Pyridine and p-Nitrophenyl Oxime Esters with Possible Photochemotherapeutic Activity: Synthesis, DNA Photocleavage and DNA Binding Studies
Abstract
:1. Introduction
2. Results and Discussion
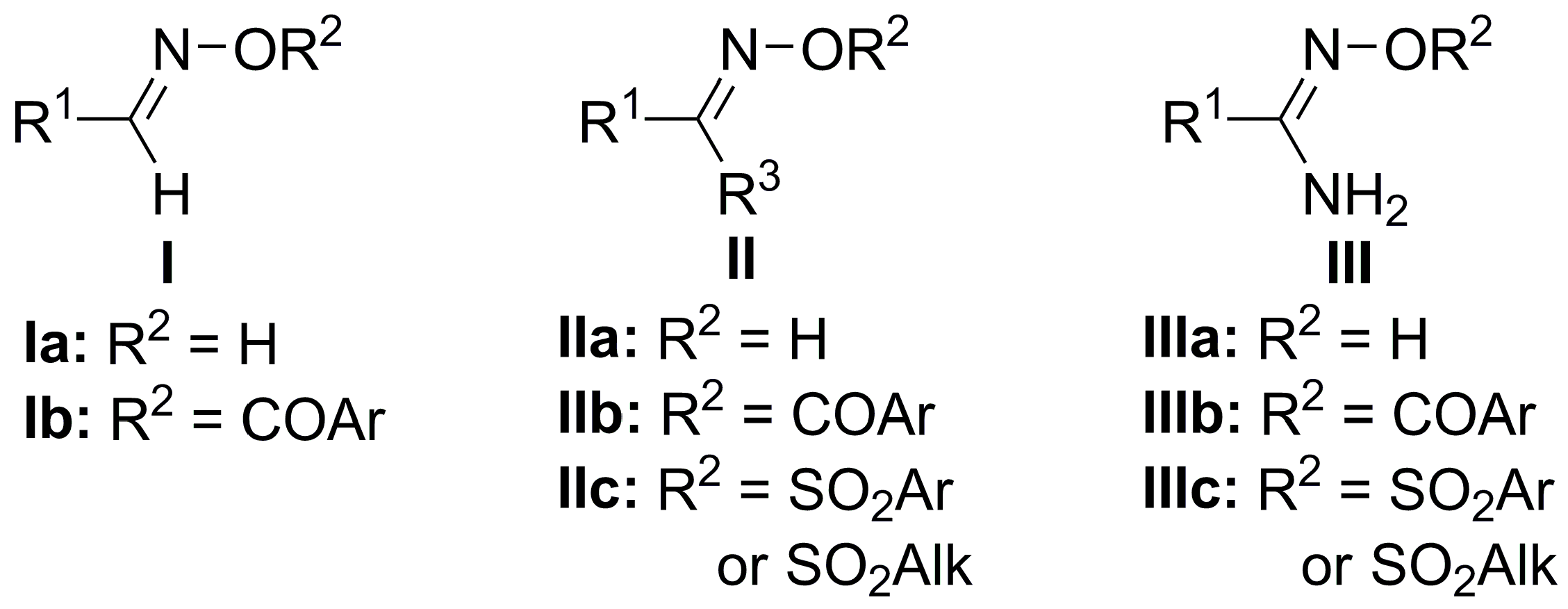
2.1. Chemistry
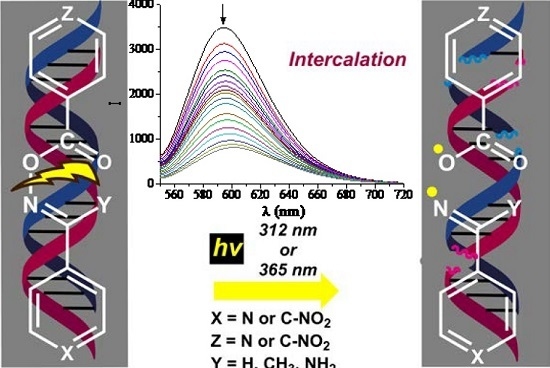
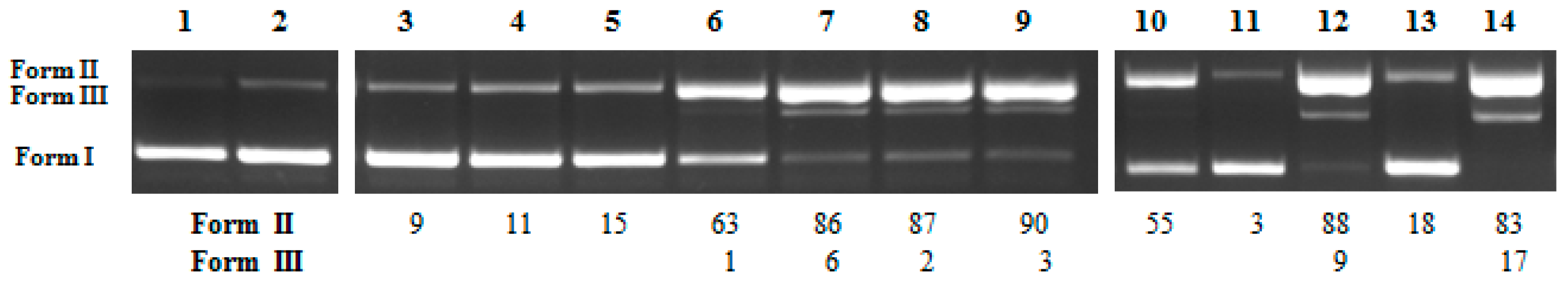
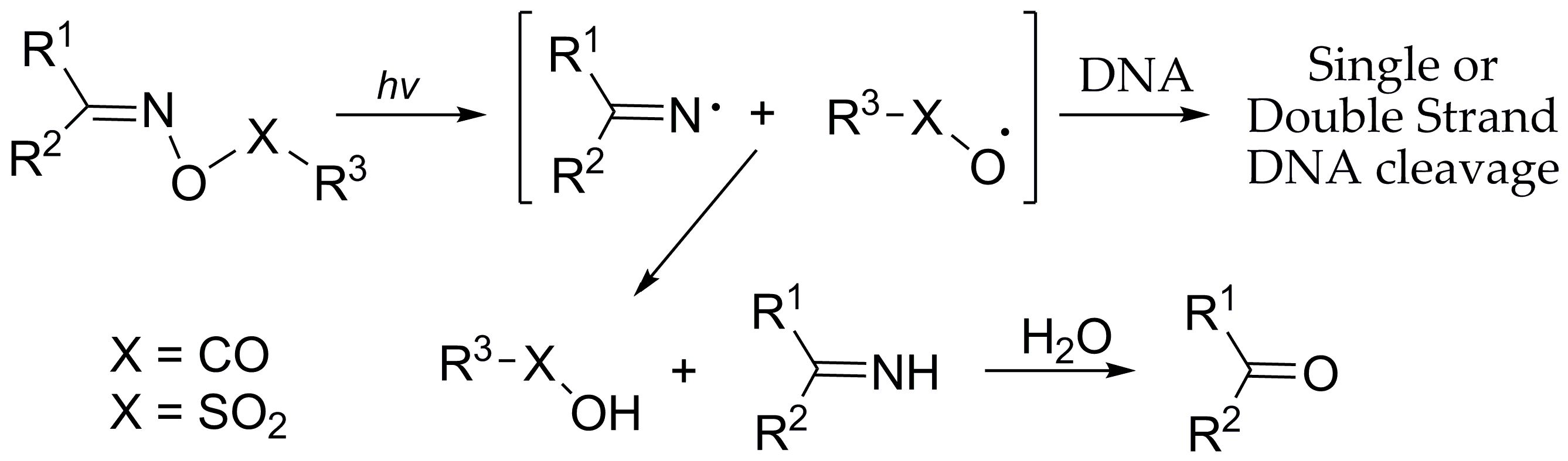
2.2. DNA Photo-Cleavage Experiments
2.3. DNA Affinity Studies
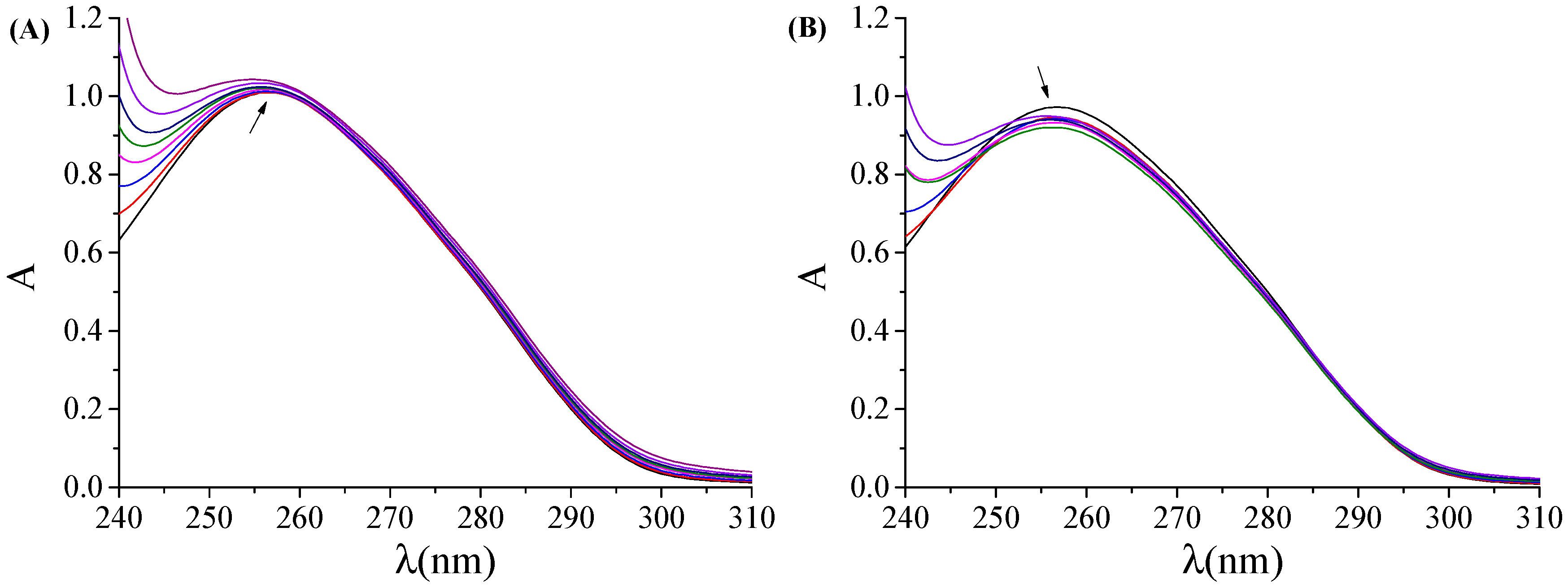
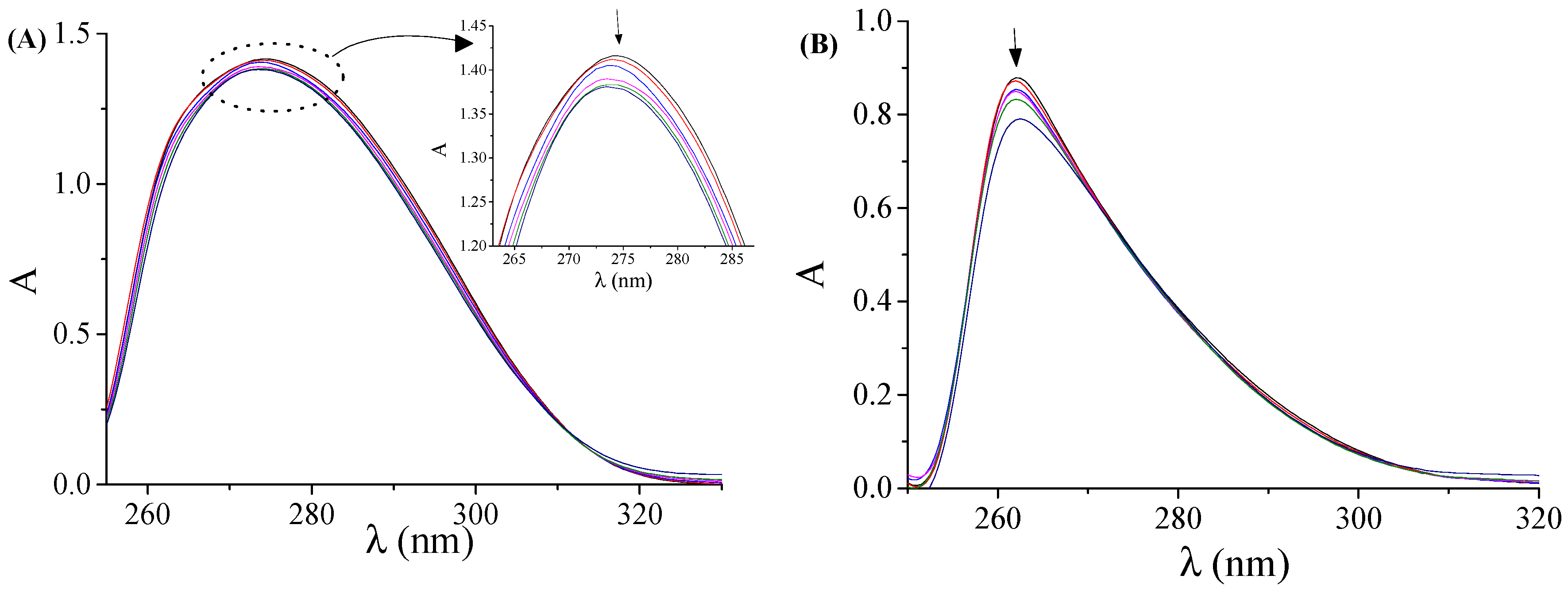
2.3.1. DNA-Binding Studies with UV Spectroscopy
2.3.2. DNA-Binding Study with Viscosity Measurements
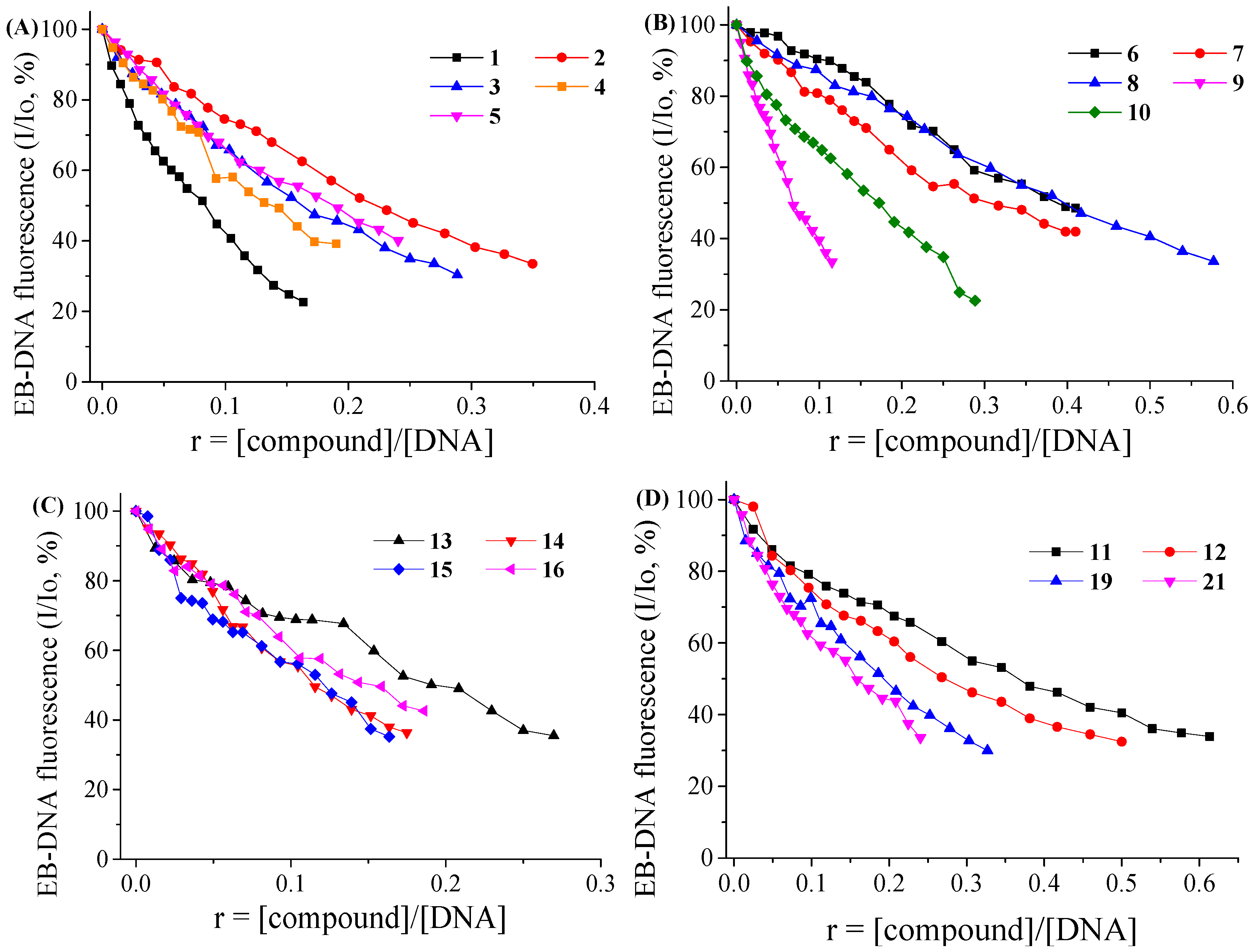
2.3.3. EB-Displacement Studies with Fluorescence Emission Spectroscopy
3. Materials and Methods
3.1. General Information
3.2. Synthesis: General Procedure for the Synthesis of Oxime Ester Conjugates
3.3. DNA Photo-Cleavage Experiments
3.3.1. Cleavage of Supercoiled Circular pBluescript KS II DNA and pBR322
3.3.2. Calculation of Single-Strand Damage (ss)% and Double-Strand Damage (ds)%
3.4. Calculation of the N-O Bond Dissociation Energies
3.5. Interaction with CT DNA
3.5.1. Study with UV Spectroscopy
3.5.2. Viscometry
3.5.3. Competitive Studies with EB
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CT | calf-thymus |
| EB | ethidium bromide |
| BDE | Bond Dissociation Energy |
| THF | tetrahydrofuran |
| DMF | Dimethyl formamide |
| DMSO | Dimethyl sulfoxide |
| DIPEA | Diisopropyl ethyl amine |
References
- Dąbrowski, J.M.; Arnaut, L.G. Photodynamic Therapy (PDT) of Cancer: From a Local to a Systemic Treatment. Photochem. Photobiol. Sci. 2015, 14, 1765–1780. [Google Scholar]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Weijer, R.; Broekgaarden, M.; Kos, M.; van Vught, R.; Rauws, E.A.J.; Breukink, E.; van Gulik, T.M.; Storm, G.; Heger, M. Enhancing photodynamic therapy of refractory solid cancers: Combining second-generation photosensitizers with multi-targeted liposomal delivery. J. Photochem. Photobiol. C 2015, 23, 103–131. [Google Scholar] [CrossRef]
- Yano, S.; Hirohara, S.; Obata, M.; Yuichiro Hagiya, Y.; Ogura, S.I.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current states and future views in photodynamic therapy. J. Photochem. Photobiol. C 2011, 12, 46–67. [Google Scholar] [CrossRef]
- Bechet, D.; Mordon, S.R.; Guillemin, F.; Barberi-Heyob, M.A. Photodynamic therapy of malignant brain tumours: A complementary approach to conventional therapies. Cancer Treat. Rev. 2014, 40, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Sirajuddin, M.; Ali, S.; Badshah, A. Drug-DNA interactions and their study by UV-Visible, fluorescence spectroscopies and cyclic voltammetry. J. Photochem. Photobiol. B: Biol. 2013, 124, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ananya, P.; Santanu, B. Chemistry and biology of DNA-binding small molecules. Curr. Sci. 2012, 102, 212–231. [Google Scholar]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Reelfs, O.; Karran, P.; Young, A.R. 4-thiothymidine sensitization of DNA to UVA offers potential for a novel Photochemotherapy. Photochem. Photobiol. Sci. 2012, 11, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Vittara, N.B.R.; Cominib, L.; Fernadeza, I.M.; Agostinia, E.; Nuñez-Montoyab, S.; Cabrerab, J.L.; Rivarola, V.A. Photochemotherapy using natural anthraquinones: Rubiadin and Soranjidiol sensitize human cancer cell to die by apoptosis. Photodiagnosis. Photodyn. Ther. 2014, 11, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Armitage, B. Photocleavage of Nucleic Acids. Chem. Rev. 1998, 98, 1171–1200. [Google Scholar] [CrossRef] [PubMed]
