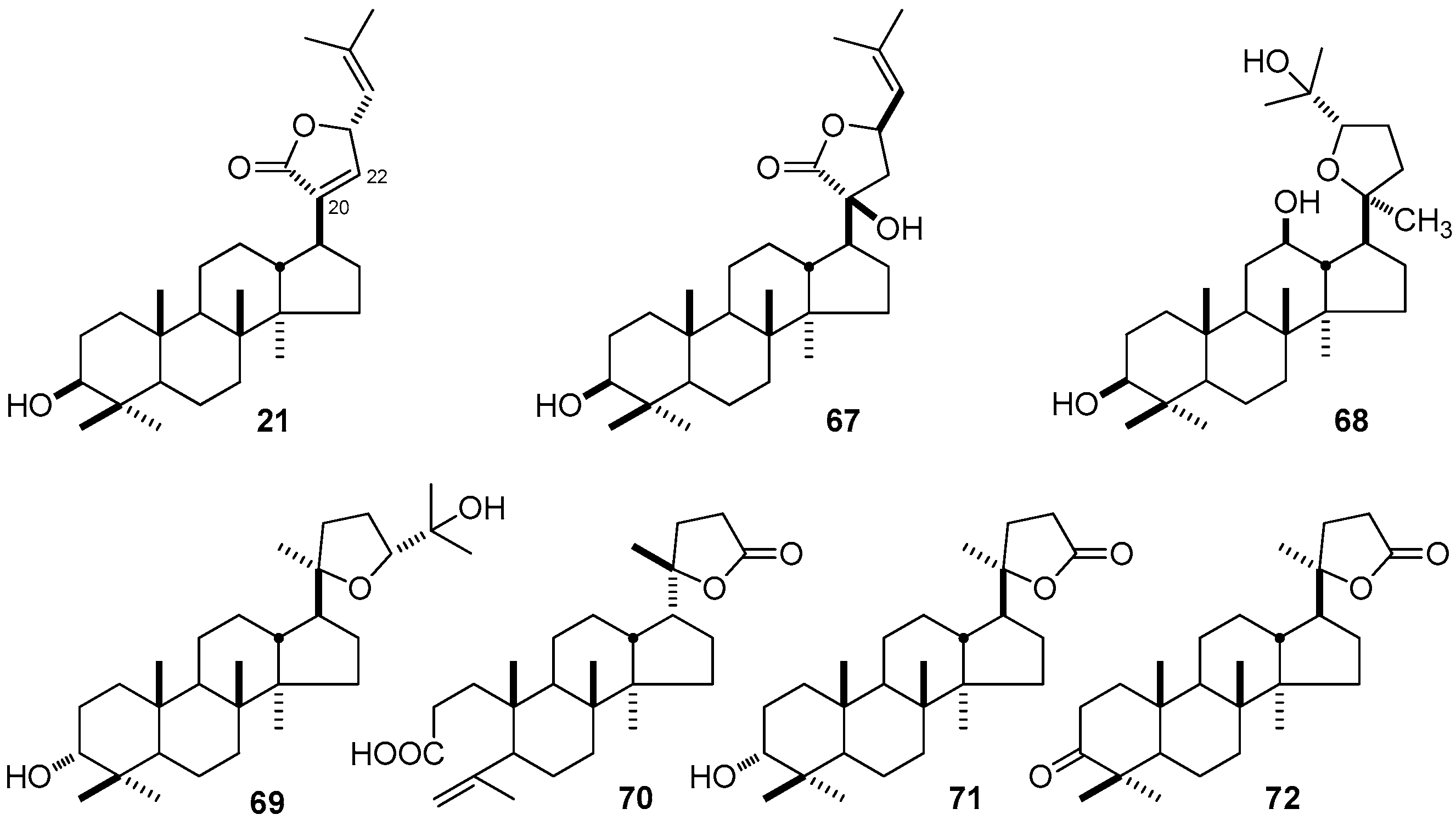

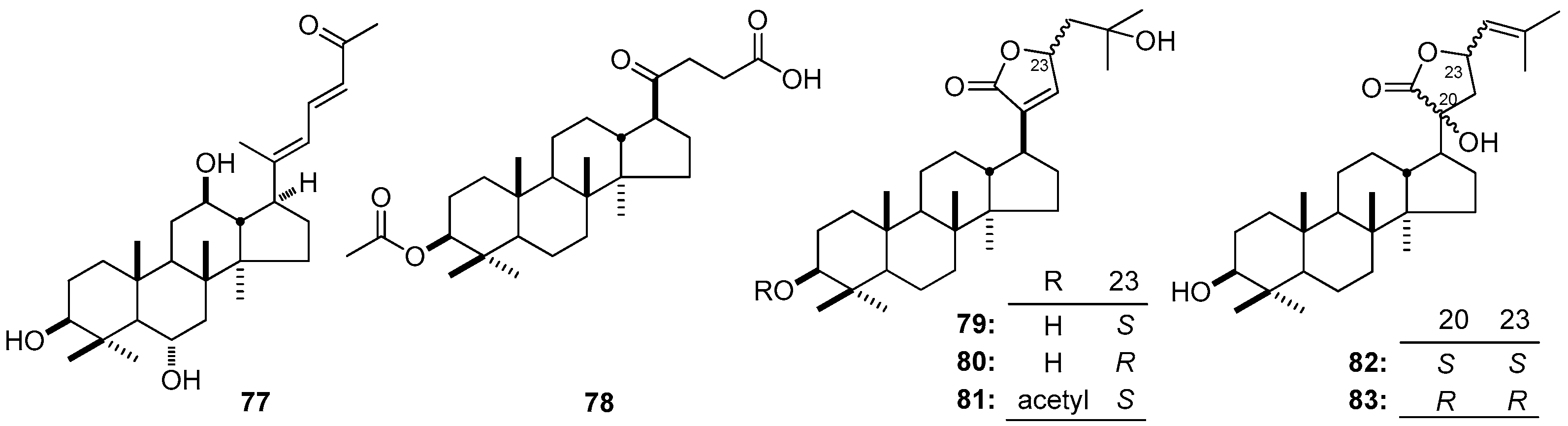
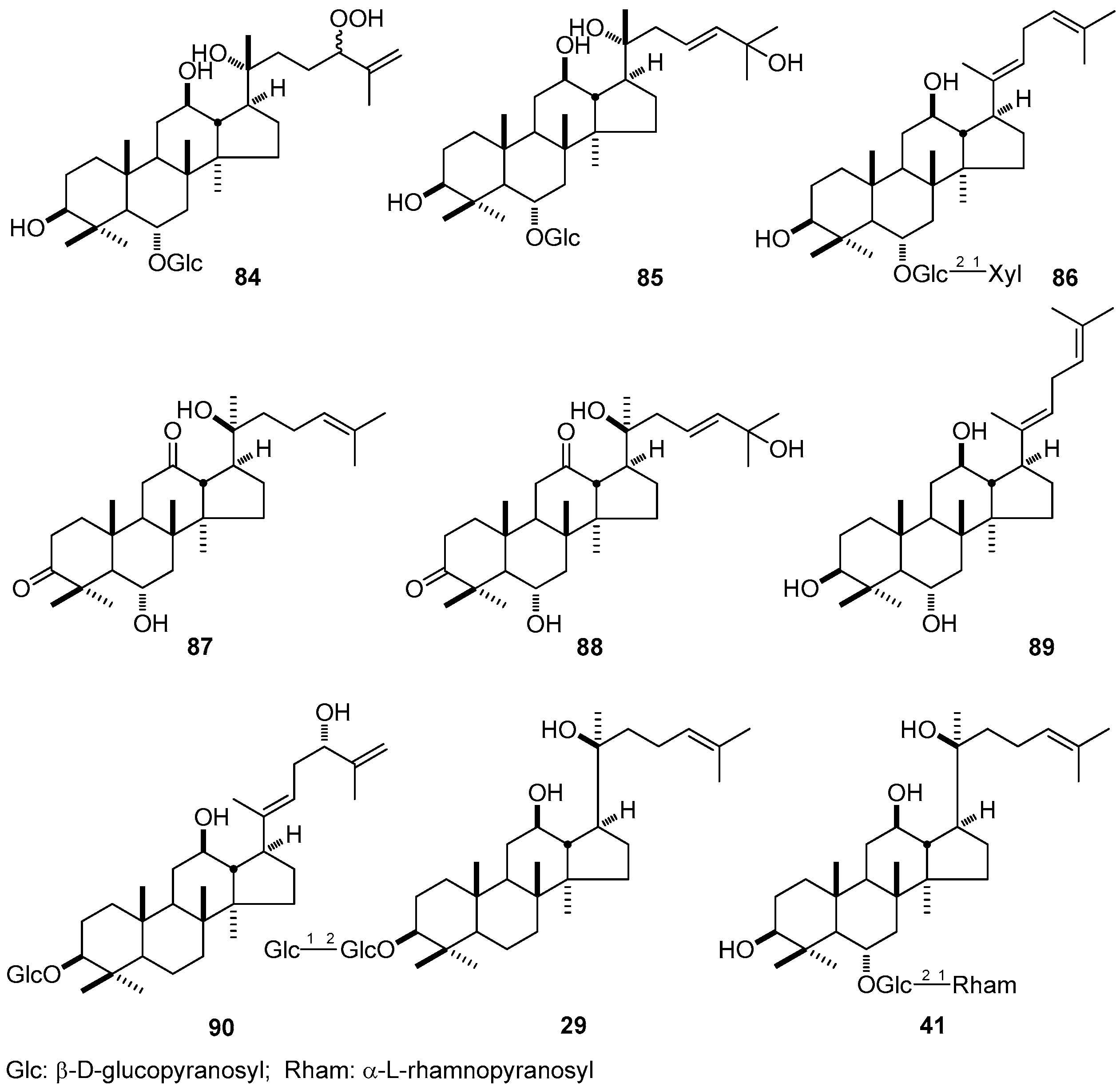
| No. | Family | Genus | Species | References |
|---|---|---|---|---|
| 1 | Anacardiaceae | Mangifera | M. indica | [4] |
| Pistacia | P. terebinthus | [5] | ||
| Rhus | R. chinensis | [6] | ||
| 2 | Apocynaceae | Nerium | N. oleander | [7] |
| Plumeria | P. obtuse | [8] | ||
| 3 | Araliaceae | Hedera | H. rhombea | [9] |
| Panax | P. japonicas | [10] | ||
| P. ginseng | [11] | |||
| P. notoginseng | [12] | |||
| P. quinquefolium | [13] | |||
| P. vietnamensis | [14] | |||
| P. vietnamensis var. fuscidiscus | [14] | |||
| Polyscias | P. fulva | [15] | ||
| Schefflera | S. arboricola | [16] | ||
| S. heptaphylla | [17] | |||
| 4 | Betulaceae | Alnus | A. nepalensis | [18] |
| A. serrulatoides | [19] | |||
| Betula | B. maximowicziana | [20] | ||
| B. pendula | [21] | |||
| B. platyphylla var. japonica | [22] | |||
| B. ovalifolia | [23] | |||
| 5 | Boraginaceae | Cordia | C. multispicata | [24] |
| C. spinescens | [25] | |||
| C. verbenacea | [26] | |||
| 6 | Burseraceae | Boswellia | B. freerana | [27] |
| Commiphora | C. confusa | [28] | ||
| C. dalzielii | [29] | |||
| C. incise | [30] | |||
| C. kua | [31] | |||
| C. myrrha | [32] | |||
| 7 | Capparaceae | Cleome | C. Africana | [33] |
| C. amblyocarpa | [34] | |||
| C. brachycarpa | [35] | |||
| C. gynandra | [36] | |||
| 8 | Caprifoliaceae | Viburnum | V. cylindricum | [37] |
| V. dilatatum | [38] | |||
| 9 | Celastraceae | Celastrus | C. rosthornianus | [39] |
| Elaeodendron | E. buchananii | [40] | ||
| Maytenus | M. macrocarpa | [41] | ||
| 10 | Combretaceae | Combretum | C. inflatum | [42] |
| C. nigricans | [43] | |||
| 11 | Commelinaceae | Commelina | C. undulate | [44] |
| 12 | Compositae | Arnica | A. lonchophylla | [45] |
