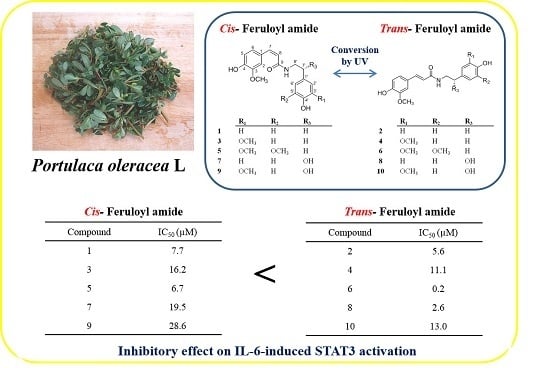
Study of the UV Light Conversion of Feruloyl Amides from Portulaca oleracea and Their Inhibitory Effect on IL-6-Induced STAT3 Activation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Elucidation of the Isolated Compounds
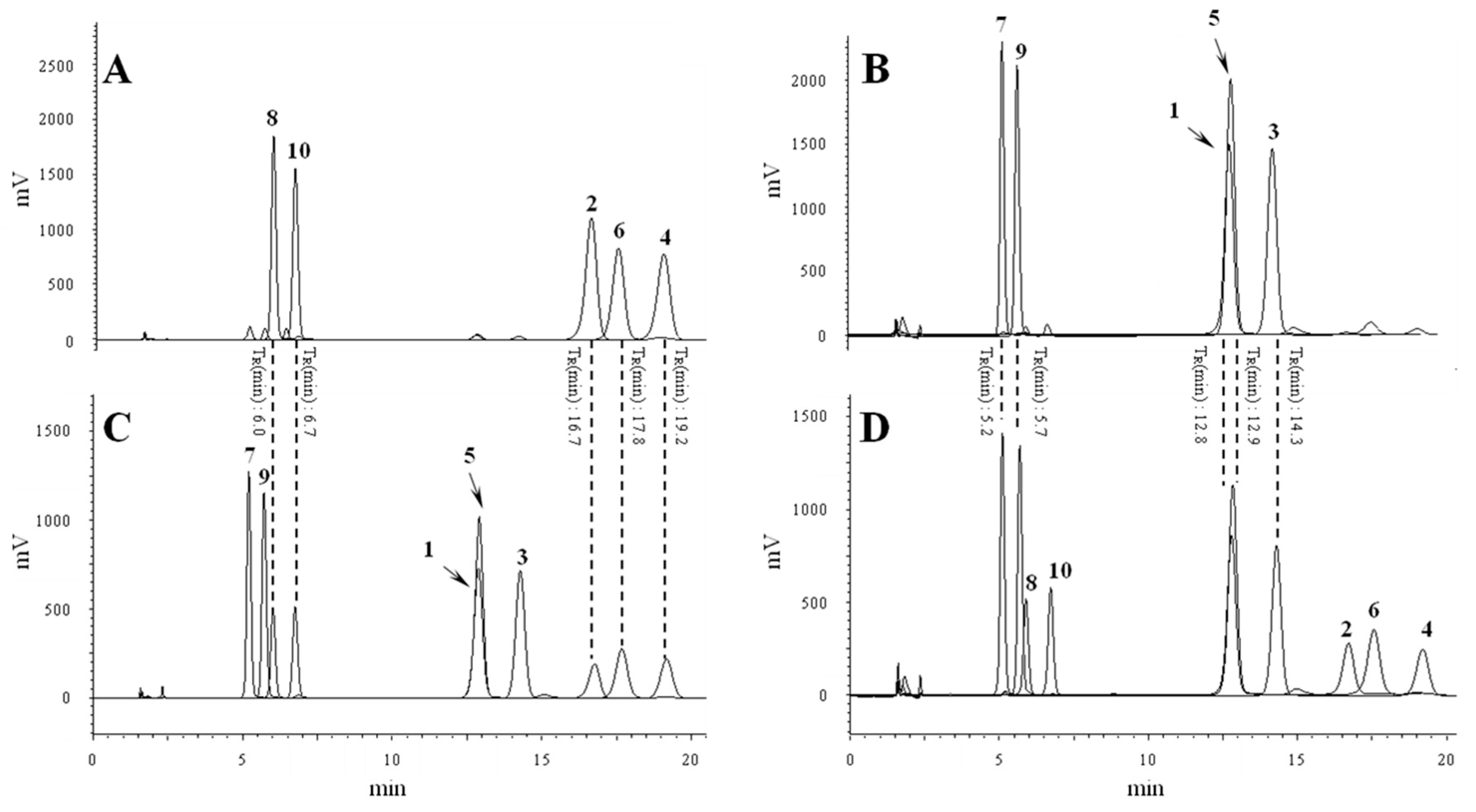
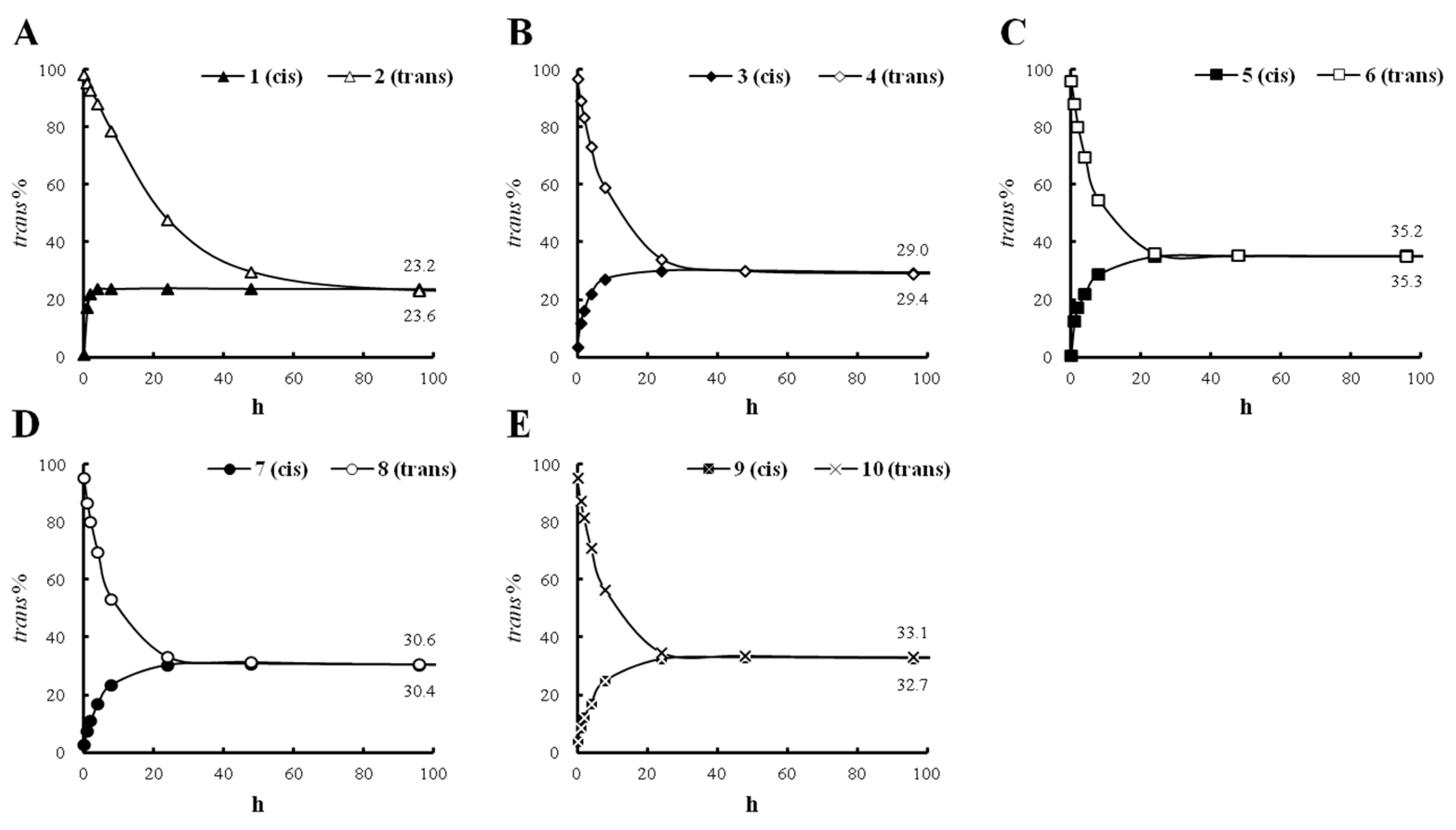
2.2. Conversion Study of Compounds 1–10
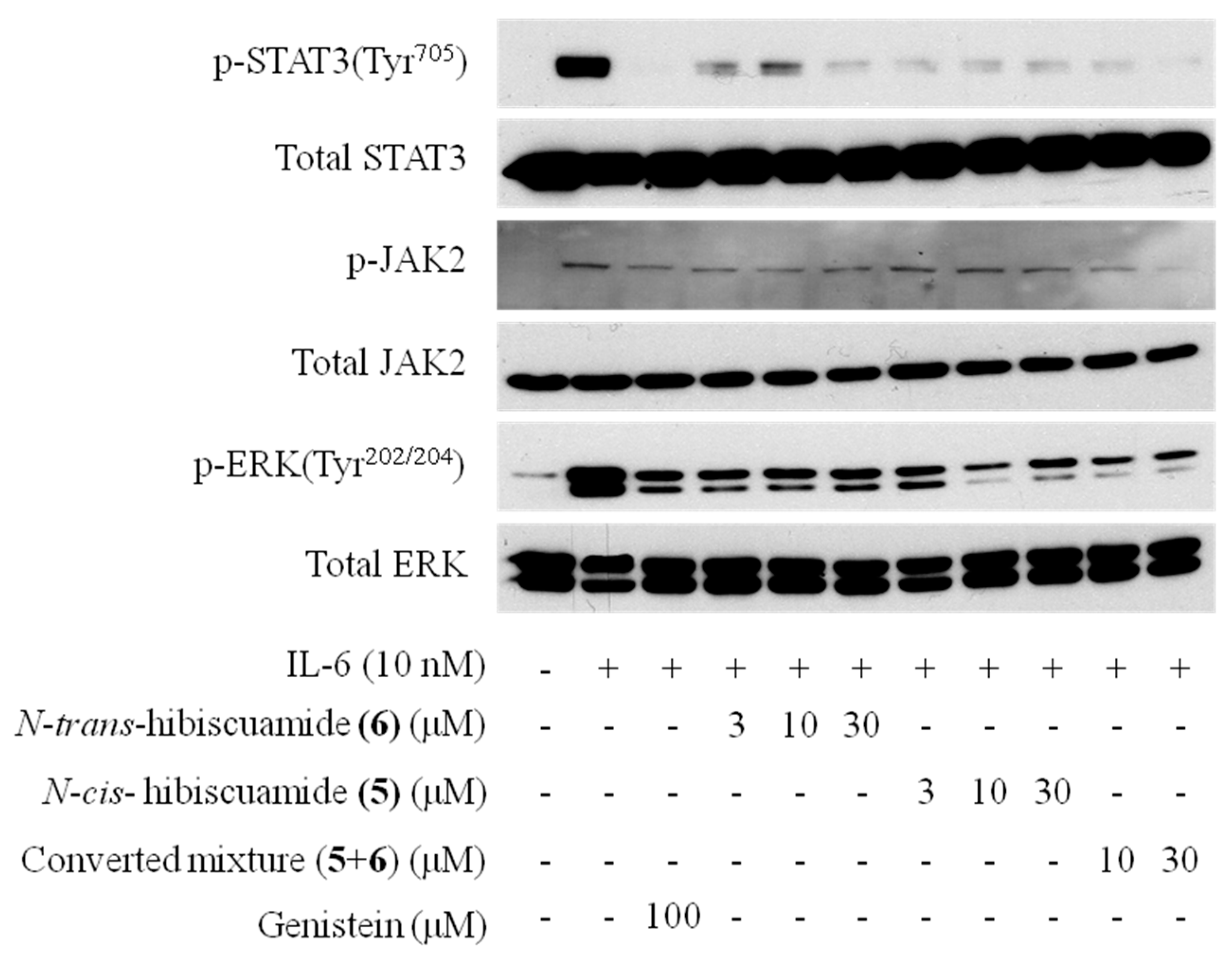
2.3. Activity of Isolated Compounds
3. Materials and Methods
3.1. Plant Material
3.2. General Procedure
3.3. Isolation and Purification Procedures
3.4. Stability and Conversion Testing of Cis and trans-Feruloyl Amides
3.5. Cell Culture
3.6. Reagents and Chemicals
3.7. Establishment of the Stable Cell Line Expressing pStat3-Luc
3.8. IL-6-Induced STAT3 Activation
3.9. Cell Viability Assay
3.10. Western Blot Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lim, Y.Y.; Quah, E.P.L. Antioxidant properties of different cultivars of Portulaca oleracea. Food Chem. 2007, 103, 734–740. [Google Scholar] [CrossRef]
- Rashed, A.N.; Afifi, F.U.; Disi, A.M. Simple evaluation of the wound healing activity of a crude extract of Portulaca oleracea L. (growing in Jordan) in Mus musculus JVI-1. J. Ethnopharmacol. 2003, 88, 131–136. [Google Scholar] [CrossRef]
- Amirul Alam, M.; Juraimi, A.S.; Rafii, M.Y.; Hamid, A.A.; Aslani, F.; Alam, M.Z. Effects of salinity and salinity-induced augmented bioactive compounds in purslane (Portulaca oleracea L.) for possible economical use. Food Chem. 2015, 169, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.E.; Kennedy, R.A. Crassulacean Acid metabolism in the succulent C (4) dicot, Portulaca oleracea L under natural environmental conditions. Plant Physiol. 