- Kar, M.; Basak, A. Design, Synthesis, and Biological Activity of Unnatural Enediynes and Related Analogues Equipped with pH-Dependent or Phototriggering Devices. Chem. Rev. 2007, 107, 2861–2890. [Google Scholar] [CrossRef] [PubMed]
- Breiner, B.; Kaya, K.; Roy, S.; Yang, W.-Y.; Alabugin, I.V. Hybrids of amino acids and acetylenic DNA-photocleavers: Optimizing efficiency and selectivity for cancer phototherapy. Org. Biomol. Chem. 2012, 10, 3974–3987. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, M. Quinones as photosensitizer for photodynamic therapy: ROS generation, mechanism and detection methods. Photodiagnosis Photodyn. Ther. 2016, 13, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.D.; Baker, T.A.; Bell, S.P.; Gann, A.; Levine, M.; Losick, R. Molecular Biology of the Gene, 5th ed.; Cold Spring Harbor Laboratory Press: Plainview, NY, USA, 2004; Chapters 9 and 10. [Google Scholar]
- Elmroth, K.; Nygren, J.; Mårtensson, S.; Ismail, I.H.; Hammarsten, O. Cleavage of cellular DNA by calicheamicin γ1. DNA Repair 2003, 2, 363–374. [Google Scholar] [CrossRef]
- Kaya, K.; Johnson, M.; Alabugin, I.V. Opening Enediyne Scissors Wider: pH-Dependent DNA Photocleavage by meta-Diyne Lysine Conjugates. Photochem. Photobiol. 2015, 91, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Abele, E.; Abele, R. Recent Advances in the Synthesis of Heterocycles from Oximes. Curr. Org. Synth. 2014, 11, 403–428. [Google Scholar] [CrossRef]
- O’Brien, C. The Rearrangement of Ketoxime O-Sulfonates to Amino Ketones (The Neber Rearrangement). Chem. Rev. 1964, 64, 81–89. [Google Scholar] [CrossRef]
- Ooi, T.; Takahashi, M.; Doda, K.; Maruoka, K. Asymmetric Induction in the Neber Rearrangement of Simple Ketoxime Sulfonates under Phase-Transfer Conditions: Experimental Evidence for the Participation of an Anionic Pathway. J. Am. Chem. Soc. 2002, 124, 7640–7641. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.-X.; An, N.; Deng, W.-P.; Eriksson, L.A. Catalysts or Initiators? Beckmann Rearrangement Revisited. J. Org. Chem. 2013, 78, 6782–6785. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, D.N.; Varella, E.A. The Chemistry of Acid Derivatives; The Chemistry of Amidoximes; Patai, S., Ed.; Interscience: New York, NY, USA, 1992; Suppl. B, Volume 2, Part 2; pp. 875–966. [Google Scholar]
- Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.E.; Varella, E.; Nicolaides, D.N. Recent Developments in the Chemistry and in the Biological Applications of Amidoximes. Curr. Pharm. Des. 2008, 14, 1001–1047. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, N.; Dutta, S.; Dasgupta, S.; Pradeep Singh, N.D.; Baidya, M.; Ghosh, S.K. Synthesis, photophysical, photochemical, DNA cleavage/binding and cytotoxic properties of pyrene oxime ester conjugates. Photochem. Photobiol. Sci. 2012, 11, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Hwu, J.R.; Tsay, S.-C.; Hong, S.C.; Leu, Y.-J.; Liu, C.-F.; Chou, S.-S.P. Oxime esters of anthraquinone as photo-induced DNA-cleaving agents for single- and double-strand scissions. Tetrahedron Lett. 2003, 44, 2957–2960. [Google Scholar] [CrossRef]
