Preparative Separation and Purification of the Total Flavonoids in Scorzonera austriaca with Macroporous Resins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening of Macroporous Resin
2.2. Maximum Adsorption Capacity of the Selected Resin
2.3. Effect of Ethanol Concentration on Ratio of Desorption in Static Desorption
2.4. Desorption Flow Rate in Dynamic Desorption
2.5. Gradient Elution in Dynamic Adsorption and Desorption
2.6. Preparation Method Validation
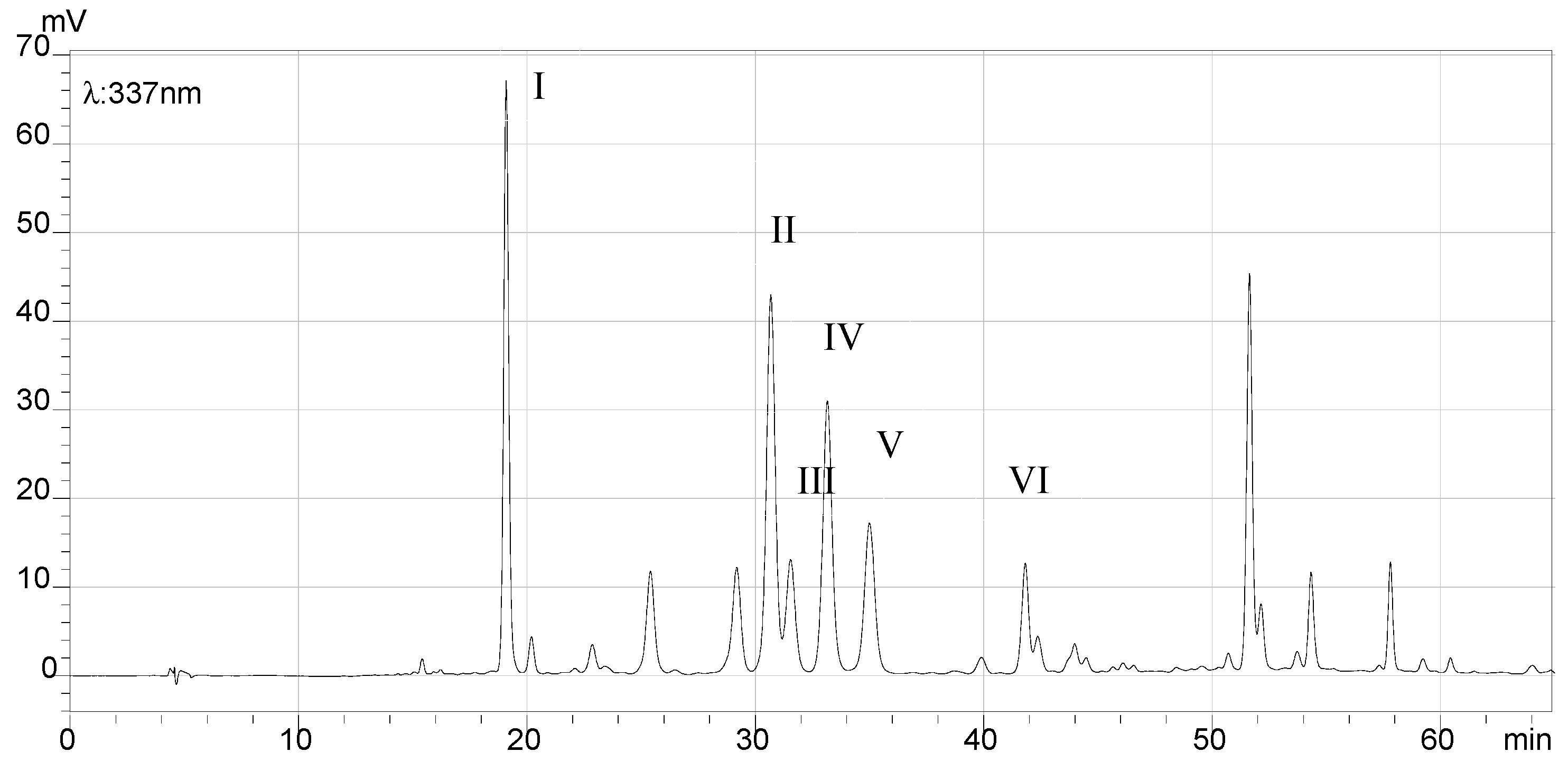
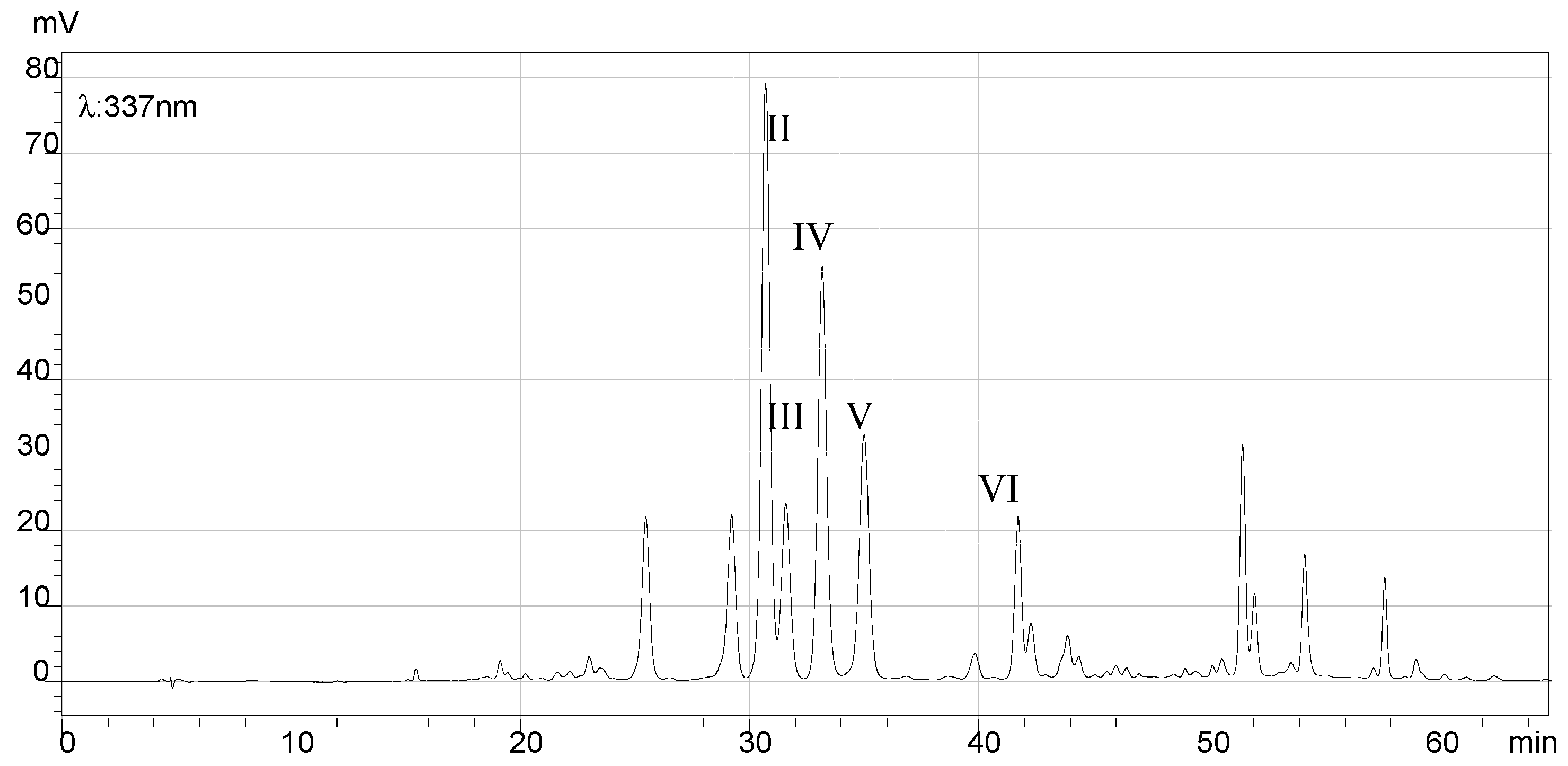
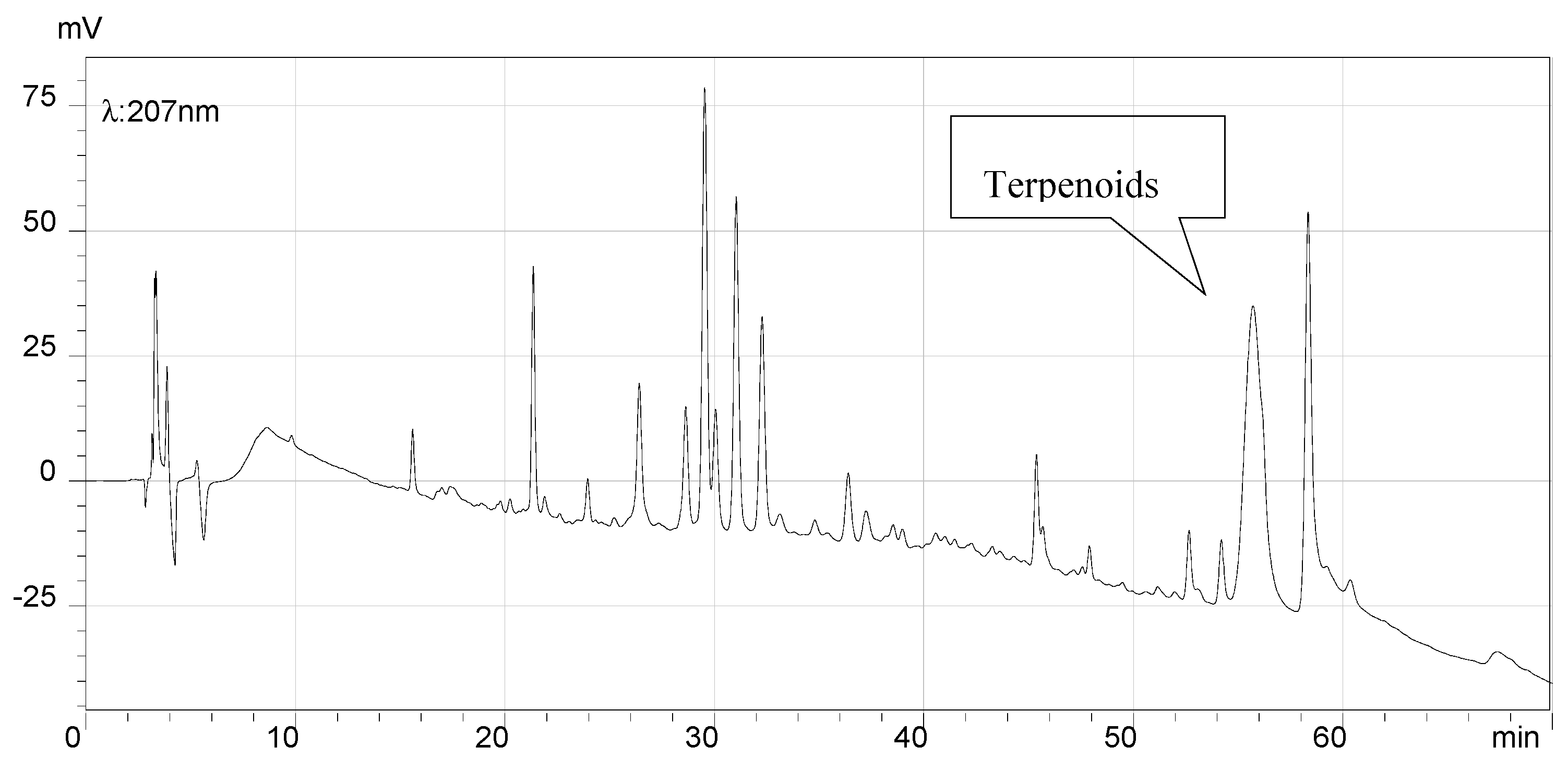
2.7. HPLC Analysis of the Final Products and Crude Flavonoid Extracts of Scorzonera austriaca
3. Experimental Section
3.1. Materials
3.2. Pretreatment of Adsorbents
3.3. Preparation of the Crude Flavonoid Extracts from Scorzonera austriaca Herbs
3.4. Ultraviolet-Visible Spectrophotometry Method for Determination of Flavonoid Compounds
3.5. HPLC/ESI/MS Analysis of Total Flavonoids