1982, 69, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Erkan, N. Antioxidant activity and phenolic compounds of fractions from Portulaca oleracea L. Food Chem. 2012, 133, 775–781. [Google Scholar] [CrossRef]
- Xiang, L.; Xing, D.; Wang, W.; Wang, R.; Ding, Y.; Du, L. Alkaloids from Portulaca oleracea L. Phytochemistry 2005, 66, 2595–2601. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Howe, P.; Zhou, Y.F.; Xu, Z.Q.; Hocart, C.; Zhan, R. Fatty acids and β-carotene in Australian purslane (Portulaca oleracea) varieties. J. Chromatogr. A 2000, 893, 207–213. [Google Scholar] [CrossRef]
- Chan, K.; Islam, M.W.; Kamil, M.; Radhakrishnan, R.; Zakaria, M.N.; Habibullah, M.; Attas, A. The analgesic and anti-inflammatory effects of Portulaca oleracea L. subsp. Sativa (Haw.) Celak. J. Ethnoparmacol. 2000, 73, 445–451. [Google Scholar] [CrossRef]
- Chen, C.J.; Wang, W.Y.; Wang, X.L.; Dong, L.W.; Yue, Y.T.; Xin, H.L.; Ling, C.Q.; Li, M. Anti-hypoxic activity of the ethanol extract from Portulaca oleracea in mice. J. Ethnopharmacol 2009, 124, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yu, L.; Chen, G. Determination of flavonoids in Portulaca oleracea L. By capillary electrophoresis with electrochemical detection. J. Pharm. Biomed. Anal. 2006, 41, 493–499. [Google Scholar] [CrossRef] [PubMed]