- Hwu, J.R.; Tsay, S.-C.; Hong, S.C.; Hsu, M.-H.; Liu, C.-F.; Chou, S.-S.P. Relationship Between Structure of Conjugated Oxime Esters and Their Ability to Cleave DNA. Bioconjug. Chem. 2013, 24, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Bindu, P.J.; Mahadevan, K.M.; Satyanarayan, N.D.; Ravikumar Naik, T.R. Synthesis and DNA cleavage studies of novel quinoline oxime esters. Bioorg. Med. Chem. 2012, 22, 898–900. [Google Scholar] [CrossRef] [PubMed]
- Hwu, J.R.; Yang, J.-R.; Tsay, S.-C.; Hsu, M.-H.; Chen, Y.-C.; Chou, S.-S.P. Photo-Induced DNA cleavage by (heterocyclo)carbonyl oxime esters of anthraquinone. Tetrahedron Lett. 2008, 49, 3312–3315. [Google Scholar] [CrossRef]
- Chou, S.-S.P.; Juan, J.-C.; Tsay, S.-C.; Huang, K.P.; Hwu, J.R. Oxime Esters of 2,6-Diazaanthracene-9,10-dione and 4,5-Diazafluoren-9-one as Photo-induced DNA-Cleaving Agents. Molecules 2012, 17, 3370–3382. [Google Scholar] [CrossRef] [PubMed]
- Karamtzioti, P.; Papastergiou, A.; Stefanakis, J.G.; Koumbis, A.E.; Anastasiou, I.; Koffa, M.; Fylaktakidou, K.C. O−Benzoyl pyridine aldoxime and amidoxime derivatives: Novel efficient DNA photo−cleavage agents. Med. Chem. Commun. 2015, 6, 719–726. [Google Scholar] [CrossRef]
- Theodorakis, E.A.; Xiang, X.; Lee, M.; Gibson, T. On the Mechanism of Photo-induced Nucleic Acid Cleavage Using N-Aroyloxy-2-thiopyridones. Tetrahedron Lett. 1998, 39, 3383–3386. [Google Scholar] [CrossRef]
- Theodorakis, E.A.; Wilcoxen, K.M. N-Aroyloxy-2-thiopyridones as efficient oxygen-radical generators: Novel time-controlled DNA photocleaving reagents. Chem. Commun. 1996, 1927–1928. [Google Scholar] [CrossRef]
- Blom, P.; Xiang, X.; Kao, D.; Theodorakis, E.A. Design, Synthesis, and Evaluation of N-Aroyloxy-2-thiopyridones as DNA Photocleaving Reagents. Bioorg. Med. Chem. 1999, 7, 727–736. [Google Scholar] [CrossRef]
- Andreou, Ν.-P.; Dafnopoulos, K.; Tortopidis, C.; Koumbis, A.E.; Koffa, M.; Psomas, G.; Fylaktakidou, K.C. Alkyl and Aryl Sulfonyl p-Pyridine Ethanone Oximes are Efficient DNA Photo-cleavage Agents. J. Photochem. Photobiol. B Biol. 2016, 158, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Papastergiou, A.; Perontsis, S.; Gritzapis, P.; Koumbis, A.E.; Koffa, M.; Psomas, G.; Fylaktakidou, K.C. Evaluation of O-Alkyl and Aryl Sulfonyl Aromatic and Heteroaromatic Amidoximes as Novel Potent DNA Photo-Cleavers. Photochem. Photobiol. Sci. 2016, 15, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Fischer, F.L.; Ribeiro, C.W.; Silveira, G.P.; Sá, M.M.; Nomeb, F.; Terenzi, H. Metal-Free artificial nucleases based on simple oxime and hydroxylamine scaffolds. Bioorg. Med. Chem. Lett. 2008, 18, 4499–4502. [Google Scholar] [CrossRef] [PubMed]
- Abele, E.; Abele, R.; Lukevics, E. Pyridine Oximes: Synthesis, Reactions, and Biological Activity. (Review). Chem. Heterocycl. Compd. 2003, 39, 825–865. [Google Scholar] [CrossRef]
