Carbon Nanotube Based Groundwater Remediation: The Case of Trichloroethylene
Abstract
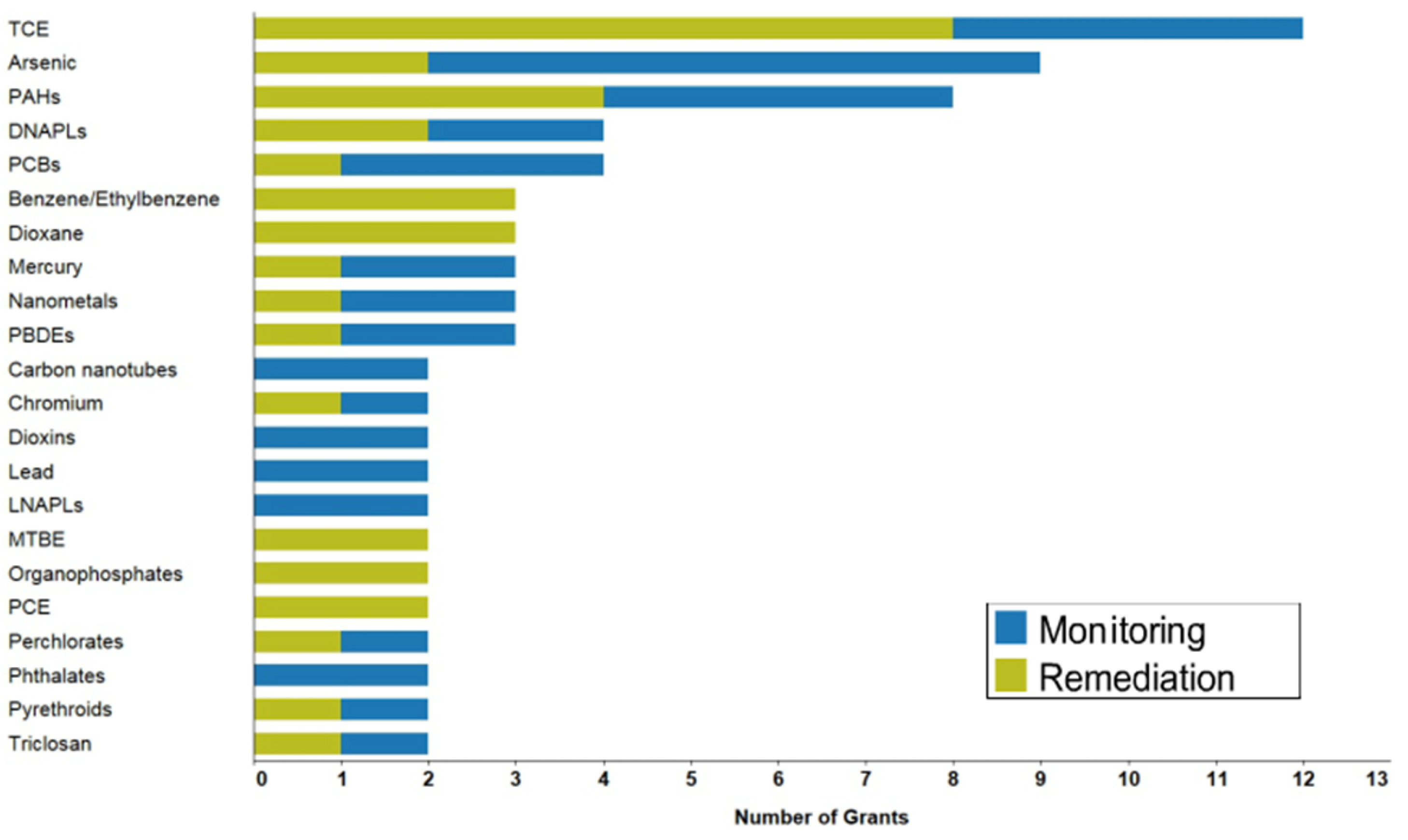
:1. Introduction
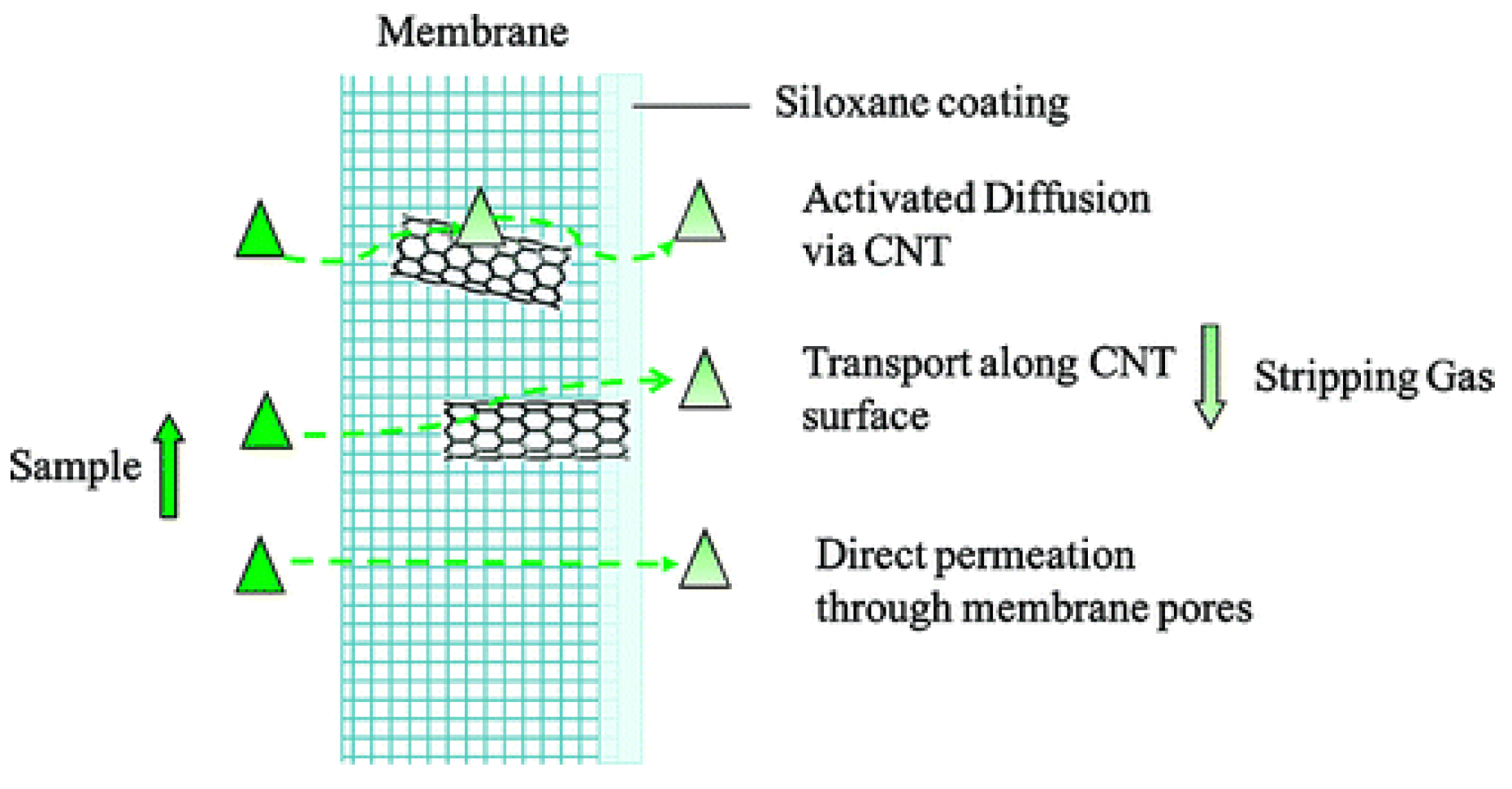
2. Strategies for TCE Removal: CNT Membranes and Hybrids
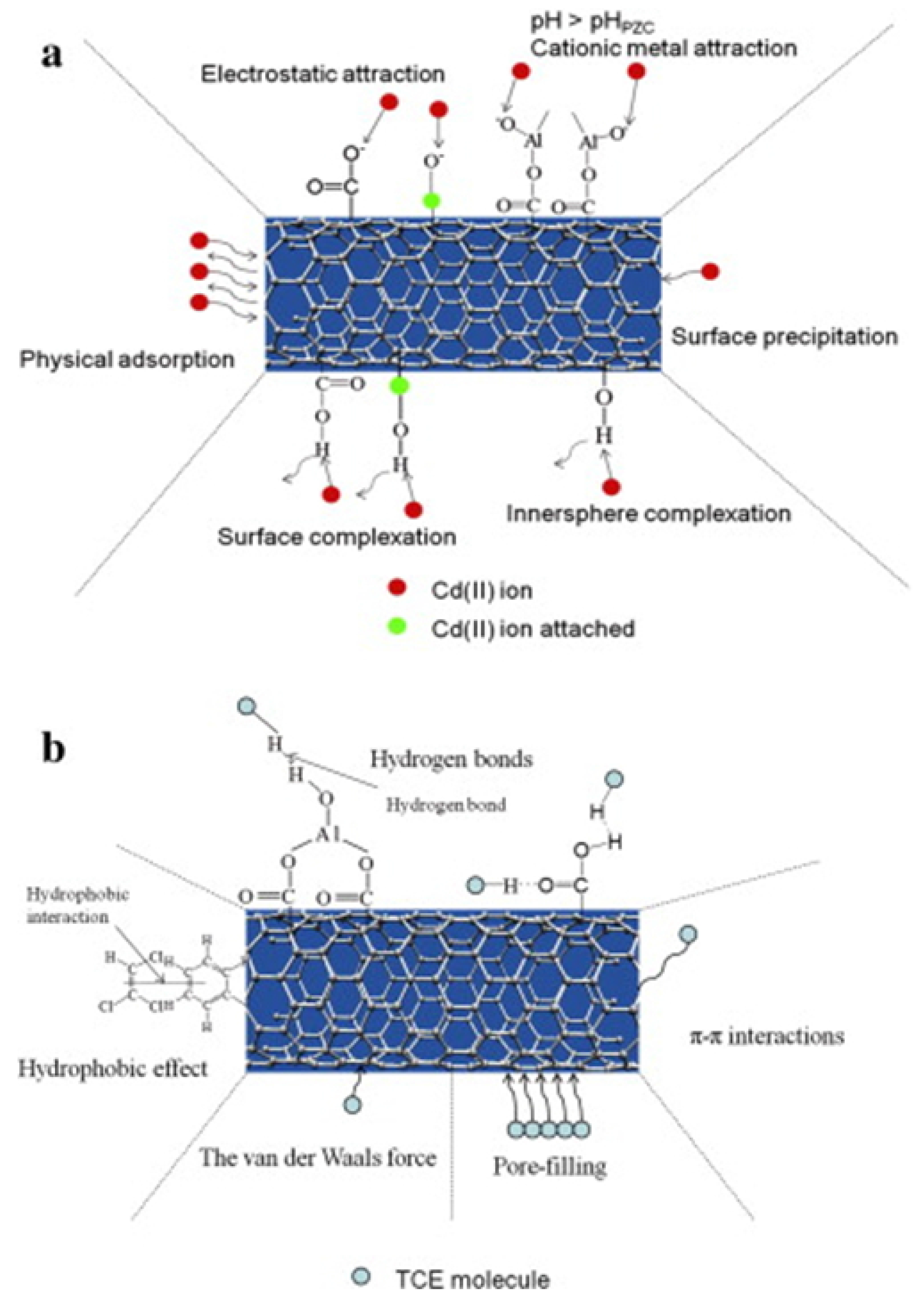
2.1. CNT Interactions with Matrix and COCs
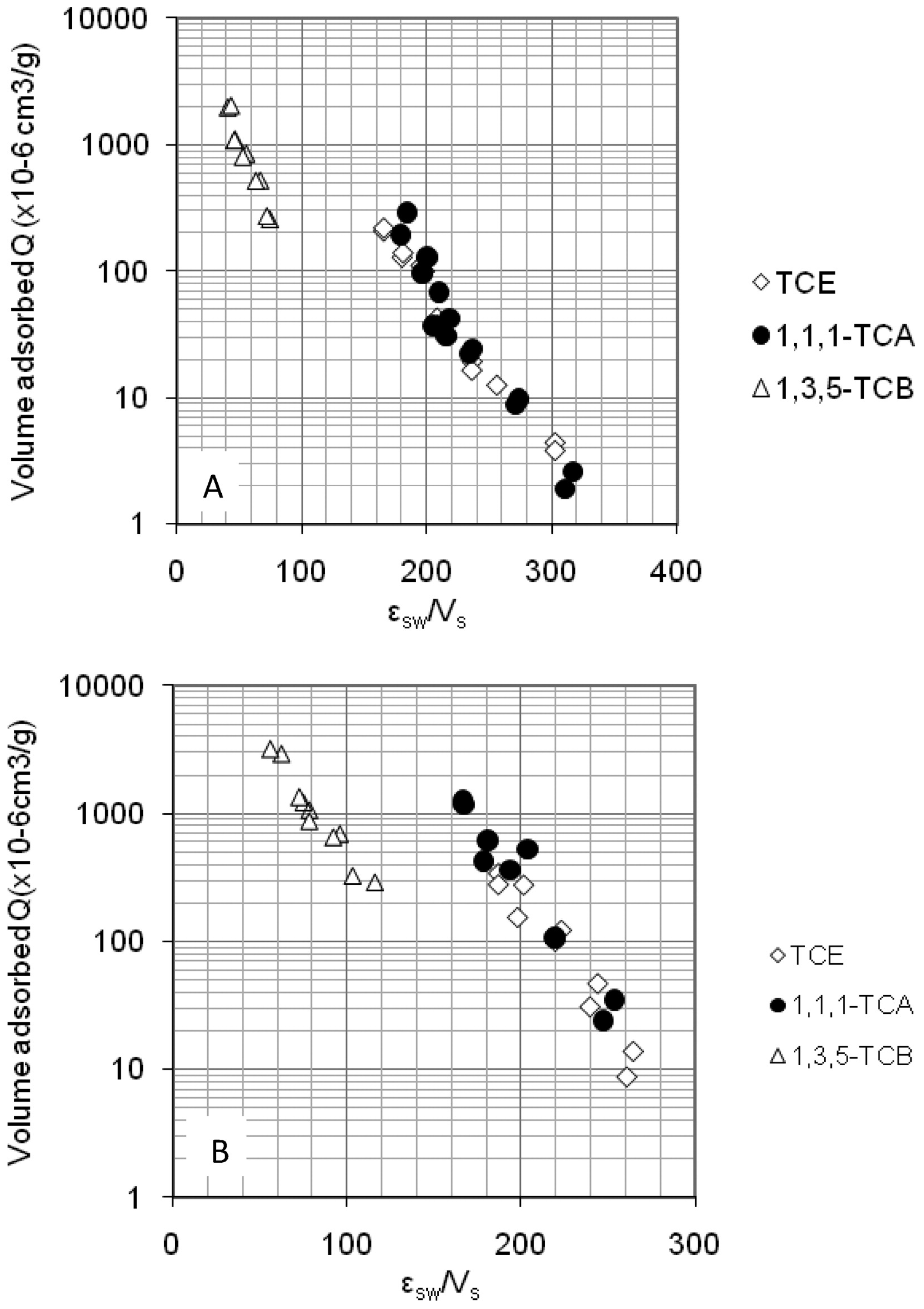
2.2. Competitive Adsorption of COCs
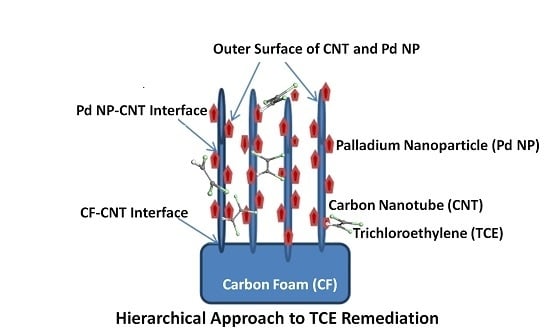
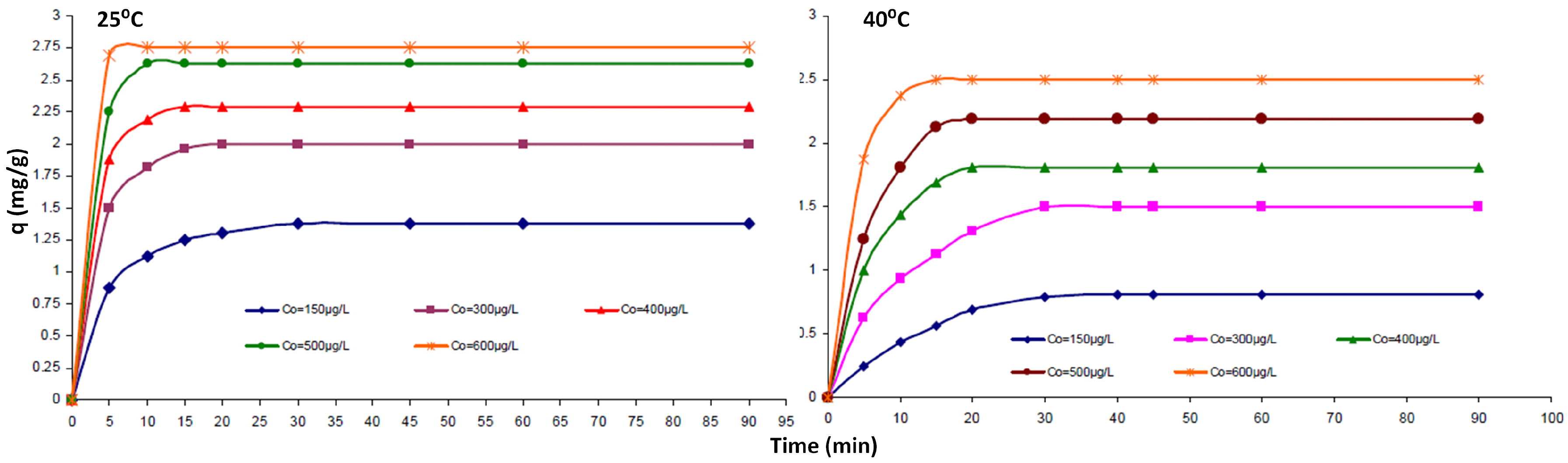
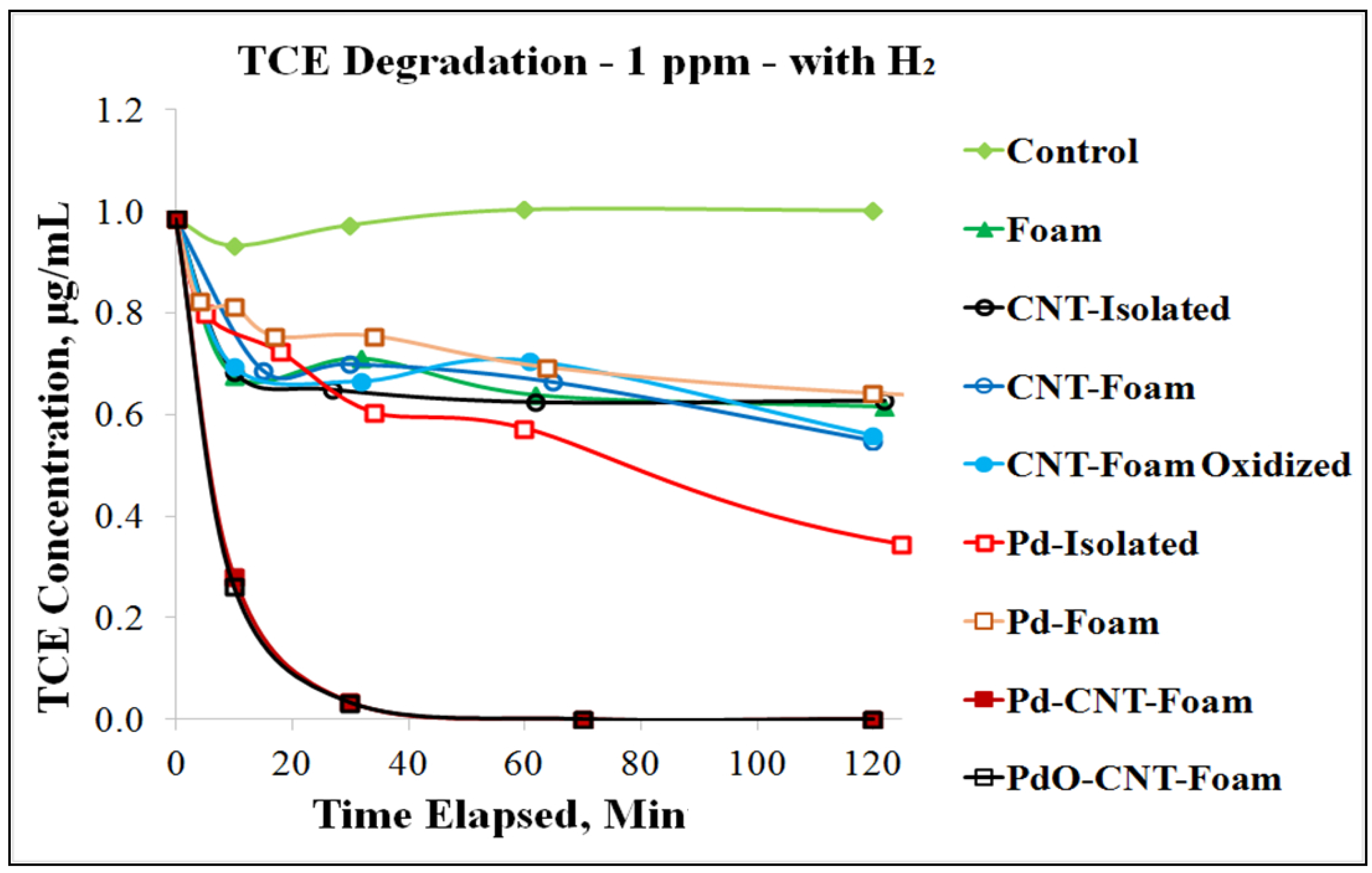
3. CNT Hierarchical Hybrid Approach for TCE Remediation
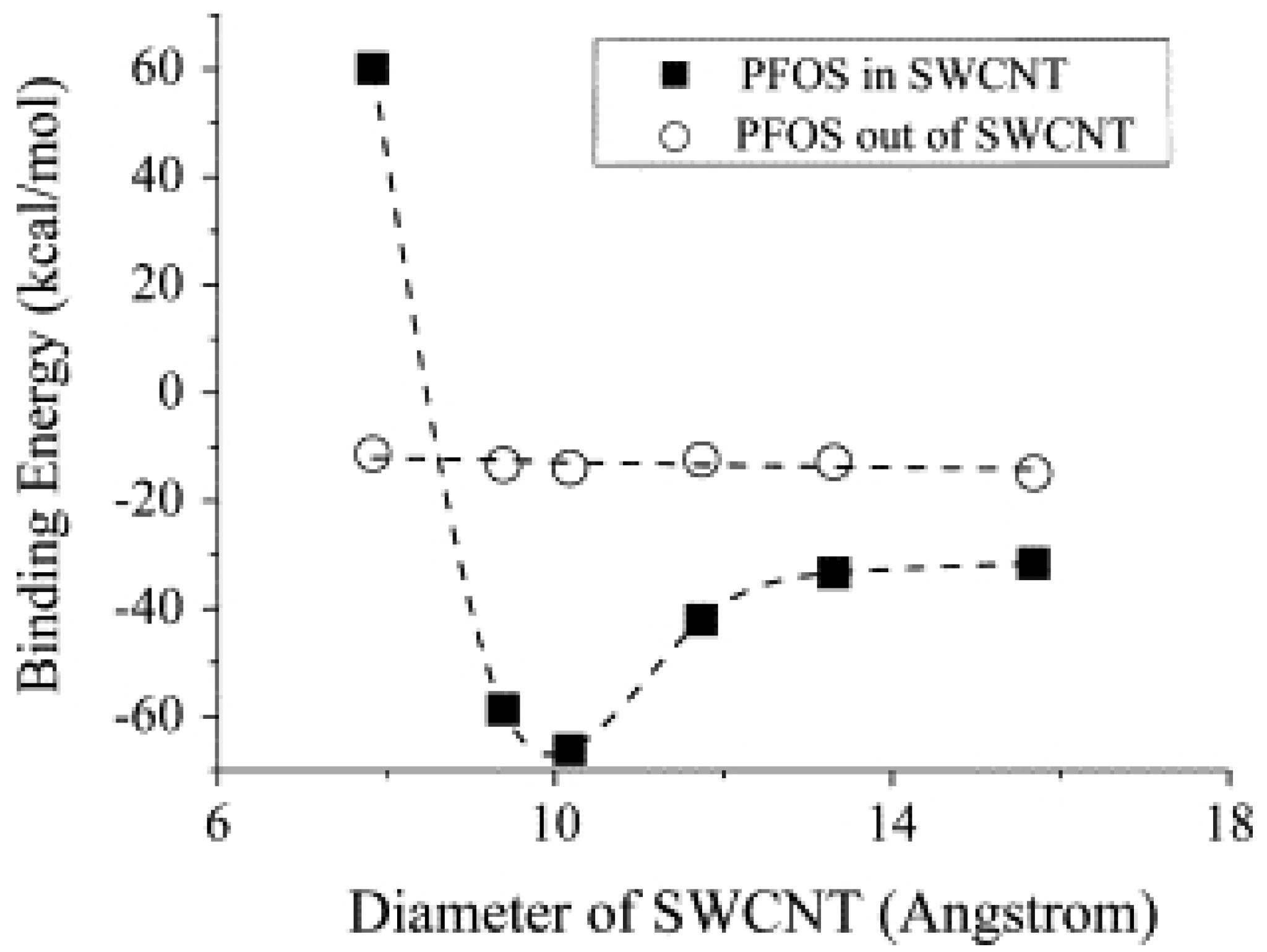
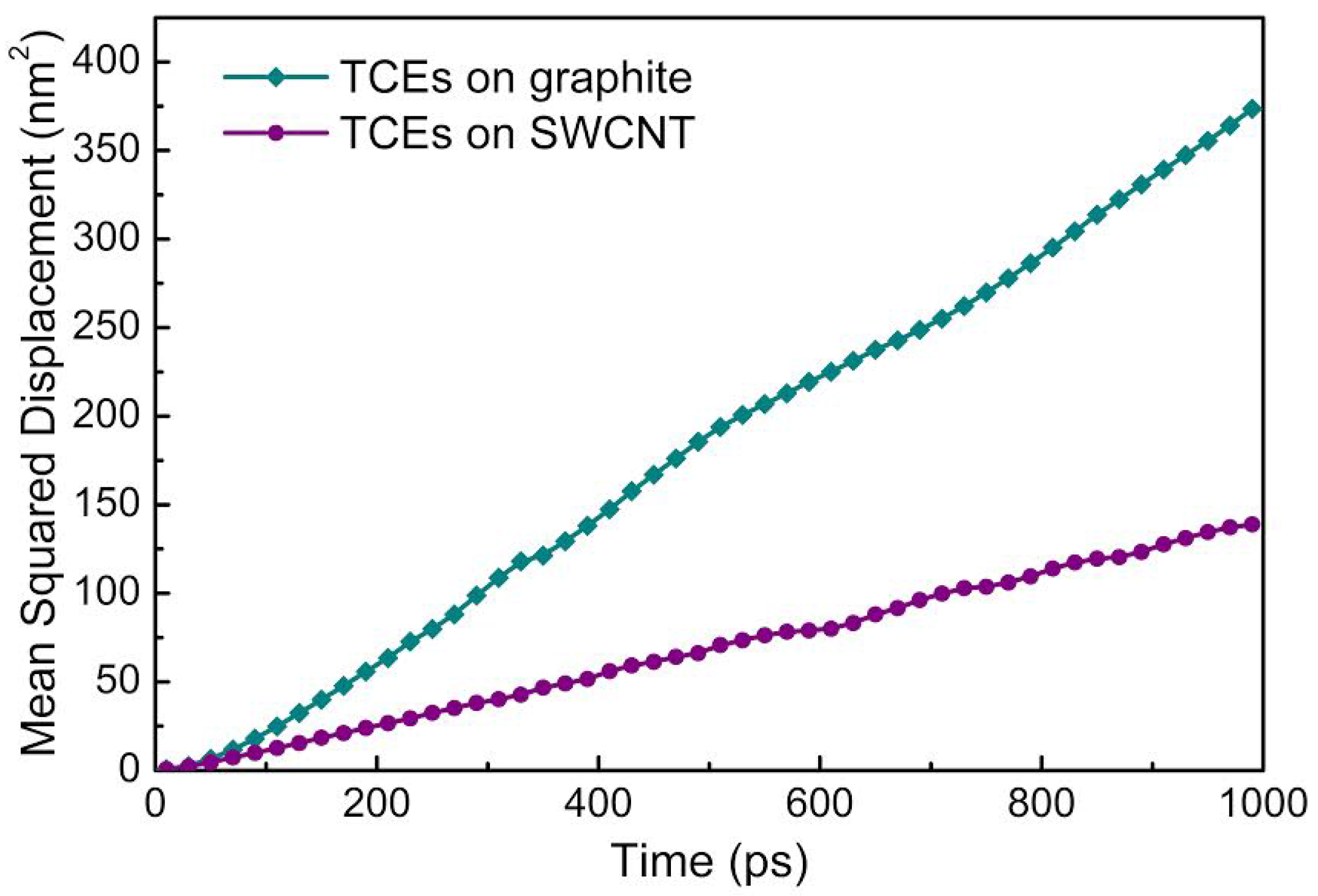
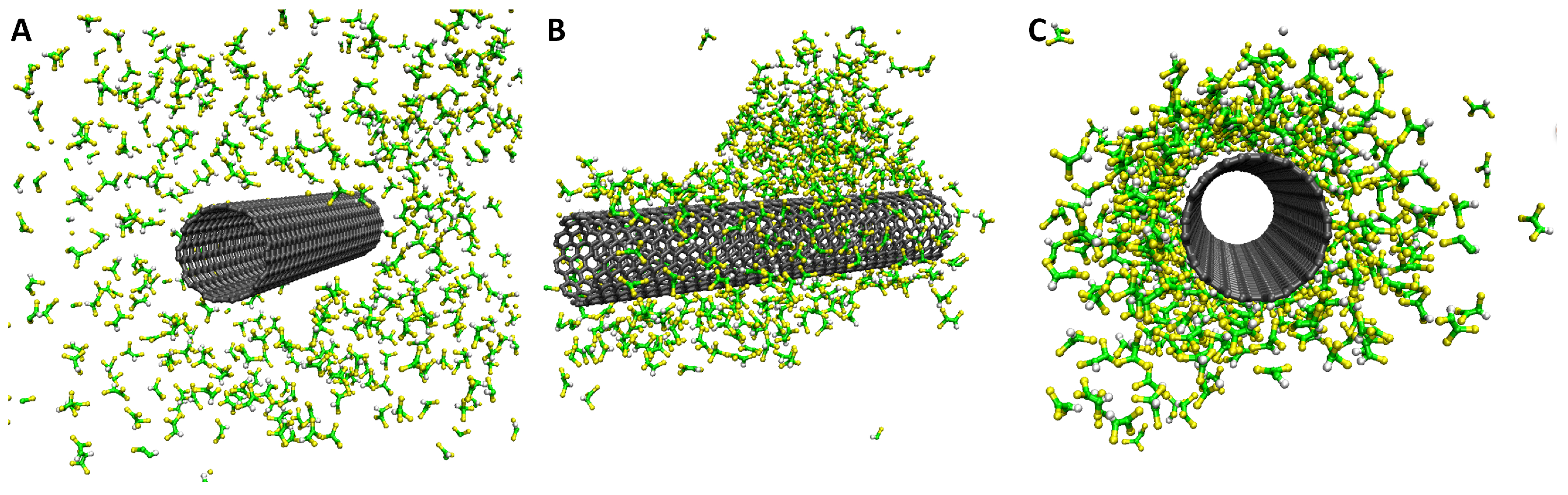
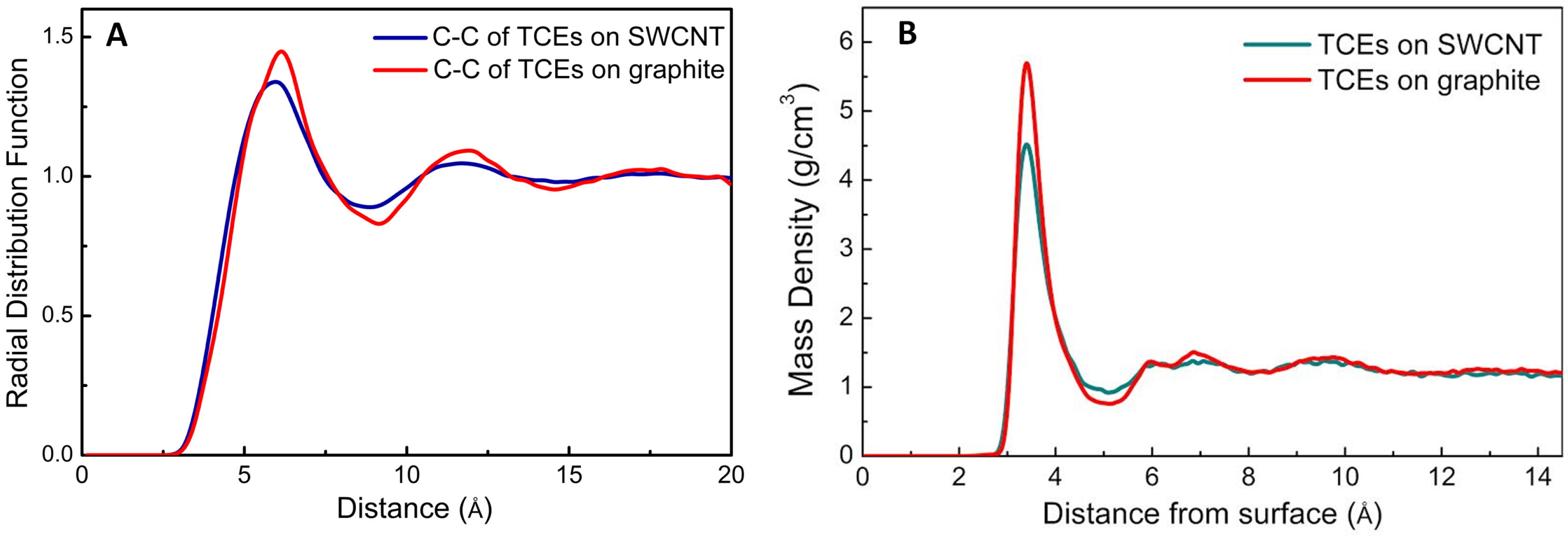
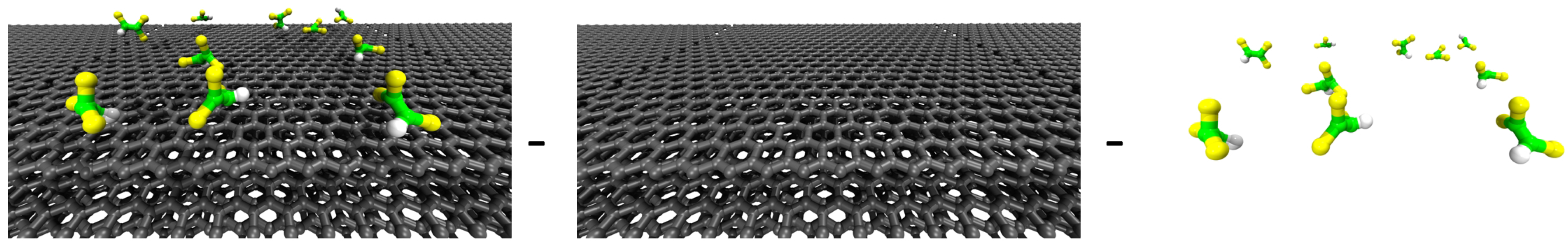
4. TCE Adsorption on CNT: Results from Molecular Modeling
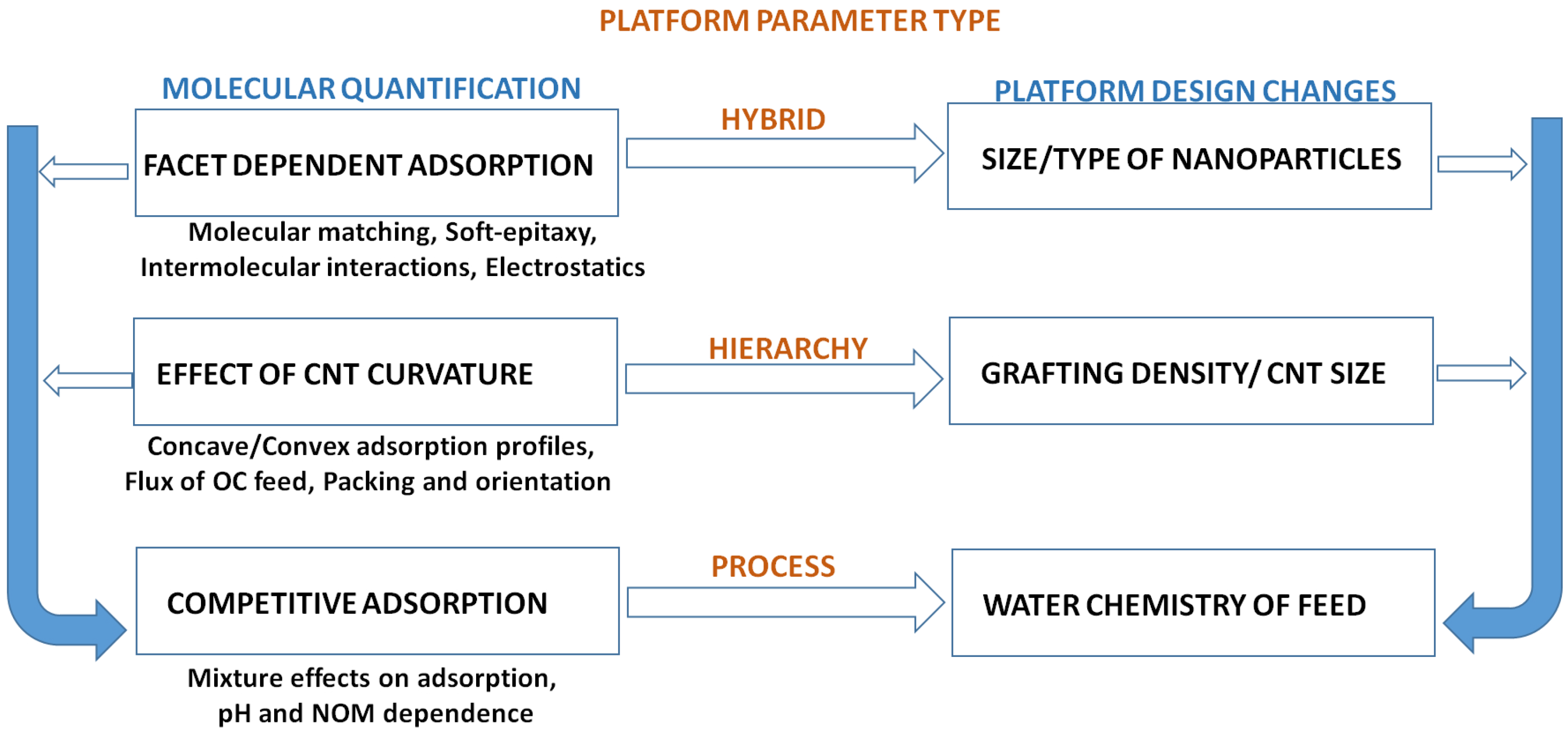
5. Conclusions & Future Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McKnight, U.S.; Funder, S.G.; Rasmussen, J.J.; Finkel, M.; Binning, P.J.; Bjerg, P.L. An integrated model for assessing the risk of TCE groundwater contamination to human receptors and surface water ecosystems. Ecol. Eng. 2010, 36, 1126–1137. [Google Scholar] [CrossRef] [Green Version]
- Einarson, M.D.; Mackay, D.M. Peer reviewed: Predicting impacts of groundwater contamination. Environ. Sci. Technol. 2001, 35, 66A–73A. [Google Scholar] [CrossRef] [PubMed]
- Chapman, S.W.; Parker, B.L.; Cherry, J.A.; Aravena, R.; Hunkeler, D. Groundwater—Surface water interaction and its role on TCE groundwater plume attenuation. J. Contam. Hydrol. 2007, 91, 203–232. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Bayer-Raich, M.; Holder, T.; Kolesar, C.; Müller, D.; Ptak, T. Quantification of groundwater contamination in an urban area using integral pumping tests. J. Contam. Hydrol. 2004, 75, 183–213. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.J.; Lee, J.Y.; Yi, M.J.; Kim, H.S.; Lee, K.K. Soil contamination with TCE in an industrial complex: Contamination levels and implication for groundwater contamination. Geosci. J. 2010, 14, 313–320. [Google Scholar] [CrossRef]
- Yoo, K.; Shukla, S.K.; Ahn, J.J.; Oh, K.; Park, J. Decision tree-based data mining and rule induction for identifying hydrogeological parameters that influence groundwater pollution sensitivity. J. Clean. Prod. 2016, 122, 277–286. [Google Scholar] [CrossRef]
- Glaze, W.H.; Kang, J.W. Advanced oxidation processes for treating groundwater contaminated with TCE and PCE: Laboratory studies. J. Am. Water Works Assoc. 1988, 80, 57–63. [Google Scholar]