- De la Mata, J.; Uy, H.L.; Guise, T.A.; Story, B.; Boyce, B.F.; Mundy, G.R.; Roodman, G.D. Interleukin-6 enhances hypercalcemia and bone resorption mediated by parathyroid hormone-related protein in vivo. J. Clin. Investig. 1995, 95, 2846–2852. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.; Zhang, X.G.; Lu, Z.Y.; Bataille, R. Interleukin-6 in human multiple myeloma. Blood 1995, 85, 863–872. [Google Scholar] [PubMed]
- Nishimura, R.; Nagao, K.; Miyayama, H.; Matsuda, M.; Baba, K.; Matsuoka, Y.; Yamashita, H.; Fukuda, M.; Mizumoto, T.; Hamamoto, R. An analysis of serum interleukin-6 levels to predict benefits of medroxyprogesterone acetate in advanced or recurrent breast cancer. Oncology 2000, 59, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Nowell, M.A.; Richards, P.J.; Fielding, C.A.; Ognjanovic, S.; Topley, N.; Williams, A.S.; Bryant-Greenwood, G.; Jones, S.A. Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: Implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2006, 54, 2084–2095. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Gollapudi, S.; Su, H.; Gupta, S. Leptin activates human B cells to secrete TNF-α, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J. Clin. Immunol. 2011, 31, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Liao, M.; Yang, F.; Xiong, S. Amide alkaloids from Acorus Tatarinowii Schott. Adv. Biomed. Eng. Res. 2014, 2, 1–4. [Google Scholar]