- Kliachyna, M.; Santoni, G.; Nussbaum, V.; Renou, J.; Sanson, B.; Colletier, J.-P.; Arboléas, M.; Loiodice, M.; Weik, M.; Jean, L.; et al. Design, synthesis and biological evaluation of novel tetrahydroacridine pyridine- aldoxime and -amidoxime hybrids as efficient uncharged reactivators of nerve agent-inhibited human acetylcholinesterase. Eur. J. Med. Chem. 2014, 78, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Mercey, G.; Renou, J.; Verdelet, T.; Kliachyna, M.; Baati, R.; Gillon, E.; Arboléas, M.; Loiodice, M.; Nachon, F.; Jean, L.; et al. Phenyltetrahydroisoquinoline-Pyridinaldoxime Conjugates as Efficient Uncharged Reactivators for the Dephosphylation of Inhibited Human Acetylcholinesterase. J. Med. Chem. 2012, 55, 10791–10795. [Google Scholar] [CrossRef] [PubMed]
- Renou, J.; Loiodice, M.; Arboléas, M.; Baati, R.; Jean, L.; Nachon, F.; Renard, P.-Y. Tryptoline-3-hydroxypyridinaldoxime conjugates as efficient reactivators of phosphylated human acetyl and butyrylcholinesterases. Chem. Commun. 2014, 50, 3947–3950. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, D.N.; Fylaktakidou, K.C.; Litinas, K.E.; Papageorgiou, G.K.; Hadjipavlou−Litina, D. 1,3-Cycloaddition Reactions of 2-oxo-2H-[1]benzopyran-4-carbonitrile N-oxide. Synthesis of Several New 4-Substituted Coumarins. J. Heterocyclic Chem. 1998, 35, 619–625. [Google Scholar] [CrossRef]
- Nicolaides, D.N.; Gautam, D.R.; Litinas, K.E.; Manouras, C.; Fylaktakidou, K.C. Reactions of 2-(Methoxyimino)benzene-1-ones with a-Alkyl-ethoxycarbonyl-methylene(triphenyl) phosphoranes. Tetrahedron 2001, 57, 9469–9474. [Google Scholar] [CrossRef]
- Gautam, D.D.R.; Protopapas, J.; Fylaktakidou, K.C.; Litinas, K.E.; Nicolaides, D.N.; Tsoleridis, K. Unexpected one−pot synthesis of new polycyclic coumarin[4,3−c]pyridine derivatives via tandem hetero−Diels−Alder and 1,3 dipolar cycloaddition reaction. Tetrahedron Lett. 2009, 50, 448–451. [Google Scholar] [CrossRef]
- Nicolaides, D.N.; Fylaktakidou, K.C.; Litinas, K.E.; Hadjipavlou-Litina, D. Synthesis and Biological Evaluation of Several Coumarin-4-Carboxamidoxime and 3-(Coumarin-4-yl)-1,2,4-oxadiazole Derivatives. Eur. J. Med. Chem. 1998, 33, 715–724. [Google Scholar] [CrossRef]
- Nicolaides, D.N.; Litinas, K.E.; Vrasidas, I.; Fylaktakidou, K.C. Thermal Transformation of Arylamidoximes in the Presence of Phosphorous Ylides. Unexpected Formation of 3-Aryl-5-Arylamino-1,2,4-Oxadiazoles. J. Heterocycl. Chem. 2004, 41, 499–503. [Google Scholar] [CrossRef]
- Nicolaides, D.N.; Litinas, K.E.; Papamehael, T.; Grzeskowiak, H.; Gautam, D.R.; Fylaktakidou, K.C. An Easy Transformation of 2-Amino-2-(hydroxyimino)acetates to Carbamoylformamidoximes. Synthesis 2005, 407–410. [Google Scholar] [CrossRef]
- Fylaktakidou, K.C.; Litinas, K.E.; Saragliadis, A.; Adamopoulos, S.G.; Nicolaides, D.N. Synthesis of oxadiazoloquinoxaline, oxathiadiazoloquinoxaline and oxadiazolobenzothiazine derivatives. J. Heterocycl. Chem. 2006, 43, 579–583. [Google Scholar] [CrossRef]
- Ispicoudi, M.; Litinas, K.E.; Fylaktakidou, K.C. A convenient synthesis of 5-amino-substituted-1,2,4-oxadiale derivatives via reactions of amidoximes with carbodiimides. Heterocycles 2008, 75, 1321–1328. [Google Scholar]