- Aieta, E.M.; Reagan, K.M.; Lang, J.S.; McReynolds, L.; Kang, J.W.; Glaze, W.H. Advanced oxidation processes for treating groundwater contaminated with TCE and PCE: Pilot-scale evaluations. J. Am. Water Works Assoc. 1988, 80, 64–72. [Google Scholar]
- Hirvonen, A.; Tuhkanen, T.; Kalliokoski, P. Treatment of TCE-and PCE contaminated groundwater using UV/H2O2 and O3/H2O2 oxidation processes. Water Sci. Technol. 1996, 33, 67–73. [Google Scholar] [CrossRef]
- Cundy, A.B.; Hopkinson, L.; Whitby, R.L. Use of iron-based technologies in contaminated land and groundwater remediation: A review. Sci. Total Environ. 2008, 400, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Lien, P.; Yang, Z.; Chang, Y.; Tu, Y.; Kao, C. Enhanced bioremediation of TCE-contaminated groundwater with coexistence of fuel oil: Effectiveness and mechanism study. Chem. Eng. J. 2016, 289, 525–536. [Google Scholar] [CrossRef]
- Han, J.; Xin, J.; Zheng, X.; Kolditz, O.; Shao, H. Remediation of trichloroethylene-contaminated groundwater by three modifier-coated microscale zero-valent iron. Environ. Sci. Pollut. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Harding-Marjanovic, K.C.; Yi, S.; Weathers, T.S.; Sharp, J.O.; Sedlak, D.L.; Alvarez-Cohen, L. Effects of aqueous film-forming foams (AFFFs) on trichloroethene (TCE) dechlorination by a dehalococcoides mccartyi-containing microbial community. Environ. Sci. Technol. 2016, 50, 3352–3361. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, D.; Pollack, G.M.; Collins, L.B.; Rusyn, I. Pharmacokinetic analysis of trichloroethylene metabolism in male B6C3F1 mice: Formation and disposition of trichloroacetic acid, dichloroacetic acid, S-(1, 2-dichlorovinyl) glutathione and S-(1, 2-dichlorovinyl)-l-cysteine. Toxicol. Appl. Pharmacol. 2009, 238, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Hua, L.Q.; Nishijima, W.; Shoto, E.; Okada, M. Biodegradation of trichloroethylene (TCE) adsorbed on granular activated carbon (GAC). Water Res. 2000, 34, 4139–4142. [Google Scholar] [CrossRef]
- Tseng, H.H.; Su, J.G.; Liang, C. Synthesis of granular activated carbon/zero valent iron composites for simultaneous adsorption/dechlorination of trichloroethylene. J. Hazard. Mater. 2011, 192, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Takasaki, J.; Kumazawa, H. Photocatalytic degradation of gaseous ammonia and trichloroethylene over TiO2 ultrafine powders deposited on activated carbon particles. Chem. Eng. Commun. 2003, 190, 322–333. [Google Scholar] [CrossRef]
- Jou, G.C.J. Application of activated carbon in a microwave radiation field to treat trichloroethylene. Carbon 1998, 36, 1643–1648. [Google Scholar] [CrossRef]