| Kalimeris | K. indica | [46] | ||
| Saussurea | S. oligantha | [47] | ||
| 13 | Convolvulaceae | Operculina | O. turpethum | [48] |
| 14 | Cucurbitaceae | Actinostemma | A. lobatum | [49] |
| Gynostemma | G. pentaphyllum | [50] | ||
| G. cardiospermum | [51] | |||
| G. compressum | [52] | |||
| G. yixingense | [53] | |||
| Luffa | L. operculata | [54] | ||
| Momordica | M. charantia | [12] | ||
| Neoalsomitra | N. integrifoliola | [55] | ||
| 15 | Cyperaceae | Cyperus | C. rotundus | [56] |
| 16 | Davidiaceae | Davidia | D. involucrata | [57] |
| 17 | Ericaceae | Gaultheria | G. yunnanensi | [58] |
| 18 | Euphorbiaceae | Homonoia | H. riparia | [59] |
| 19 | Fagaceae | Castanea | C. mollissima | [60] |
| 20 | Flacourtiaceae | Oncoba | O. manii | [61] |
| 21 | Gentianaceae | Gentiana | G. rigescens | [62] |
| 22 | Hippocrateaceae | Salacia | S. chinensis | [63] |
| 23 | Juglandaceae | Cyclocarya | C. paliurus | [64] |
| 24 | Labiatae | Glechoma | G. longituba | [65] |
| Salvia | S. aspera | [66] | ||
| S. barrelieri | [67] | |||
| S. hierosolymitana | [68] | |||
| Phlomis | P. umbrosa | [69] | ||
| 25 | Lauraceae | Machilus | M. yaoshansis | [70] |
| 26 | Leguminosae | Astragalus | A. membranaceus | [12] |
| Erythrophleum | E. fordii | [71] | ||
| 27 | Martyniaceae | Ibicella | I. lutea | [72] |
| Probosidea | P. Louisiana | [72] | ||
| 28 | Meliaceae | Aglaia | A. elliptica | [73] |
| A. erythrosperma | [74] | |||
| A. eximia | [75] | |||
| A. forbesii | [76] | |||
| A. foveolata | [77] | |||
| A. lawii | [78] | |||
| A. odorata | [79] | |||
| A. silvestris | [80] | |||
| A. smithii | [81] | |||
| A. tomentosa | [78] | |||
| Amoora | A. yunnanensis | [82] | ||
| Chisocheton | C. cumingianus | [83] | ||
| C. penduliflorus | [84] | |||
| C. polyandrous | [85] | |||