- Ispicoudi, M.; Amvrazis, M.; Kontogiorgis, C.; Koumbis, A.E.; Litinas, K.E.; Hadjipavlou−Litina, D.J.; Fylaktakidou, K.C. Convenient Synthesis and Biological Profile of 5-Amino-substituted 1,2,4-oxadiazole Derivatives. Eur. J. Med. Chem. 2010, 45, 5635–5645. [Google Scholar] [CrossRef] [PubMed]
- Doulou, I.; Kontogiorgis, C.; Koumbis, A.E.; Evgenidou, E.; Hadjipavlou−Litina, D.; Fylaktakidou, K.C. Synthesis of stable aromatic and heteroaromatic sulfonyl-amidoximes and evaluation of their antioxidant and lipid peroxidation activity. Eur. J. Med. Chem. 2014, 80, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.E.; Jeppesen, C.; Egholm, M.; Buchardt, O. Photochemical cleavage of DNA by nitrobenzamides linked to 9-Aminoacridine. Biochemistry 1988, 27, 6338–6343. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.E.; Egholm, M.; Koch, T.; Christensen, J.B.; Buchardt, O. Photolytic cleavage of DNA by nitrobenzamido ligands linked to 9-aminoacridines gives DNA polymerase substrates in a wavelength-dependent reaction. Bioconjugate. Chem. 1991, 2, 57–66. [Google Scholar] [CrossRef]
- Hurley, R.; Testa, A.C. Triplet-State yield of aromatic nitro compounds. J. Am. Chem. Soc. 1968, 90, 1949–1952. [Google Scholar] [CrossRef]
- Saito, I.; Takayama, M. Photoactivatable DNA-Cleaving Amino Acids: Highly Sequence-Selective DNA Photocleavage by Novel l-Lysine Derivatives. J. Am. Chem. Soc. 1995, 117, 5590–5591. [Google Scholar] [CrossRef]
- Wondrak, G.T.; Jacobson, M.K.; Jacobson, E.L. Endogenous UVA-photosensitizers: Mediators of skin photodamage and novel targets for skin photoprotection. Photochem. Photobiol. Sci. 2006, 5, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Lalevée, J.; Allonas, X.; Fouassier, J.P.; Tachi, H.; Izumitani, A.; Shirai, M.; Tsunooka, M. Investigation of the photochemical properties of an important class of photobase generators: The O-acyloximes. J. Photochem. Photobiol. A 2002, 151, 27–37. [Google Scholar] [CrossRef]
- Pearson, R.C. Electronic Spectra and Chemical Reactivity. J. Am. Chem. Soc. 1988, 110, 2092–2097. [Google Scholar] [CrossRef]
- Varras, P.C.; Zarkadis, A.K. Ground- and Triplet Excited-State Properties Correlation: A Computational CASSCF/CASPT2 Approach Based on the Photodissociation of Allylsilanes. J. Phys. Chem. A 2012, 116, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Budyka, M.F.; Zyubina, T.S.; Zarkadis, A.K. Correlating ground and excited state properties: A quantum chemical study of the photodissociation of the C-N bond in N-substituted anilines. J. Mol. Struct. (Theochem) 2002, 594, 113–125. [Google Scholar] [CrossRef]
- Budyka, M.F.; Zyubina, T.S.; Zarkadis, A.K. Quantum chemical study of the Si–C bond photodissociation in benzylsilane derivatives: A specific ‘excited-state’ silicon effect. J. Mol. Struct. (Theochem) 2004, 668, 1–11. [Google Scholar] [CrossRef]
- Marazzi, M.; Wibowo, M.; Gattuso, H.; Dumont, E.; Roca-Sanjuán, D.; Monari, A. Hydrogen abstraction by photoexcited benzophenone: Consequences for DNA photosensitization. Phys. Chem. Chem. Phys. 2016, 18, 7829–7836. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Pierre, V.C.; Barton, J.K. Metallo-Intercalators and metallo-insertors. Chem. Commun. 2007, 4565–4579. [Google Scholar] [CrossRef] [PubMed]