3.6. Static Adsorption and Desorption Tests
3.7. Dynamic Adsorption and Desorption Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- The Editorial Board of Zhong Hua Ben Cao of State Administration of Traditional Chinese Medicine of the People’s Republic of China. Zhong Hua Ben Cao, 1st ed.; Scientific and Technical Publishers: Shanghai, China, 1999; Volume 7, pp. 939–941. [Google Scholar]
- Zhang, T.W.; Xie, Y.; Zhang, Z.; Wang, G.S. Study on hepatoprotective effects of total flavonoids in Scorzonera austriaca Wild in vivo and in vitro. Chin. J. Biochem. Pharm. 2015, 35, 6–9. [Google Scholar]
- Wang, G.S.; Yang, X.H.; Zhou, X.P. Aplication of the extract of Scorzonera austriaca in preparing medicine to treat hepatitis. China Patent 2011102552351, 24 July 2013. [Google Scholar]
- Xie, Y.; Wang, J.; Geng, Y.M.; Qu, Y.F.; Zhang, Z.; Wang, G.S. Experimental studies on inhibitory effects of total flavonoids in Scorzonera austriaca Wild on hepatitis B virus in vitro. Chin. J. Biochem. Pharm. 2015, 35, 41–44. [Google Scholar]
- Xiao, X.H.; Si, X.X.; Tong, X.; Li, G.K. Preparation of flavonoids and diarylheptanoid from Alpinia katsumadai Hayata by microwave-assisted extraction and high-speed counter-current chromatography. Sep. Purif. Technol. 2011, 81, 265–269. [Google Scholar] [CrossRef]
- Qi, Y.Y.; Sun, A.L.; Liu, R.M.; Meng, Z.L.; Xie, H.Y. Isolation and purification of flavonoid and isoflavonoid compounds from the pericarp of Sophora japonica L. by adsorption chromatography on 12% cross-linked agarose gel media. J. Chromatogr. A 2007, 1140, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Luan, N.; Li, D. Study on supercritical CO2 extraction of flavonoids from Cynomorium songaricum. J. Chin. Med. Mater. 2010, 33, 1167–1171. [Google Scholar]
- Chang, L.; Wei, Y.; Bi, P.Y.; Shao, Q. Recovery of liquiritin and glycyrrhizic acid from Glycyrrhiza uralensis Fisch by aqueous two-phase flotation and multi-stage preparative high performance liquid chromatography. Sep. Purif. Technol. 2014, 134, 204–209. [Google Scholar]