| Dysoxylum | D. binectariferum | [86] | ||
| D. cauliflorum | [87] | |||
| D. densiflorum | [88] | |||
| D. hainanense | [89] | |||
| D. hongkongense | [90] | |||
| D. malabaricum | [91] | |||
| D. mollissimum | [92] | |||
| D. muelleri | [93] | |||
| D. richii | [94] | |||
| Walsura | W. chrysogyne | [95] | ||
| 29 | Moraceae | Ficus | F. pumila | [96] |
| 30 | Myricaceae | Myrica | M. rubra | [97] |
| 31 | Myrsinaceae | Rapanea | R. umbellate | [98] |
| R. lancifolia | [98] | |||
| R. guyanensis | [98] | |||
| 32 | Oleaceae | Forsythia | F. suspense | [99] |
| F. koreana | [100] | |||
| Ligustrum | L. lucidum | [101] | ||
| 33 | Palmae | Borassus | B. flabellifer | [102] |
| 34 | Polypodiaceae | Pyrrosia | P. lingua | [103] |
| Polypodiodes | P. niponica | [104] | ||
| 35 | Pterobryaceae | Esenbeckia | E. yaxhoob | [105] |
| 36 | Rhamnaceae | Colubrina | C. elliptica | [106] |
| Gouania | G. lupuloides | [107] | ||
| Hovenia | H. acerba | [108] | ||
| H. dulcis | [109] | |||
| Zizyphus | Z. glabrata | [110] | ||
| Z. joazeiro | [111] | |||
| Z. jujuba | [112] | |||
| Z. lotus | [113] | |||
| Z. spinosi | [114] | |||
| Z. xylopyra | [115] | |||
| 37 | Rhizophoraceae | Bruguiera | B. gymnorrhiza | [116] |
| Ceriops | C. tagal | [117] | ||
| 38 | Rhoipteleaceae | Rhoiptelea | R. chiliantha | [118] |
| 39 | Rosaceae | Cerasus | C. yedoensis | [119] |
| 40 | Rubiaceae | Gardenia | G. aubryi | [120] |
| G. collinsae | [121] | |||
| G. urvillei | [122] | |||
| 41 | Sapindaceae | Eurycorymbus | E. cavaleriei | [123] |
| Sapindus | S. mukorossi | [124] | ||
| 42 | Scrophulariaceae | Bacopa | B. monnieri | [125] |
| 43 | Simaroubaceae | Ailanthus | A. altissim | [126] |
| A. excelsa | [127] | |||
| Brucea | B. javanica | [128] | ||
| 44 | Sinopteridaceae | Notholaena | N. greggii | [129] |