- Pyle, A.M.; Rehmann, J.P.; Meshoyrer, R.; Kumar, C.V.; Turro, N.J.; Barton, J.K. Mixed-Ligand complexes of ruthenium(II): Factors governing binding to DNA. J. Am. Chem. Soc. 1989, 111, 3051–3058. [Google Scholar] [CrossRef]
- Pratviel, G.; Bernadou, J.; Meunier, B. DNA and RNA cleavage by metal complexes. Adv. Inorg. Chem. 1998, 45, 251–262. [Google Scholar]
- Wolfe, A.; Shimer, G.; Meehan, T. Polycyclic aromatic hydrocarbons physically intercalate into duplex regions of denatured DNA. Biochemistry 1987, 26, 6392–6396. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakopoulou, A.; Dendrinou-Samara, C.; Pantazaki, A.A.; Alexiou, M.; Nordlander, E.; Kessissoglou, D.P. Synthesis, structure and interactions with DNA of novel tetranuclear, [Mn4(II/II/II/IV)] mixed valence complexes. J. Inorg. Biochem. 2008, 102, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gimenez, J.L.; Gonzalez-Alvarez, M.; Liu-Gonzalez, M.; Macias, B.; Borras, J.; Alzuet, G. Toward the development of metal-based synthetic nucleases: DNA binding and oxidative DNA cleavage of a mixed copper(II) complex with N-(9H-purin-6-yl)benzenesulfonamide and 1,10-phenantroline. Antitumor activity in human Caco-2 cells and Jurkat T lymphocytes. Evaluation of p53 and Bcl-2 proteins in the apoptotic mechanism. J. Inorg. Biochem. 2009, 103, 923–934. [Google Scholar] [PubMed]
- Liu, J.; Zhang, H.; Chen, C.; Deng, H.; Lu, T.; Li, L. Interaction of macrocyclic copper(II) complexes with calf-thymus DNA: Effects of the side chains of the ligands on the DNA-binding behaviors. Dalton Trans. 2003, 114–119. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Plenum Press: New York, NY, USA, 2006. [Google Scholar]
- Wilson, W.D.; Ratmeyer, L.; Zhao, M.; Strekowski, L.; Boykin, D. The search for structure-specific nucleic acid-interactive drugs: Effects of compound structure on RNA versus DNA interaction strength. Biochemistry 1993, 32, 4098–4104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Lin, H.; Zhu, S.; Sun, H.; Chen, Y. Dinuclear palladium(II) complexes containing two monofunctional [Pd(en)(pyridine)Cl]+ units bridged by Se or S. Synthesis, characterization, cytotoxicity and kinetic studies of DNA-binding. J. Inorg. Biochem. 1998, 70, 219–226. [Google Scholar] [PubMed]
- Heller, D.P.; Greenstock, C.L. Fluorescence lifetime analysis of DNA intercalated ethidium bromide and quenching by free dye. Biophys. Chem. 1994, 50, 305–312. [Google Scholar] [CrossRef]
- Marmur, J. A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J. Mol. Biol. 1961, 3, 208–218. [Google Scholar] [CrossRef]
- Reichmann, M.F.; Rice, S.A.; Thomas, C.A.; Doty, P. A Further Examination of the Molecular Weight and Size of Desoxypentose Nucleic Acid. J. Am. Chem. Soc. 1954, 76, 3047–3053. [Google Scholar] [CrossRef]
- Buyle, R. Sur les esters activés I. Aminolyse des dérivés de oximes et amidoximes. Helv. Chim. Acta 1964, 47, 2444–2448. [Google Scholar] [CrossRef]
- Image J; version 1.50.I. Available online: http://rsb.info.nih.gov/ij/download.html (accessed on 30 June 2016).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; et al. Gaussian 09; Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Sample Availability: Samples of all compounds are available from the authors.