- Song, X.L.; Li, J.H.; Wang, J.T.; Chen, L.X. Quercetin molecularly imprinted polymers: Preparation, recognition characteristics and properties as sorbent for solid-phase extraction. Talanta 2009, 80, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.S.; Tang, J.; Ho, C.T. Selection and optimization of macroporous resin for separation of stilbene glycoside from Polygonum multiflorum Thunb. J. Chem. Technol. Biotechnol. 2008, 83, 1422–1427. [Google Scholar] [CrossRef]
- Li, C.; Zheng, Y.Y.; Wang, X.F.; Feng, S.L.; Di, D.L. Simultaneous separation and purification of flavonoids and oleuropein from Olea europaea L. (olive) leaves using macroporous resin. J. Sci. Food Agric 2011, 91, 2826–2834. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Zhao, H.F.; Dong, Y.; Yang, B.; Zhao, M.M. Macroporous resin purification behavior of phenolics and rosmarinic acid from Rabdosia serra (Maxim.) Hara leaf. Food Chem. 2012, 130, 417–424. [Google Scholar] [CrossRef]
- Lin, X.Q.; Wu, J.L.; Fan, J.S.; Qian, W.B.; Zhou, X.Q.; Qian, C.; Jin, X.H.; Wang, L.L.; Bai, J.X.; Ying, H.J. Adsorption of butanol from aqueous solution onto a new type of macroporous adsorption resin: studies of adsorption isotherms and kinetics simulation. J. Chem. Technol. Biotechnol. 2012, 87, 924–931. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Zu, Y.G.; Fu, Y.J.; Ma, W.; Zhang, D.F.; Kong, Y.; Li, X.J. Preliminary enrichment and separation of genistein and apigenin from extracts of pigeon pea roots by macroporous resins. Bioresour. Technol. 2010, 101, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, H.H.; Zhang, H.Y.; Wang, Y.R.; Hu, P. Development of a process for separation of mogroside V from Siraitia grosvenorii by macroporous resins. Molecules 2011, 16, 7288–7301. [Google Scholar] [CrossRef] [PubMed]
- He, Z.Y.; Xia, W.S. Preparative separation and purification of phenolic compounds from Canarium album L. by macroporous resins. J. Sci. Food Agric. 2008, 88, 493–498. [Google Scholar] [CrossRef]