| N. rigida | [130] | |||
| 45 | Tiliaceae | Corchorus | C. capsularis | [131] |
| 46 | Umbelliferae | Centella | C. asiatica | [132] |
| No. | 1 a [133] | 1 b [134] | 2 a [133] | 2 b [134] | 3 b [135] | 4 a [59] | 5 a [136] | 6 a [136] | 7 b [72] | 8 b [134] | 9 b [134] | 10 b [72] | 11 a [137] | 12 a [138] | 13 b [119] | 14 a [59] | 15 a [135] | 16 b [139] | 17 a [59] | 18 a [140] | 19 a [140] | 20 a [55] | 21 b [141] | 22 b [142] | 23 a [143] | 24 a [143] | 25 a [144] | 26 a [144] | 27 a [133] | 28 a [145] | 29 a [133] | 30 a [145] | 31 a [146] | 32 a [133] | 33 a [133] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39.2 | 39.1 | 39.5 | 39.0 | 39.0 | 39.8 | 40.3 | 40.2 | 33.6 | 33.5 | 39.0 | 76.1 | 39.6 | 41.7 | 39.9 | 40.6 | 39.0 | 38.5 | 39.6 | 34.2 | 34.3 | 33.5 | 39.0 | 38.9 | 39.5 | 39.5 | 39.4 | 39.4 | 39.3 | 39.4 | 39.2 | 39.4 | 38.9 | 39.3 | 39.2 |
| 2 | 28.0 | 26.7 | 28.2 | 27.4 | 27.4 | 28.5 | 28.0 | 27.8 | 25.4 | 25.3 | 27.4 | 35.9 | 25.9 | 28.7 | 34.1 | 28.5 | 28.1 | 27.1 | 28.6 | 26.5 | 26.5 | 25.4 | 27.3 | 27.5 | 28.2 | 28.3 | 28.3 | 28.3 | 26.8 | 27.9 | 26.9 | 28.7 | 26.8 | 26.8 | 26.6 |
| 3 | 78.3 | 78.4 | 77.9 | 78.8 | 78.9 | 78.7 | 79.5 | 79.5 | 76.2 | 76.1 | 78.9 | 76.6 | 78.1 | 78.0 | 218.2 | 78.1 | 77.6 | 78.6 | 78.8 | 75.3 | 75.3 | 76.2 | 78.8 | 78.9 | 78.0 | 77.9 | 78.0 | 78.0 | 88.9 | 78.6 | 89.0 | 78.7 | 88.9 | 88.8 | 88.8 |
| 4 | 40.2 | 39.1 | 39.5 | 39.0 | 39.1 | 40.7 | 40.0 | 40.5 | 37.6 | 37.6 | 39.0 | 37.4 | 39.5 | 40.3 | 47.4 | 38.8 | 39.7 | 38.9 | 40.8 | 38.1 | 38.1 | 37.5 | 37.5 | 38.9 | 39.6 | 39.6 | 40.0 | 40.0 | 39.8 | 40.4 | 39.7 | 40.2 | 39.7 | 39.7 | 39.6 |
| 5 | 61.7 | 61.0 | 56.3 | 56.0 | 55.9 | 62.1 | 57.3 | 62.1 | 49.5 | 49.5 | 55.9 | 48.1 | 54.2 | 56.9 | 55.3 | 66.3 | 54.1 | 55.7 | 62.2 | 49.7 | 49.4 | 49.5 | 55.7 | 55.9 | 56.4 | 56.4 | 56.4 | 56.4 | 56.5 | 61.5 | 56.5 | 61.4 | 56.4 | 56.5 | 56.3 |