- Sakoda, A.; Kawazoe, K.; Suzuki, M. Adsorption of tri-and tetra-chloroethylene from aqueous solutions on activated carbon fibers. Water Res. 1987, 21, 717–722. [Google Scholar] [CrossRef]
- Miyake, Y.; Sakoda, A.; Yamanashi, H.; Kaneda, H.; Suzuki, M. Activated carbon adsorption of trichloroethylene (TCE) vapor stripped from TCE-contaminated water. Water Res. 2003, 37, 1852–1858. [Google Scholar] [CrossRef]
- Kilduff, J.E.; Karanfil, T.; Weber, W.J. Competitive effects of nondisplaceable organic compounds on trichloroethylene uptake by activated carbon. I. Thermodynamic predictions and model sensitivity analyses. J. Colloid Interface Sci. 1998, 205, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Kilduff, J.E.; Karanfil, T.; Weber, W.J. Competitive effects of nondisplaceable organic compounds on trichloroethylene uptake by activated carbon. II. Model verification and applicability to natural organic matter. J. Colloid Interface Sci. 1998, 205, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Kim, E.S. Co-adsorption of trichloroethylene and arsenate by iron-impregnated granular activated carbon. Water Environ. Res. 2016, 88, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Lee, S.S.; Rajapaksha, A.U.; Vithanage, M.; Zhang, M.; Cho, J.S.; Lee, S.E.; Ok, Y.S. Trichloroethylene adsorption by pine needle biochars produced at various pyrolysis temperatures. Bioresour. Technol. 2013, 143, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Juuti, S.; Norokorpi, Y.; Ruuskanen, J. Trichloroacetic acid (TCA) in pine needles caused by atmospheric emissions of kraft pulp mills. Chemosphere 1995, 30, 439–448. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef] [PubMed]
- Mauter, M.S.; Elimelech, M. Environmental applications of carbon-based nanomaterials. Environ. Sci. Technol. 2008, 42, 5843–5859. [Google Scholar] [CrossRef] [PubMed]
- Savage, N.; Diallo, M.S. Nanomaterials and water purification: Opportunities and challenges. J. Nanopart. Res. 2005, 7, 331–342. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Sae-Khow, O.; Mitra, S. Carbon nanotube immobilized composite hollow fiber membranes for pervaporative removal of volatile organics from water. J. Phys. Chem. C 2010, 114, 16351–16356. [Google Scholar] [CrossRef]
- Krause, R.W.; Mamba, B.B.; Dlamini, L.N.; Durbach, S.H. Fe–Ni nanoparticles supported on carbon nanotube-co-cyclodextrin polyurethanes for the removal of trichloroethylene in water. J. Nanopart. Res. 2010, 12, 449–456. [Google Scholar] [CrossRef]