- King, R.R.; Calhoun, L.A. Characterization of cross-linked hydroxycinnamic acid amides isolated from potato common scab lesions. Phytochemistry 2005, 66, 2468–2473. [Google Scholar] [CrossRef] [PubMed]
- Al-Taweel, A.M.; Perveen, S.; El-Shafae, A.M.; Fawzy, G.A.; Malik, A.; Afza, N.; Iqbal, L.; Latif, M. Bioactive phenolic amides from Celtis Africana. Molecules 2012, 17, 2675–2682. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chang, F.; Yen, H.; Wu, Y. Amides from stems of annona Cherimola. Phytochemistry 1998, 49, 1443–1447. [Google Scholar] [CrossRef]
- Seo, G.W.; Cho, J.; Moon, J.; Park, K. Isolation and identification of Cinnamic acid amides as antioxidants from Allium fistulosum L. and their free radical scavenging activity. Food Sci. Biotechnol. 2011, 20, 555–560. [Google Scholar] [CrossRef]
- Chen, J.J.; Huang, S.Y.; Duh, C.Y.; Chen, I.S.; Wang, T.C.; Fang, H.Y. A new cytotoxic amide from the stem wood of Hibiscus tiliaceus. Planta Med. 2006, 72, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.B.; Jia, W.; Gao, W.Y.; Yao, Z.; Teng, J.; Zhao, A.H.; Duan, H.Q. Antitumor constituents from Alternanthera philoxeroides. J. Asian Nat. Prod. Res. 2007, 9, 511–515. [Google Scholar] [CrossRef] [PubMed]
- DellaGreca, M.; Previtera, L.; Purcaro, R.; Zarrelli, A. Cinnamic acid amides and lignanamides from Aptenia cordifolia. Tetrahedron 2006, 62, 2877–2882. [Google Scholar] [CrossRef]
- Holzbach, J.C.; Lopes, L.M. Aristolactams and alkamides of Aristolochia gigantea. Molecules 2010, 15, 9462–9472. [Google Scholar] [CrossRef] [PubMed]