- Li, J.; Yu, D.Q. Chemical constituents from herbs of Erigeron breviscapus. Chin. J. Chin. Mater. Med. 2011, 36, 1458–1461. [Google Scholar]
- Liu, X.; Zhang, Z.L.; Wang, Z.; He, L.; Peng, C.; Wang, G.S. Isolation and structure identification of flavonoid glycosides from Scorzonera austriaca Wild. J. Jilin Univ. Med. Ed. 2012, 38, 595–597. [Google Scholar]
- Li, Q.M.; Wang, G.S. Isolation and structure identification of flavonoid glycosides from Scorzonera ruprechtiana Lipsch et Krasch. J. Jilin Univ. Med. Ed. 2010, 36, 10–13. [Google Scholar]
- Sample Availability: Samples of the compounds II–VI are available from the authors.
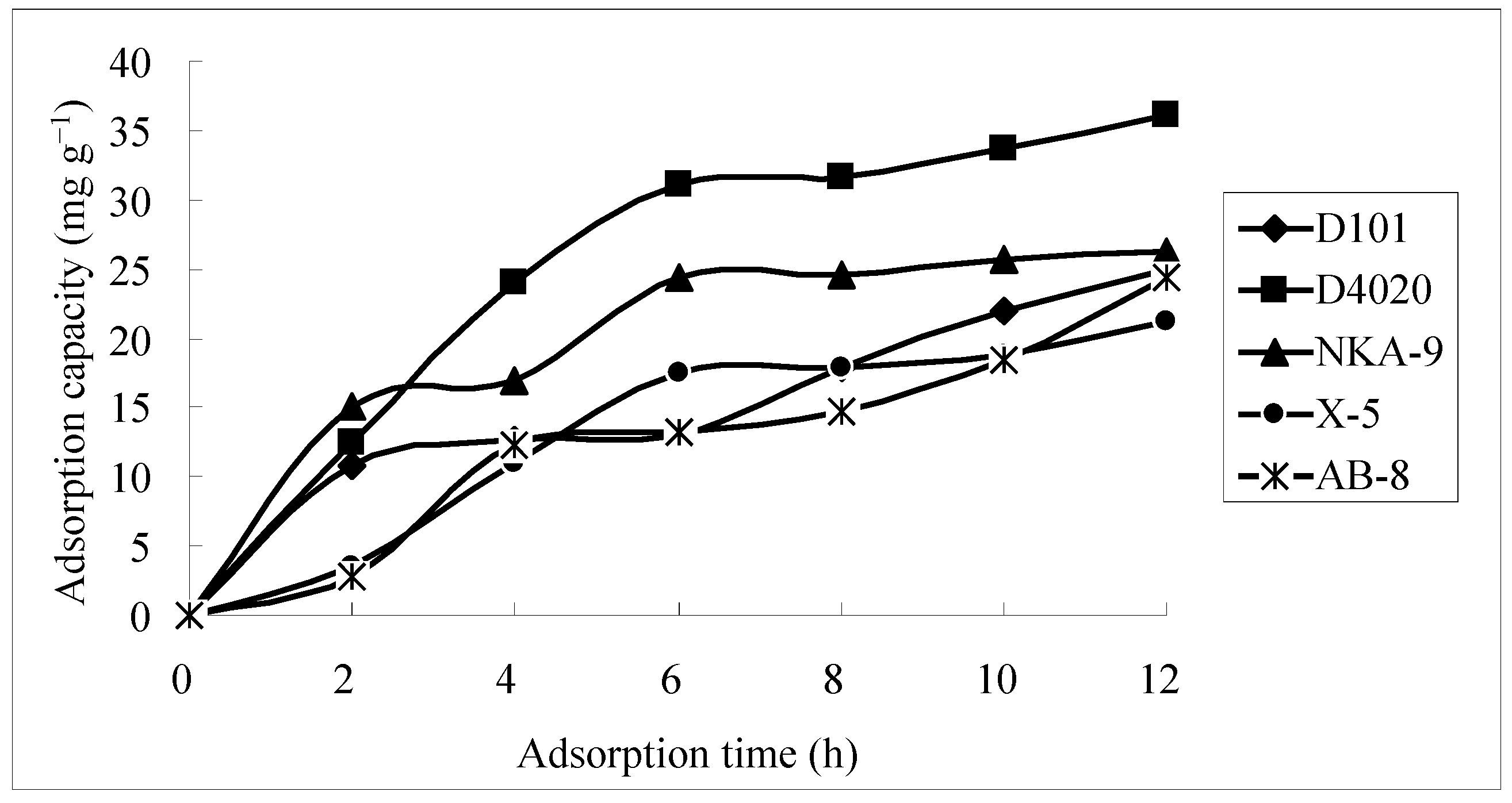
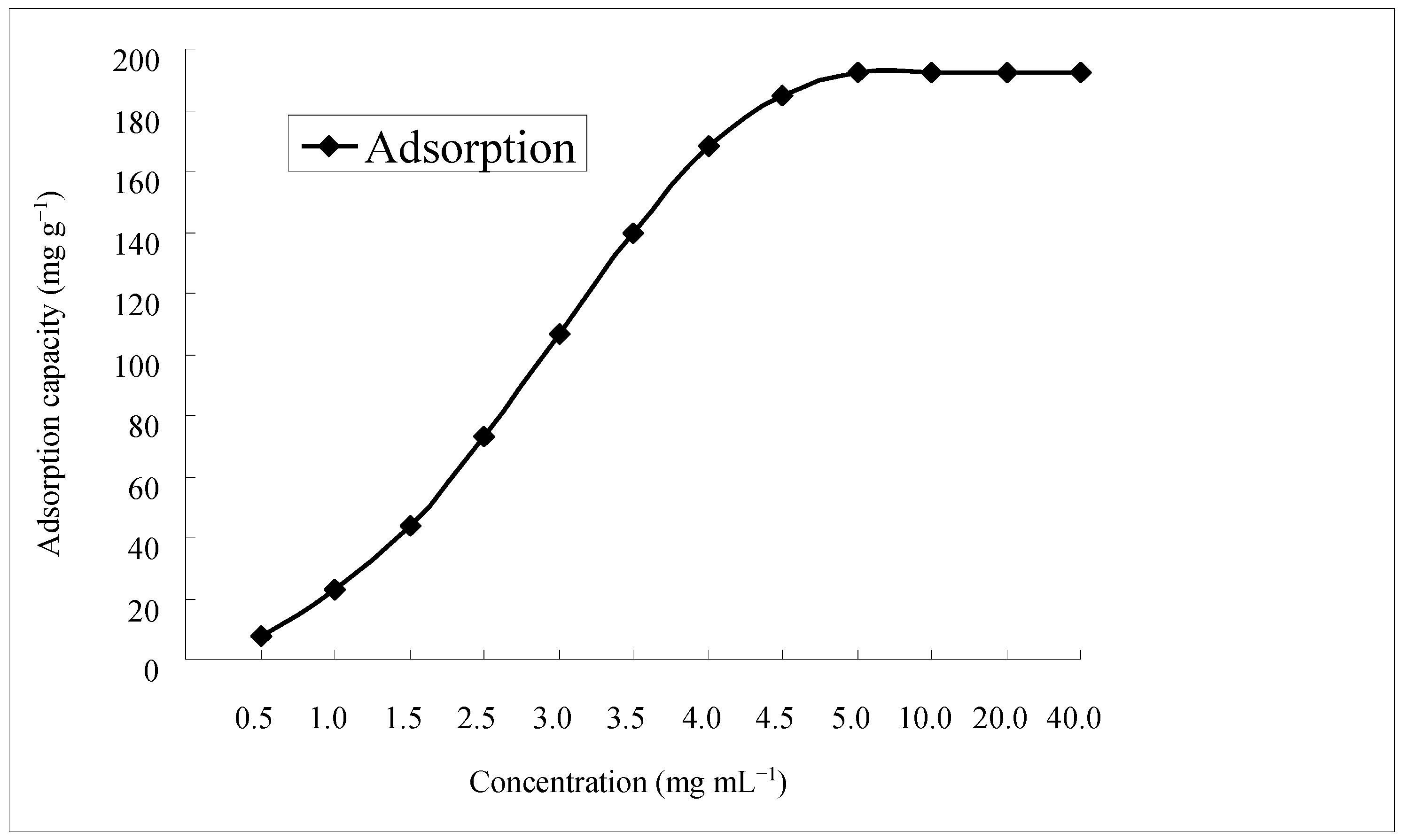

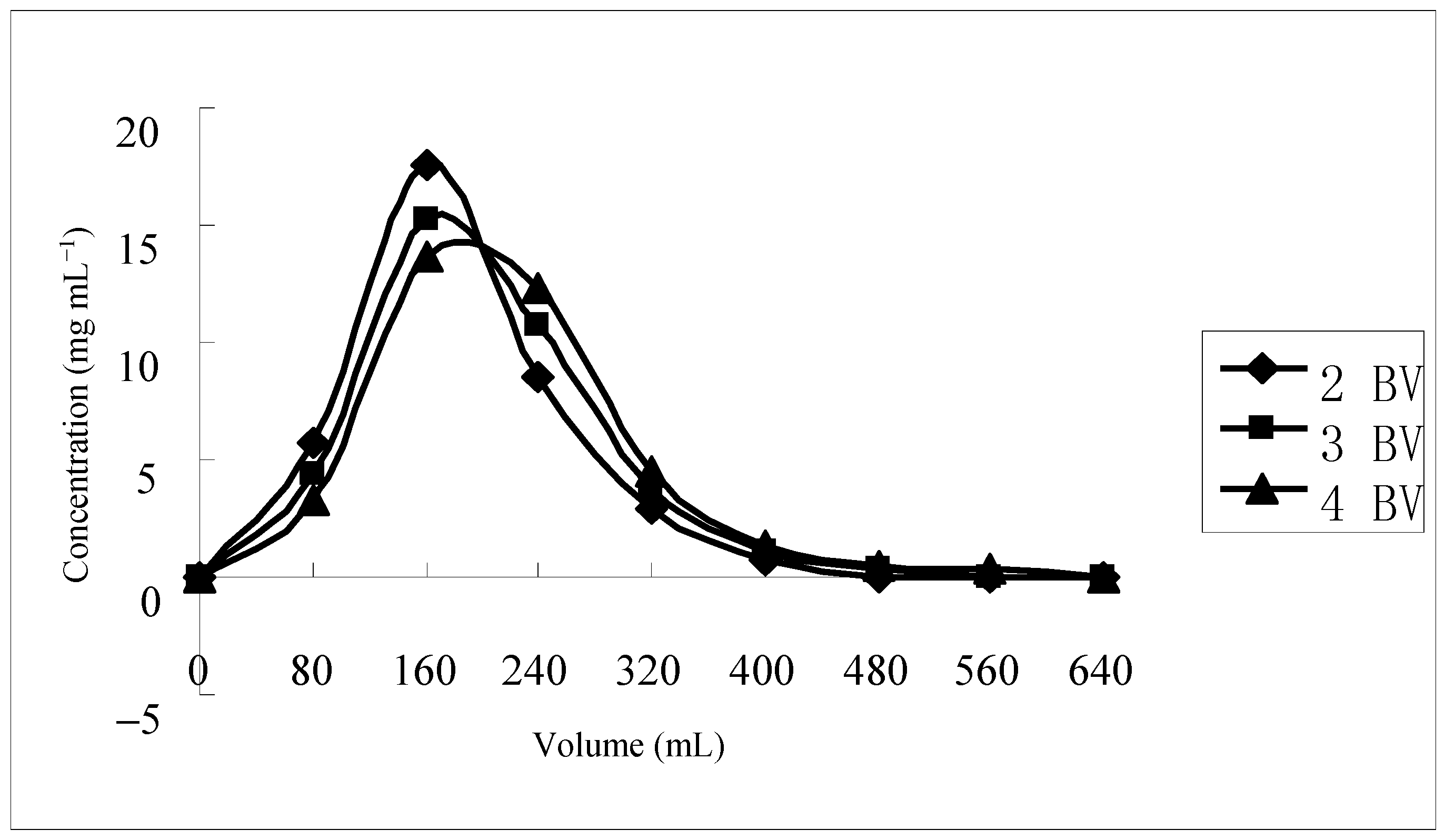

| Trade Name | Qa a (mg·g−1) | Qd a (mg·g−1) | Rd a (%) |
|---|---|---|---|
| D101 | 26.4 ± 2.6 | 20.7 ± 2.1 | 78.4 ± 2.3 |