| 6 | 67.6 | 68.4 | 18.7 | 18.3 | 18.3 | 67.9 | 18.9 | 69.9 | 18.2 | 18.2 | 18.3 | 18.1 | 27.6 | 18.7 | 19.6 | 212.3 | 37.1 | 18.3 | 67.9 | 18.6 | 18.6 | 18.2 | 18.2 | 18.3 | 18.8 | 18.8 | 18.8 | 18.8 | 18.6 | 80.1 | 18.5 | 79.9 | 18.4 | 18.5 | 18.4 |
| 7 | 47.4 | 46.8 | 35.2 | 34.8 | 35.2 | 48.3 | 35.9 | 47.3 | 34.7 | 35.1 | 34.8 | 34.6 | 74.7 | 36.6 | 34.5 | 53.7 | 214.0 | 33.9 | 48.4 | 35.2 | 35.2 | 34.8 | 35.3 | 34.9 | 35.4 | 35.4 | 35.2 | 35.2 | 35.3 | 45.2 | 35.2 | 45.1 | 35.0 | 35.2 | 35.1 |
| 8 | 41.1 | 40.8 | 40.0 | 39.8 | 40.4 | 41.9 | 40.9 | 42.0 | 39.9 | 40.5 | 39.8 | 40.5 | 46.0 | 41.1 | 40.2 | 47.3 | 56.0 | 40.2 | 40.8 | 40.2 | 40.2 | 40.0 | 40.8 | 39.8 | 40.2 | 40.3 | 37.4 | 37.4 | 40.2 | 41.1 | 40.0 | 41.2 | 39.9 | 40.1 | 40.0 |
| 9 | 50.1 | 49.5 | 50.4 | 50.2 | 50.6 | 50.6 | 50.9 | 50.7 | 49.9 | 45.3 | 50.1 | 50.7 | 50.4 | 56.1 | 50.0 | 51.1 | 51.1 | 53.4 | 50.9 | 50.6 | 50.5 | 49.8 | 50.6 | 49.9 | 51.0 | 50.9 | 50.6 | 50.5 | 49.3 | 50.2 | 50.4 | 50.1 | 50.2 | 50.3 | 50.1 |
| 10 | 39.3 | 39.2 | 37.3 | 37.1 | 37.1 | 39.7 | 38.2 | 40.2 | 37.2 | 36.9 | 37.1 | 43.3 | 37.6 | 39.6 | 36.8 | 44.5 | 37.2 | 37.5 | 39.9 | 37.7 | 37.7 | 37.3 | 37.1 | 37.1 | 37.5 | 37.5 | 39.6 | 39.6 | 37.1 | 39.7 | 37.0 | 39.7 | 37.0 | 37.0 | 36.9 |
| 11 | 31.9 | 30.9 | 32.0 | 31.2 | 21.5 | 22.0 | 32.0 | 32.0 | 30.9 | 29.0 | 31.2 | 34.3 | 32.3 | 70.5 | 22.0 | 22.6 | 31.2 | 39.1 | 22.0 | 31.7 | 32.5 | 30.4 | 21.2 | 30.6 | 32.6 | 32.3 | 32.6 | 32.3 | 32.3 | 32.1 | 32.0 | 32.1 | 27.9 | 30.9 | 30.6 |
| 12 | 70.9 | 70.5 | 70.9 | 70.8 | 25.4 | 27.7 | 71.9 | 71.9 | 71.0 | 68.4 | 70.7 | 72.1 | 71.1 | 40.8 | 27.5 | 27.4 | 70.8 | 214.1 | 28.0 | 71.1 | 70.7 | 70.6 | 24.7 | 69.9 | 72.5 | 72.6 | 71.0 | 71.0 | 70.9 | 71.0 | 71.0 | 71.0 | 78.5 | 70.2 | 70.1 |