- Brent, C.T.; Andrew, L. Resveratrol: Isomeric molar absorptivities and stability. J. Agric. Food Chem. 1996, 44, 1253–9472. [Google Scholar]
- Song, Li.; Lanyi, J.K.; Yang, D.; Ei-Sayed, M.A. Retinal isomer composition in some bacteriorhodopsin mutants under light and dark adaptation conditions. J. Phys. Chem. 1995, 99, 10052–10055. [Google Scholar] [CrossRef]
- Karaköse, H.; Jaiswal, R.; Deshpande, S.; Kuhnert, N. Investigation of the photochemical changes of chlorogenic acids induced by ultraviolet light in model systems and in agricultural practice with Stevia rebaudiana cultivation as an example. J. Agric. Food Chem. 2015, 63, 3339–3347. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.M.; Lee, S.W.; Yun, B.R.; Hwang, B.S.; Kim, S.N.; Park, C.S.; Jeoung, S.H.; Kim, H.K.; Lee, W.S.; Rho, M.C. Vigna angularis inhibits IL-6-induced cellular signaling and ameliorates collagen-induced arthritis. Rheumatology 2014, 53, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.
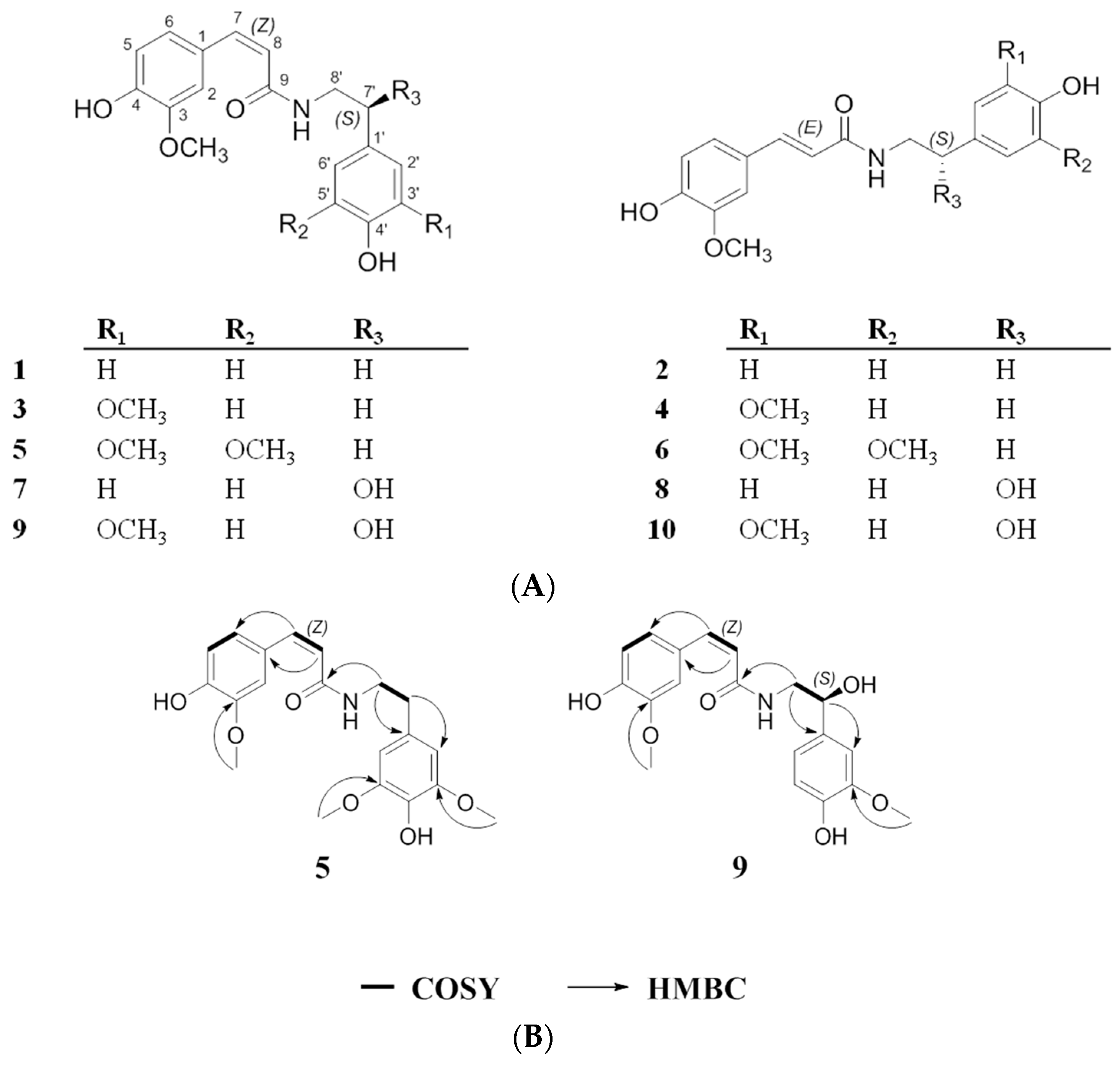

| Positions | 5 (cis, Z) | 6 (trans, E) | 9 (cis, Z) | 10 (trans, E) | ||||
|---|---|---|---|---|---|---|---|---|
| δH | δc | δH | δc | δH | δc | δH | δc | |
| 1 | - | 128.0 | - | 127.6 | - | 126.6 | - | 128.5 |
| 2 | 7.34 (d, 2.0) 2 | 116.2 | 7.07 (d, 2.0) | 111.6 | 7.38 (d, 1.8) | 112.7 | 7.09 (d, 1.8) | 111.8 |
| 3 | - | 149.3 | - | 149.4 | - | 147.4 | - | 149.2 |
| 4 | - | 149.8 | - | 149.4 | - | 148.2 | - | 150.1 |
| 5 | 6.69 (d, 8.0) | 114.0 | 6.74 (d, 8.0) | 117.0 | 6.72 (d, 8.4) | 115.9 | 6.77 (d, 7.8) | 116.7 |