| D4020 | 38.7 ± 2.1 | 34.8 ± 3.0 | 90.1 ± 2.6 |
| NKA-9 | 28.1 ± 3.5 | 25.1 ± 3.1 | 89.4 ± 3.3 |
| X-5 | 22.8 ± 1.7 | 15.6 ± 1.9 | 68.5 ± 2.5 |
| AB-8 | 26.2 ± 2.3 | 23.0 ± 2.6 | 87.7 ± 4.3 |
| Eeluate | Water | 5% | 10% | 15% | 20% | 25% | 30% | 35% | 40% | 60% |
|---|---|---|---|---|---|---|---|---|---|---|
| Volume needed (BV) | 5 | 5 | 5 | 4 | 5 | 4 | 5 | 3 | 2 | 2 |
| Colour of TLC spots | purple | purple, brown | yellow | yellow | yellow | yellow | yellow | brown | brown | brown |
| No. | 1 | 2 | 3 | 4 | 5 | 6 | mean | RSD/% |
|---|---|---|---|---|---|---|---|---|
| a Recovery yield (%) | 63.40 | 65.55 | 64.46 | 62.50 | 61.72 | 63.83 | 63.58 | 1.37 |
| Content (%) | 93.68 | 90.95 | 92.54 | 94.28 | 96.50 | 93.18 | 93.52 | 1.85 |
| Peak No. | Retention Time (min) | HRMS (m/z) [M + H]+ | MS (m/z) Caculated | Structural Identification | Reference |
|---|---|---|---|---|---|
| I | 19.08 | 355.1027 | 355.1024 | Chlorogenic acid | [17] |
| II | 30.67 | 581.1527 | 581.1501 | 2″-O-β-d-xylopyranosyl isoorientin | [18] |
| III | 31.54 | 565.1580 | 565.1552 | 6-C-α-l-arabipyranosyl orientin | [18] |
| IV | 33.15 | 449.1089 | 449.1078 | Orientin | [19] |
| V | 34.99 | 449.1093 | 449.1078 | Isoorientin | [19] |
| VI | 41.81 | 433.1156 | 433.1129 | Vitexin | [18] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Guo, Q.-S.; Wang, G.-S. Preparative Separation and Purification of the Total Flavonoids in Scorzonera austriaca with Macroporous Resins. Molecules 2016, 21, 768. https://doi.org/10.3390/molecules21060768
Xie Y, Guo Q-S, Wang G-S. Preparative Separation and Purification of the Total Flavonoids in Scorzonera austriaca with Macroporous Resins. Molecules. 2016; 21(6):768. https://doi.org/10.3390/molecules21060768
Chicago/Turabian StyleXie, Yang, Qiu-Shi Guo, and Guang-Shu Wang. 2016. "Preparative Separation and Purification of the Total Flavonoids in Scorzonera austriaca with Macroporous Resins" Molecules 21, no. 6: 768. https://doi.org/10.3390/molecules21060768