- Singh, G.; Rana, D.; Matsuura, T.; Ramakrishna, S.; Narbaitz, R.M.; Tabe, S. Removal of disinfection byproducts from water by carbonized electrospun nanofibrous membranes. Sep. Purif. Technol. 2010, 74, 202–212. [Google Scholar] [CrossRef]
- Yu, J.G.; Zhao, X.H.; Yang, H.; Chen, X.H.; Yang, Q.; Yu, L.Y.; Jiang, J.H.; Chen, X.Q. Aqueous adsorption and removal of organic contaminants by carbon nanotubes. Sci. Total Environ. 2014, 482, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Sae-Khow, O.; Mitra, S. Simultaneous extraction and concentration in carbon nanotube immobilized hollow fiber membranes. Anal. Chem. 2010, 82, 5561–5567. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Zhu, Z.; Li, F.; Liu, S.; Wang, L.; Tang, X.; Rudolph, V. Investigation of Gas permeability in carbon nanotube (CNT)-polymer matrix membranes via modifying CNTs with functional groups/metals and controlling modification location. J. Phys. Chem. C 2011, 115, 6661–6670. [Google Scholar] [CrossRef]
- Xiao, S.; Shen, M.; Guo, R.; Huang, Q.; Wang, S.; Shi, X. Fabrication of multiwalled carbon nanotube-reinforced electrospun polymer nanofibers containing zero-valent iron nanoparticles for environmental applications. J. Mater. Chem. 2010, 20, 5700–5708. [Google Scholar] [CrossRef]
- Ajmani, G.S.; Goodwin, D.; Marsh, K.; Fairbrother, D.H.; Schwab, K.J.; Jacangelo, J.G.; Huang, H. Modification of low pressure membranes with carbon nanotube layers for fouling control. Water Res. 2012, 46, 5645–5654. [Google Scholar] [CrossRef] [PubMed]
- Upadhyayula, V.K.; Deng, S.; Mitchell, M.C.; Smith, G.B. Application of carbon nanotube technology for removal of contaminants in drinking water: A review. Sci. Total Environ. 2009, 408, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.D.; Shen, L.; Sheridan, J.; Liu, J.Z.; Chen, C.; Zheng, Q. Friction of water slipping in carbon nanotubes. Phys. Rev. E 2011, 83, 036316. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Du, G.Q.; Chen, L.J.; Ren, J.G.; Sun, J.X.; Bai, F.W.; Yang, S.T. A carbon nanotube filled polydimethylsiloxane hybrid membrane for enhanced butanol recovery. Sci. Rep. 2014, 4, 5925. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liu, J.; Yuan, X.; Dong, H.; Zeng, G.; Wu, H.; Wang, H.; Liu, J.; Hua, S.; Zhang, S.; et al. Facile synthesis of alumina-decorated multi-walled carbon nanotubes for simultaneous adsorption of cadmium ion and trichloroethylene. Chem. Eng. J. 2015, 273, 101–110. [Google Scholar] [CrossRef]