| 13 | 48.1 | 47.2 | 48.5 | 47.7 | 42.3 | 41.0 | 49.5 | 49.6 | 47.7 | 45.3 | 48.5 | 46.7 | 48.7 | 41.1 | 42.3 | 41.3 | 49.9 | 56.2 | 41.2 | 48.4 | 49.4 | 48.9 | 46.7 | 49.2 | 52.4 | 50.4 | 48.6 | 48.6 | 48.7 | 48.3 | 48.6 | 48.3 | 46.8 | 49.5 | 49.4 |
| 14 | 51.5 | 51.3 | 51.6 | 51.6 | 50.3 | 48.3 | 52.6 | 52.5 | 51.7 | 48.8 | 51.6 | 51.2 | 52.0 | 50.6 | 50.2 | 48.4 | 50.2 | 54.7 | 45.8 | 52.2 | 52.3 | 51.7 | 49.8 | 51.2 | 51.2 | 51.0 | 51.8 | 51.7 | 51.9 | 51.7 | 51.8 | 51.7 | 52.2 | 51.5 | 51.3 |
| 15 | 31.3 | 30.9 | 31.8 | 31.1 | 31.2 | 44.6 | 32.0 | 31.4 | 31.1 | 31.3 | 31.1 | 30.9 | 36.1 | 31.3 | 31.1 | 44.5 | 33.2 | 30.8 | 50.5 | 32.3 | 32.2 | 31.3 | 31.2 | 31.1 | 33.8 | 32.6 | 31.5 | 31.4 | 31.5 | 31.4 | 31.4 | 31.2 | 31.3 | 30.8 | 30.6 |
| 16 | 26.8 | 25.4 | 26.8 | 25.5 | 27.6 | 74.2 | 27.1 | 27.1 | 26.5 | 24.1 | 26.4 | 26.1 | 28.5 | 25.5 | 24.8 | 73.9 | 27.0 | 24.7 | 220.6 | 25.5 | 25.7 | 26.8 | 28.7 | 25.2 | 30.8 | 28.7 | 27.0 | 26.9 | 26.8 | 26.8 | 26.8 | 26.8 | 27.2 | 26.7 | 26.6 |
| 17 | 54.6 | 53.5 | 54.7 | 53.6 | 49.9 | 52.2 | 51.3 | 50.9 | 53.5 | 46.9 | 49.9 | 54.4 | 54.2 | 50.3 | 49.8 | 52.5 | 53.8 | 46.1 | 58.5 | 49.9 | 49.9 | 50.6 | 37.5 | 54.7 | 48.2 | 50.9 | 54.6 | 54.8 | 54.8 | 54.8 | 54.8 | 54.8 | 54.1 | 51.7 | 51.5 |
| 18 | 17.5 | 17.2 | 16.2 | 16.2 | 16.2 | 17.8 | 16.3 | 17.6 | 16.5 | 15.9 | 16.2 | 15.9 | 10.7 | 17.0 | 15.2 | 16.7 | 15.3 | 15.9 | 18.0 | 16.5 | 16.6 | 15.5 | 15.5 | 15.6 | 15.8 | 15.9 | 16.5 | 16.5 | 16.5 | 17.4 | 15.9 | 17.4 | 16.3 | 16.3 | 16.2 |
| 19 | 17.4 | 17.2 | 15.8 | 15.7 | 15.5 | 18.8 | 16.8 | 17.7 | 15.7 | 15.2 | 15.7 | 11.7 | 16.7 | 17.0 | 16.0 | 17.6 | 15.9 | 15.8 | 17.9 | 15.6 | 15.7 | 16.0 | 15.3 | 16.1 | 16.3 | 16.5 | 16.3 | 16.3 | 16.0 | 17.7 | 16.4 | 17.6 | 15.7 | 16.0 | 16.0 |