| 6 | 6.90 (dd, 8.0, 2.0) | 125.2 | 6.98 (dd, 8.0, 2.0) | 123.6 | 6.91 (dd, 8.4, 1.8) | 123.8 | 7.00 (d, 7.8) | 123.5 |
| 7 | 6.57 (d, 13.2) | 138.6 | 7.41 (d, 15.6) | 142.4 | 6.59 (d, 12.6) | 137.7 | 7.42 (d, 15.6) | 142.5 |
| 8 | 5.78 (d, 13.2) | 121.2 | 6.36 (d, 15.6) | 118.3 | 5.79 (d, 12.6) | 118.6 | 6.45 (d, 15.6) | 118.9 |
| 9 | - | 170.6 | - | 169.5 | - | 169.2 | - | 169.8 |
| 1′ | - | 131.3 | - | 131.4 | - | 133.9 | - | 135.7 |
| 2′ | 6.45 (s) | 107.1 | 6.50 (s) | 106.9 | 6.93 (d, 1.8) | 109.4 | 6.97 (d, 1.8) | 111.1 |
| 3′ | - | 149.3 | - | 151.5 | - | 147.6 | - | 149.6 |
| 4′ | - | 135.2 | - | 135.2 | - | 145.9 | - | 147.4 |
| 5′ | - | 149.0 | - | 149.9 | 6.69 (d, 8.4) | 114.4 | 6.75 (d, 7.8) | 116.2 |
| 6′ | 6.45 (s) | 107.0 | 6.50 (s) | 107.2 | 6.76 (dd, 8.4, 1.8) | 118.6 | 6.81 (dd, 7.8, 1.8) | 120.2 |
| 7′ | 2.70 (t, 7.2) | 36.6 | 2.75 (t, 7.2) | 36.8 | 4.66 (dd, 7.8, 4.8) | 72.2 | 4.71 (dd, 7.8, 4.8) | 73.8 |
| 8′ α | 3.42 (t, 7.6) | 42.4 | 3.47 (t, 7.6) | 42.6 | 3.39 (dd, 13.8, 5.4) | 46.8 | 3.43 (dd, 13.8, 5.4) | 50.0 |
| 8′ β | 3.42 (t, 7.6) | 42.4 | 3.47 (t, 7.6) | 42.6 | 3.46 (dd, 13.2, 7.8) | 46.8 | 3.52 (dd, 13.2, 7.8) | 50.0 |
| 3-OCH3 | 3.75 (s) | 56.8 | 3.80 (s) | 56.9 | 3.79 (s) | 55.0 | 3.82 (s) | 55.6 |
| 3′-OCH3 | 3.75 (s) | 56.8 | 3.80 (s) | 56.9 | 3.80 (s) | 55.0 | 3.84 (s) | 55.5 |
| 5′-OCH3 | 3.79 (s) | 56.4 | 3.85 (s) | 56.5 | - | - | - | - |
| Compounds | IC50 (μM) |
|---|---|
| N-cis-feruloyltyramine (1) | 19.3 |
| N-trans-feruloyltyramine (2) | 5.6 |