- Matilainen, A.; Sillanpää, M. Removal of natural organic matter from drinking water by advanced oxidation processes. Chemosphere 2010, 80, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tanada, S.; Shinoda, S.; Nakamura, T.; Harada, T.; Ohtsu, J. Studies on the adsorption characteristics of trichloroethylene onto activated carbon fiber in gaseous phase. Eisei Kagaku 1992, 38, 93–98. [Google Scholar] [CrossRef]
- Ma, X.; Anand, D.; Zhang, X.; Talapatra, S. Adsorption and desorption of chlorinated compounds from pristine and thermally treated multiwalled carbon nanotubes. J. Phys. Chem. C 2011, 115, 4552–4557. [Google Scholar] [CrossRef]
- Naghizadeh, A.; Nasseri, S.; Nazmara, S. Removal of trichloroethylene from water by adsorption on to multiwall carbon nanotubes. J. Environ. Health Sci. Eng. 2011, 8, 317–324. [Google Scholar]
- Li, Y.; Niu, J.; Shen, Z.; Feng, C. Size effect of single-walled carbon nanotube on adsorption of perfluorooctanesulfonate. Chemosphere 2013, 91, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shao, T.; Karanfil, T. The effects of dissolved natural organic matter on the adsorption of synthetic organic chemicals by activated carbons and carbon nanotubes. Water Res. 2011, 45, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Ersan, G.; Kaya, Y.; Apul, O.G.; Karanfil, T. Adsorption of organic contaminants by graphene nanosheets, carbon nanotubes and granular activated carbons under natural organic matter preloading conditions. Sci. Total Environ. 2016, 565, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, J.; Xing, B. Sorption of organic contaminants by carbon nanotubes: Influence of adsorbed organic matter. Environ. Sci. Technol. 2008, 42, 3207–3212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shao, T.; Bekaroglu, S.S.K.; Karanfil, T. Adsorption of synthetic organic chemicals by carbon anotubes: Effects of background solution chemistry. Water Res. 2010, 44, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Naghizadeh, A.; Nasseri, S.; Rashidi, A.; Kalantary, R.R.; Nabizadeh, R.; Mahvi, A. Adsorption kinetics and thermodynamics of hydrophobic natural organic matter (NOM) removal from aqueous solution by multi-wall carbon nanotubes. Water Sci. Technol. Water Supply 2013, 13, 273–285. [Google Scholar] [CrossRef]
- Hnaien, M.; Lagarde, F.; Bausells, J.; Errachid, A.; Jaffrezic-Renault, N. A new bacterial biosensor for trichloroethylene detection based on a three-dimensional carbon nanotubes bioarchitecture. Anal. Bioanal. Chem. 2011, 400, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Crock, C.A.; Rogensues, A.R.; Shan, W.; Tarabara, V.V. Polymer nanocomposites with graphene-based hierarchical fillers as materials for multifunctional water treatment membranes. Water Res. 2013, 47, 3984–3996. [Google Scholar] [CrossRef] [PubMed]