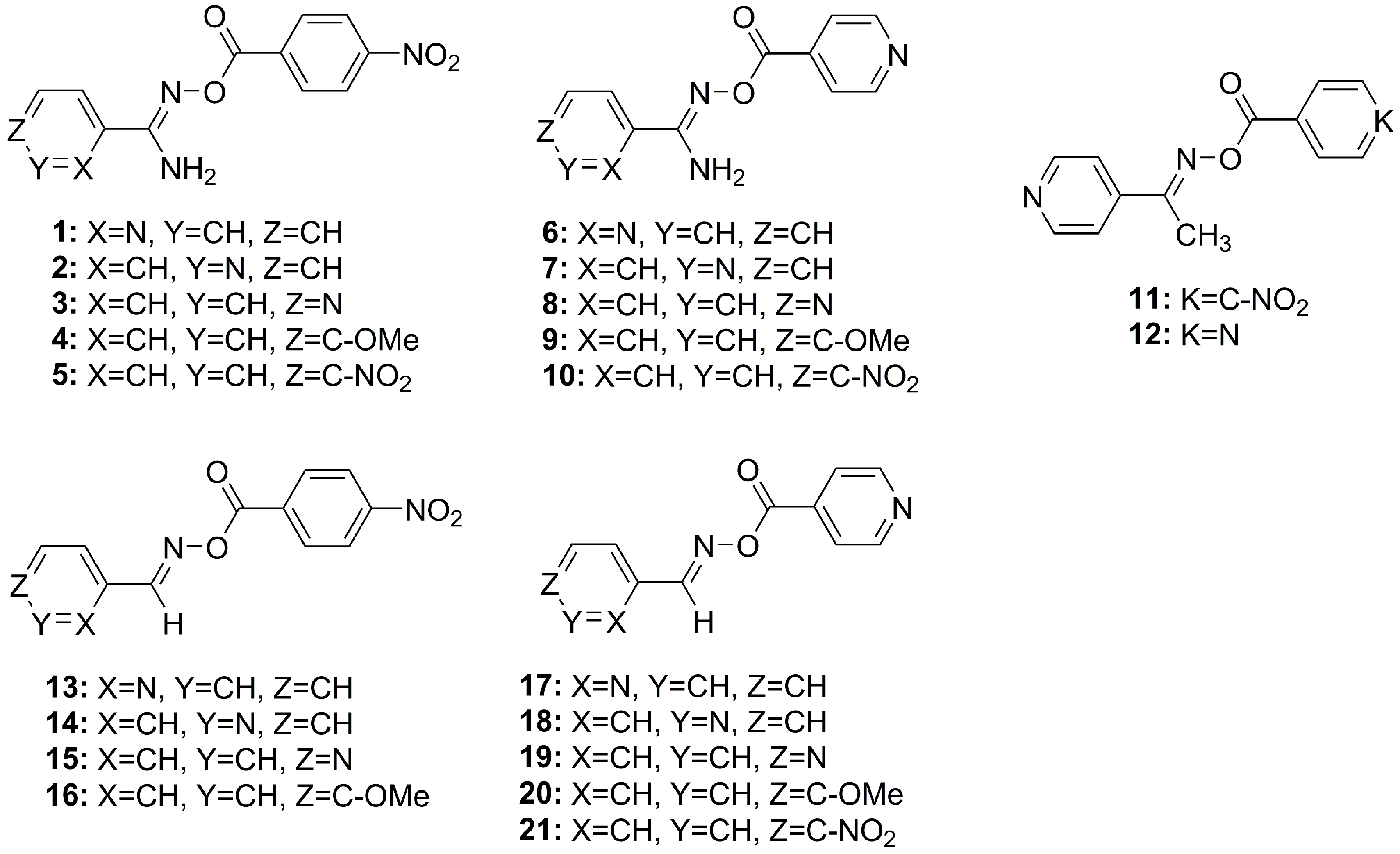



| Compound | Imine Type 1 | Imine Substituent | Conjugate | 312 nm % (ss + ds) DNA 3 Cleavage (STDEV) | 365 nm % (ss + ds) DNA 4 Cleavage (STDEV) | BDE (kcal/mol) |
|---|---|---|---|---|---|---|
| 1 | AM | o-pyr | p-NO2Ph | 90 (±4) 2 | 43 (±7) | 52.81 |
| 2 | AM | m-pyr | p-NO2Ph | 90 (±3) 2 | 79 (±7) | 51.34 |
| 3 | AM | p-pyr | p-NO2Ph | 82 (±8) 2 | 79 (±8) | 50.83 |
| 4 | AM | p-MeOPh | p-NO2Ph | 30 (±10) | NUV | 51.13 |
| 5 | AM | p-NO2Ph | p-NO2Ph | 83 (±9) | 50 (±9) | 50.67 |
| 6 | AM | o-pyr | p-pyr | 7 (±4) | NUV | 52.41 |
| 7 | AM | m-pyr | p-pyr | 8 (±4) | NUV | 51.05 |
| 8 | AM | p-pyr | p-pyr | 15 (±3) | NUV | 50.57 |
| 9 | AM | p-MeOPh | p-pyr | 6 (±4) | NUV | 50.71 |
| 10 | AM | p-NO2Ph | p-pyr | 61 (±5) | 56 (±4) | 50.51 |
| 11 | EO | p-pyr | p-NO2Ph | 90 (±5) | NUV | 46.07 |
| 12 | EO | p-pyr | p-pyr | 84 (±7) | NUV | 45.89 |
| 13 | AL | o-pyr | p-NO2Ph | 93 (±4) 2 | NUV | 47.18 |
| 14 | AL | m-pyr | p-NO2Ph | 91 (±6) 2 | NUV | 47.18 |
| 15 | AL | p-pyr | p-NO2Ph | 98 (±2) 2 | NUV | 47.00 |
| 16 | AL | p-MeOPh | p-NO2Ph | NC | NUV | 47.60 |
| 17 | AL | o-pyr | p-pyr | 50 (±10) | NUV | 46.88 |
| 18 | AL | m-pyr | p-pyr | 5 (±2) | NUV | 46.95 |
| 19 | AL | p-pyr | p-pyr | 83 (±9) | 15 (±2) | 46.81 |
| 20 | AL | p-MeOPh | p-pyr | 12 (±6) | NUV | 47.19 |
| 21 | AL | p-NO2Ph | p-pyr | 92 (±10) | 0 | 46.98 |
| Compound | Band (nm) (ΔA/Ao(%) 1, Δλ(nm)) 2 | Kb (M−1) |
|---|---|---|
| 1 | 272 (−2.0, 0) | 1.62(±0.18) × 106 |
| 2 | 266 (−7.0, 0) | 2.06(±0.04) × 105 |
| 3 | 267 (−2.5, +2) | 3.73(±0.27) × 104 |
| 4 | 265 (−3.0, +3) | 5.88(±0.41) × 104 |
| 5 | 273 (−1.5, −1) | 1.25(±0.29) × 106 |
| 6 | 277 (−1.0, 0) | 3.90(±0.16) × 105 |
| 7 | 273 (−1.0, 0) | 1.59(±0.16) × 106 |
| 8 | 264 (−5.0, +2) | 3.60(±0.17) × 105 |
| 9 | 275 (−2.5, 0) | 7.31(±0.16) × 105 |
| 10 | 272 (−2.5, 0) | 1.52(±0.10) × 105 |
| 11 | 272 (−1.0, 0) | 4.79(±0.08) × 104 |
| 12 | 274 (−12.0, −3) | 6.02(±0.17) × 105 |
| 13 | 268 (−5.0, +1) | 3.04(±0.22) × 105 |
| 14 | 272 (−2.0, 0) | 8.50(±0.27) × 105 |
| 15 | 267 (−2.5, +1) | 3.23(±0.03) × 105 |
| 16 | 277 (+1.0, 0) | 3.47(±0.27) × 105 |
| 19 | 263 (−10.0, 0) | 1.29(±0.09) × 106 |