| 20 | 73.9 | 73.9 | 72.9 | 74.0 | 75.4 | 75.1 | 74.7 | 74.7 | 74.4 | 75.3 | 74.5 | 73.4 | 73.3 | 73.9 | 75.4 | 75.1 | 73.3 | 73.1 | 74.6 | 86.7 | 87.1 | 86.4 | 140.3 | 76.7 | 155.5 | 140.1 | 73.0 | 73.1 | 73.0 | 73.0 | 73.0 | 73.0 | 73.0 | 83.4 | 83.3 |
| 21 | 26.9 | 26.7 | 26.9 | 26.8 | 24.8 | 27.0 | 22.4 | 22.4 | 27.0 | 26.9 | 21.8 | 26.2 | 27.3 | 25.8 | 25.4 | 27.1 | 27.1 | 26.4 | 27.0 | 26.9 | 26.9 | 21.3 | 173.9 | 19.4 | 108.1 | 13.2 | 27.5 | 27.4 | 27.2 | 27.1 | 27.1 | 27.0 | 26.9 | 22.5 | 22.3 |
| 22 | 35.7 | 34.5 | 35.8 | 34.8 | 40.5 | 44.3 | 44.0 | 44.0 | 34.7 | 36.3 | 42.3 | 35.3 | 35.2 | 41.8 | 40.4 | 44.2 | 35.6 | 37.8 | 41.3 | 32.8 | 32.5 | 39.1 | 145.7 | 35.8 | 32.7 | 123.6 | 32.1 | 32.1 | 36.0 | 35.8 | 35.9 | 35.8 | 36.5 | 36.2 | 36.1 |
| 23 | 22.9 | 22.3 | 22.9 | 22.4 | 22.6 | 23.6 | 19.4 | 18.9 | 22.4 | 22.4 | 21.8 | 22.4 | 23.2 | 23.3 | 22.5 | 23.6 | 23.0 | 22.4 | 23.6 | 28.7 | 28.6 | 25.9 | 78.0 | 16.3 | 27.1 | 27.4 | 30.8 | 30.6 | 23.1 | 23.0 | 23.0 | 23.0 | 23.0 | 23.3 | 23.1 |
| 24 | 126.2 | 125.0 | 126.2 | 125.2 | 124.7 | 126.4 | 45.4 | 45.4 | 125.0 | 124.7 | 124.6 | 125.3 | 126.5 | 126.0 | 124.6 | 126.4 | 126.4 | 124.9 | 125.9 | 85.6 | 88.3 | 86.4 | 121.9 | 36.5 | 125.3 | 123.9 | 76.4 | 76.0 | 126.2 | 126.4 | 126.4 | 126.3 | 126.6 | 126.0 | 126.0 |
| 25 | 130.5 | 131.4 | 130.6 | 131.4 | 131.6 | 131.1 | 71.5 | 71.5 | 131.8 | 131.9 | 131.9 | 131.2 | 130.8 | 130.7 | 131.7 | 131.2 | 131.0 | 131.5 | 131.5 | 70.1 | 70.0 | 70.2 | 137.8 | 73.1 | 131.2 | 131.2 | 150.2 | 150.0 | 130.0 | 130.8 | 130.8 | 130.8 | 130.9 | 131.0 | 130.8 |
| 26 | 25.8 | 25.7 | 25.8 | 25.8 | 25.7 | 26.2 | 29.4 | 29.1 | 25.7 | 25.7 | 25.8 | 25.8 | 25.9 | 26.1 | 25.7 | 26.2 | 26.0 | 25.7 | 26.2 | 27.3 | 26.5 | 24.6 | 25.7 | 33.0 | 25.8 | 25.7 | 110.1 | 109.8 | 25.9 | 25.8 | 25.9 | 25.8 | 25.8 | 25.8 | 25.7 |