- Kedem, S.; Rozen, D.; Cohen, Y.; Paz, Y. Enhanced stability effect in composite polymeric nanofibers containing titanium dioxide and carbon nanotubes. J. Phys. Chem. C 2009, 113, 14893–14899. [Google Scholar] [CrossRef]
- Di, J.; Li, S.; Zhao, Z.; Huang, Y.; Jia, Y.A.; Zheng, H. Biomimetic CNT@TiO2 composite with enhanced photocatalytic properties. Chem. Eng. J. 2015, 281, 60–68. [Google Scholar] [CrossRef]
- Mahdavi, H.; Rahimi, A.; Shahalizade, T. Catalytic polymeric membranes with palladium nanoparticle/multi-wall carbon nanotubes as hierarchical nanofillers: Preparation, characterization and application. J. Polym. Res. 2016, 23, 1–12. [Google Scholar] [CrossRef]
- Bhushan, B. Biomimetics: Bioinspired Hierarchical-Structured Surfaces for Green Science and Technology; Springer Science & Business Media: Berlin, Germany; Heidelberg, Germany, 2012. [Google Scholar]
- Wang, G.G.; Zhu, L.Q.; Liu, H.C.; Li, W.P. Self-assembled biomimetic superhydrophobic CaCO3 coating inspired from fouling mineralization in geothermal water. Langmuir 2011, 27, 12275–12279. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.M.; Karumuri, A.; Barney, I.T. Hierarchical nanostructures by nanotube grafting on porous cellular surfaces. J. Phys. D Appl. Phys. 2009, 42, 195503. [Google Scholar] [CrossRef]
- Vijwani, H.; Mukhopadhyay, S.M. Palladium nanoparticles on hierarchical carbon surfaces: A new architecture for robust nano-catalysts. Appl. Surf. Sci. 2012, 263, 712–721. [Google Scholar] [CrossRef]
- Karumuri, A.K.; Oswal, D.P.; Hostetler, H.A.; Mukhopadhyay, S.M. Silver nanoparticles attached to porous carbon substrates: Robust materials for chemical-free water disinfection. Mater. Lett. 2013, 109, 83–87. [Google Scholar] [CrossRef]
- Karumuri, A.K.; Oswal, D.P.; Hostetler, H.A.; Mukhopadhyay, S.M. Silver nanoparticles supported on carbon nanotube carpets: Influence of surface functionalization. Nanotechnology 2016, 27, 145603. [Google Scholar] [CrossRef] [PubMed]
- Vijwani, H. Hierarchical Porous Structures with Aligned Carbon Nanotubes as Efficient Adsorbents and Metal-Catalyst Supports. Ph.D. Thesis, Wright State University, Fairborn, OH, USA, 2015. [Google Scholar]
- Vijwani, H.; Agrawal, A.; Mukhopadhyay, S.M. Dechlorination of environmental contaminants using a hybrid nanocatalyst: Palladium nanoparticles supported on hierarchical carbon nanostructures. J. Nanotechnol. 2012, 2012, 478381. [Google Scholar] [CrossRef]