| Converted mixture (1 + 2) | 15.2 |
| N-cis-feruloyl-3′-methoxytyramine (3) | 16.2 |
| N-trans-feruloyl-3′-methoxytyramine (4) | 6.0 |
| Converted mixture (3 + 4) | 6.5 |
| N-cis-hibiscusamide (5) | 6.7 |
| N-trans-hibiscusamide (6) | 0.2 |
| Converted mixture (5 + 6) | 5.9 |
| (7′S)-N-cis-feruloyloctopamine (7) | 19.5 |
| (7′S)-N-trans-feruloyloctopamine (8) | 2.6 |
| Converted mixture (7 + 8) | 6.7 |
| (7′S)-N-cis-feruloylnormetanephrine (9) | 28.6 |
| (7′S)-N-trans-feruloylnormetanephrine (10) | 13.0 |
| Converted mixture (9 + 10) | 13.7 |
| Oleanolic acid acetate 1 | 0.7 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.T.; Kim, Y.; Jang, H.-J.; Oh, H.-M.; Lim, C.-H.; Lee, S.W.; Rho, M.-C. Study of the UV Light Conversion of Feruloyl Amides from Portulaca oleracea and Their Inhibitory Effect on IL-6-Induced STAT3 Activation. Molecules 2016, 21, 865. https://doi.org/10.3390/molecules21070865
Hwang JT, Kim Y, Jang H-J, Oh H-M, Lim C-H, Lee SW, Rho M-C. Study of the UV Light Conversion of Feruloyl Amides from Portulaca oleracea and Their Inhibitory Effect on IL-6-Induced STAT3 Activation. Molecules. 2016; 21(7):865. https://doi.org/10.3390/molecules21070865
Chicago/Turabian StyleHwang, Joo Tae, Yesol Kim, Hyun-Jae Jang, Hyun-Mee Oh, Chi-Hwan Lim, Seung Woong Lee, and Mun-Chual Rho. 2016. "Study of the UV Light Conversion of Feruloyl Amides from Portulaca oleracea and Their Inhibitory Effect on IL-6-Induced STAT3 Activation" Molecules 21, no. 7: 865. https://doi.org/10.3390/molecules21070865