| 27 | 17.7 | 17.7 | 17.6 | 17.8 | 17.7 | 18.1 | 29.1 | 29.4 | 17.7 | 17.7 | 17.8 | 17.7 | 17.8 | 17.7 | 17.7 | 18.1 | 17.8 | 17.7 | 18.1 | 27.6 | 29.0 | 28.0 | 18.2 | 27.1 | 17.7 | 17.7 | 18.2 | 18.4 | 17.5 | 17.7 | 17.7 | 17.7 | 17.7 | 17.8 | 17.8 |
| 28 | 31.9 | 30.9 | 28.6 | 28.1 | 28.0 | 32.4 | 28.6 | 31.9 | 28.3 | 28.3 | 28.1 | 28.0 | 28.8 | 29.0 | 26.7 | 28.5 | 28.0 | 28.0 | 32.4 | 29.4 | 29.4 | 28.3 | 27.9 | 28.0 | 28.7 | 28.8 | 28.7 | 28.7 | 28.2 | 31.7 | 28.2 | 32.1 | 28.1 | 28.2 | 28.0 |
| 29 | 16.4 | 15.5 | 16.4 | 15.5 | 15.4 | 16.9 | 16.2 | 16.1 | 22.1 | 22.2 | 15.4 | 22.1 | 16.6 | 16.6 | 21.0 | 16.6 | 15.6 | 15.3 | 16.9 | 22.4 | 22.4 | 22.1 | 15.6 | 15.3 | 16.6 | 16.3 | 15.8 | 15.9 | 16.9 | 16.4 | 16.6 | 16.8 | 16.6 | 16.8 | 16.7 |
| 30 | 17.0 | 16.9 | 17.0 | 16.9 | 16.5 | 18.2 | 17.4 | 17.4 | 17.0 | 19.4 | 17.2 | 16.5 | 17.2 | 16.8 | 16.3 | 18.9 | 18.8 | 17.5 | 17.5 | 18.2 | 18.0 | 17.0 | 16.1 | 17.1 | 17.0 | 17.0 | 17.1 | 17.1 | 16.9 | 16.8 | 17.1 | 16.8 | 17.4 | 17.4 | 17.5 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, J.; Zheng, C.; Qu, L.; Liu, Y.; Han, L.; Yu, H.; Zhang, Y.; Wang, T. Plant Resources, 13C-NMR Spectral Characteristic and Pharmacological Activities of Dammarane-Type Triterpenoids. Molecules 2016, 21, 1047. https://doi.org/10.3390/molecules21081047
Ruan J, Zheng C, Qu L, Liu Y, Han L, Yu H, Zhang Y, Wang T. Plant Resources, 13C-NMR Spectral Characteristic and Pharmacological Activities of Dammarane-Type Triterpenoids. Molecules. 2016; 21(8):1047. https://doi.org/10.3390/molecules21081047
Chicago/Turabian StyleRuan, Jingya, Chang Zheng, Lu Qu, Yanxia Liu, Lifeng Han, Haiyang Yu, Yi Zhang, and Tao Wang. 2016. "Plant Resources, 13C-NMR Spectral Characteristic and Pharmacological Activities of Dammarane-Type Triterpenoids" Molecules 21, no. 8: 1047. https://doi.org/10.3390/molecules21081047