- Vijwani, H.; Nadagouda, M.N.; Namboodiri, V.; Mukhopadhyay, S.M. Hierarchical hybrid carbon nano-structures as robust and reusable adsorbents: Kinetic studies with model dye compound. Chem. Eng. J. 2015, 268, 197–207. [Google Scholar] [CrossRef]
- Bahr, J.L.; Mickelson, E.T.; Bronikowski, M.J.; Smalley, R.E.; Tour, J.M. Dissolution of small diameter single-wall carbon nanotubes in organic solvents? Chem. Commun. 2001. [Google Scholar] [CrossRef]
- Hamilton, C.E.; Lomeda, J.R.; Sun, Z.; Tour, J.M.; Barron, A.R. High-yield organic dispersions of unfunctionalized graphene. Nano Lett. 2009, 9, 3460–3462. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tsige, M.; Uddin, S.; Talapatra, S. Application of carbon nanotubes for removing organic contaminants from water. Mater. Express 2011, 1, 183–200. [Google Scholar] [CrossRef]
- Ma, X.; Anand, D.; Zhang, X.; Tsige, M.; Talapatra, S. Carbon nanotube-textured sand for controlling bioavailability of contaminated sediments. Nano Res. 2010, 3, 412–422. [Google Scholar] [CrossRef]
- Alarcón, L.; de Oca, J.M.; Accordino, S.; Fris, J.R.; Appignanesi, G. Hydrophobicity and geometry: Water at curved graphitic-like surfaces and within model pores in self-assembled monolayers. Fluid Phase Equilib. 2014, 362, 81–86. [Google Scholar] [CrossRef]
- Alarcón, L.; Malaspina, D.; Schulz, E.; Frechero, M.; Appignanesi, G. Structure and orientation of water molecules at model hydrophobic surfaces with curvature: From graphene sheets to carbon nanotubes and fullerenes. Chem. Phys. 2011, 388, 47–56. [Google Scholar] [CrossRef]
- Touriño, I.L.; Naranjo, A.C.; Negri, V.; Cerdán, S.; Ballesteros, P. Coarse-grained molecular dynamics simulation of water diffusion in the presence of carbon nanotubes. J. Mol. Gr. Model. 2015, 62, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Uddin, N.M.; Capaldi, F.M.; Farouk, B. Molecular dynamics simulations of carbon nanotube dispersions in water: Effects of nanotube length, diameter, chirality and surfactant structures. Comput. Mater. Sci. 2012, 53, 133–144. [Google Scholar] [CrossRef]
- Renner, M.; Domanov, Y.; Sandrin, F.; Izeddin, I.; Bassereau, P.; Triller, A. Lateral diffusion on tubular membranes: Quantification of measurements bias. PLoS ONE 2011, 6, e25731. [Google Scholar] [CrossRef] [PubMed]
- Ndoro, T.V.; Böhm, M.C.; Müller-Plathe, F. Interface and interphase dynamics of polystyrene chains near grafted and ungrafted silica nanoparticles. Macromolecules 2011, 45, 171–179. [Google Scholar] [CrossRef]
- Jana, A.K.; Tiwari, M.K.; Vanka, K.; Sengupta, N. Unraveling origins of the heterogeneous curvature dependence of polypeptide interactions with carbon nanostructures. Phys. Chem. Chem. Phys. 2016, 18, 5910–5924. [Google Scholar] [CrossRef] [PubMed]