| 21 | 265 (−6.0, 0) | 1.85(±0.18) × 106 |
| Compound | ΔI/Io (%) | Ksv (M−1) | kq (M−1s−1) |
|---|---|---|---|
| 1 | 77.4 | 1.94(±0.05) × 105 | 8.43(±0.20) × 1012 |
| 2 | 66.5 | 8.27(±0.27) × 104 | 3.60(±0.12) × 1012 |
| 3 | 69.6 | 1.80(±0.05) × 105 | 7.82(±0.22) × 1012 |
| 4 | 60.2 | 1.34(±0.05) × 105 | 5.82(±0.21) × 1012 |
| 5 | 59.9 | 1.20(±0.03) × 105 | 5.20(±0.11) × 1012 |
| 6 | 51.4 | 4.46(±0.15) × 104 | 1.94(±0.07) × 1012 |
| 7 | 58.1 | 6.08(±0.14) × 104 | 2.64(±0.06) × 1012 |
| 8 | 66.4 | 6.85(±0.25) × 104 | 2.98(±0.11) × 1012 |
| 9 | 66.6 | 3.23(±0.11) × 105 | 1.40(±0.05) × 1013 |
| 10 | 77.4 | 1.50(±0.05) × 105 | 6.52(±0.20) × 1012 |
| 11 | 66.1 | 7.15(±0.16) × 104 | 3.11(±0.07) × 1012 |
| 12 | 67.5 | 1.06(±0.03) × 105 | 4.60(±0.11) × 1012 |
| 13 | 64.5 | 1.25(±0.03) × 105 | 5.43(±0.12) × 1012 |
| 14 | 63.4 | 1.53(±0.04) × 105 | 6.65(±0.16) × 1012 |
| 15 | 64.7 | 3.11(±0.06) × 105 | 1.35(±0.03) × 1013 |
| 16 | 57.4 | 1.24(±0.04) × 105 | 5.40(±0.15) × 1012 |
| 19 | 70.0 | 7.27(±0.19) × 104 | 3.16(±0.08) × 1012 |
| 21 | 66.4 | 1.33(±0.02) × 105 | 5.80(±0.07) × 1012 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasolli, M.; Dafnopoulos, K.; Andreou, N.-P.; Gritzapis, P.S.; Koffa, M.; Koumbis, A.E.; Psomas, G.; Fylaktakidou, K.C. Pyridine and p-Nitrophenyl Oxime Esters with Possible Photochemotherapeutic Activity: Synthesis, DNA Photocleavage and DNA Binding Studies. Molecules 2016, 21, 864. https://doi.org/10.3390/molecules21070864
Pasolli M, Dafnopoulos K, Andreou N-P, Gritzapis PS, Koffa M, Koumbis AE, Psomas G, Fylaktakidou KC. Pyridine and p-Nitrophenyl Oxime Esters with Possible Photochemotherapeutic Activity: Synthesis, DNA Photocleavage and DNA Binding Studies. Molecules. 2016; 21(7):864. https://doi.org/10.3390/molecules21070864
Chicago/Turabian StylePasolli, Milena, Konstantinos Dafnopoulos, Nicolaos-Panagiotis Andreou, Panagiotis S. Gritzapis, Maria Koffa, Alexandros E. Koumbis, George Psomas, and Konstantina C. Fylaktakidou. 2016. "Pyridine and p-Nitrophenyl Oxime Esters with Possible Photochemotherapeutic Activity: Synthesis, DNA Photocleavage and DNA Binding Studies" Molecules 21, no. 7: 864. https://doi.org/10.3390/molecules21070864