- Rajarajeswari, M.; Iyakutti, K.; Kawazoe, Y. Effect of chirality and curvature of single-walled carbon nanotubes on the adsorption of uracil. Phys. Status Solidi 2011, 248, 1431–1436. [Google Scholar] [CrossRef]
- Balamurugan, K.; Subramanian, V. Adsorption of chlorobenzene onto (5, 5) armchair single-walled carbon nanotube and graphene sheet: Toxicity versus adsorption strength. J. Phys. Chem. C 2013, 117, 21217–21227. [Google Scholar] [CrossRef]
- Du, S.; Gao, H.; Seidel, C.; Tsetseris, L.; Ji, W.; Kopf, H.; Chi, L.; Fuchs, H.; Pennycook, S.J.; Pantelides, S.T. Selective nontemplated adsorption of organic molecules on nanofacets and the role of bonding patterns. Phys. Rev. Lett. 2006, 97, 156105. [Google Scholar] [CrossRef] [PubMed]
- Yin, A.X.; Min, X.Q.; Zhang, Y.W.; Yan, C.H. Shape-selective synthesis and facet-dependent enhanced electrocatalytic activity and durability of monodisperse sub-10 nm Pt- Pd tetrahedrons and cubes. J. Am. Chem. Soc. 2011, 133, 3816–3819. [Google Scholar] [CrossRef] [PubMed]
- Jha, K.C.; Liu, H.; Bockstaller, M.R.; Heinz, H. Facet recognition and molecular ordering of ionic liquids on metal surfaces. J. Phys. Chem. C 2013, 117, 25969–25981. [Google Scholar] [CrossRef]
- Heinz, H.; Jha, K.C.; Luettmer-Strathmann, J.; Farmer, B.L.; Naik, R.R. Polarization at metal-biomolecular interfaces in solution. J. R. Soc. Interface 2010. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.Y.; Wu, H.; Yao, Z.; Zhou, F.; Zhang, H.; Ozolins, V.; Huang, Y. Facet-selective adsorption on noble metal crystals guided by electrostatic potential surfaces of aromatic molecules. J. Am. Chem. Soc. 2013, 135, 15489–15500. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.



© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jha, K.C.; Liu, Z.; Vijwani, H.; Nadagouda, M.; Mukhopadhyay, S.M.; Tsige, M. Carbon Nanotube Based Groundwater Remediation: The Case of Trichloroethylene. Molecules 2016, 21, 953. https://doi.org/10.3390/molecules21070953
Jha KC, Liu Z, Vijwani H, Nadagouda M, Mukhopadhyay SM, Tsige M. Carbon Nanotube Based Groundwater Remediation: The Case of Trichloroethylene. Molecules. 2016; 21(7):953. https://doi.org/10.3390/molecules21070953
Chicago/Turabian StyleJha, Kshitij C., Zhuonan Liu, Hema Vijwani, Mallikarjuna Nadagouda, Sharmila M. Mukhopadhyay, and Mesfin Tsige. 2016. "Carbon Nanotube Based Groundwater Remediation: The Case of Trichloroethylene" Molecules 21, no. 7: 953. https://doi.org/10.3390/molecules21070953