New 30-Noroleanane Triterpenoid Saponins from Holboellia coriacea Diels
Abstract
:1. Introduction
2. Results and Discussion
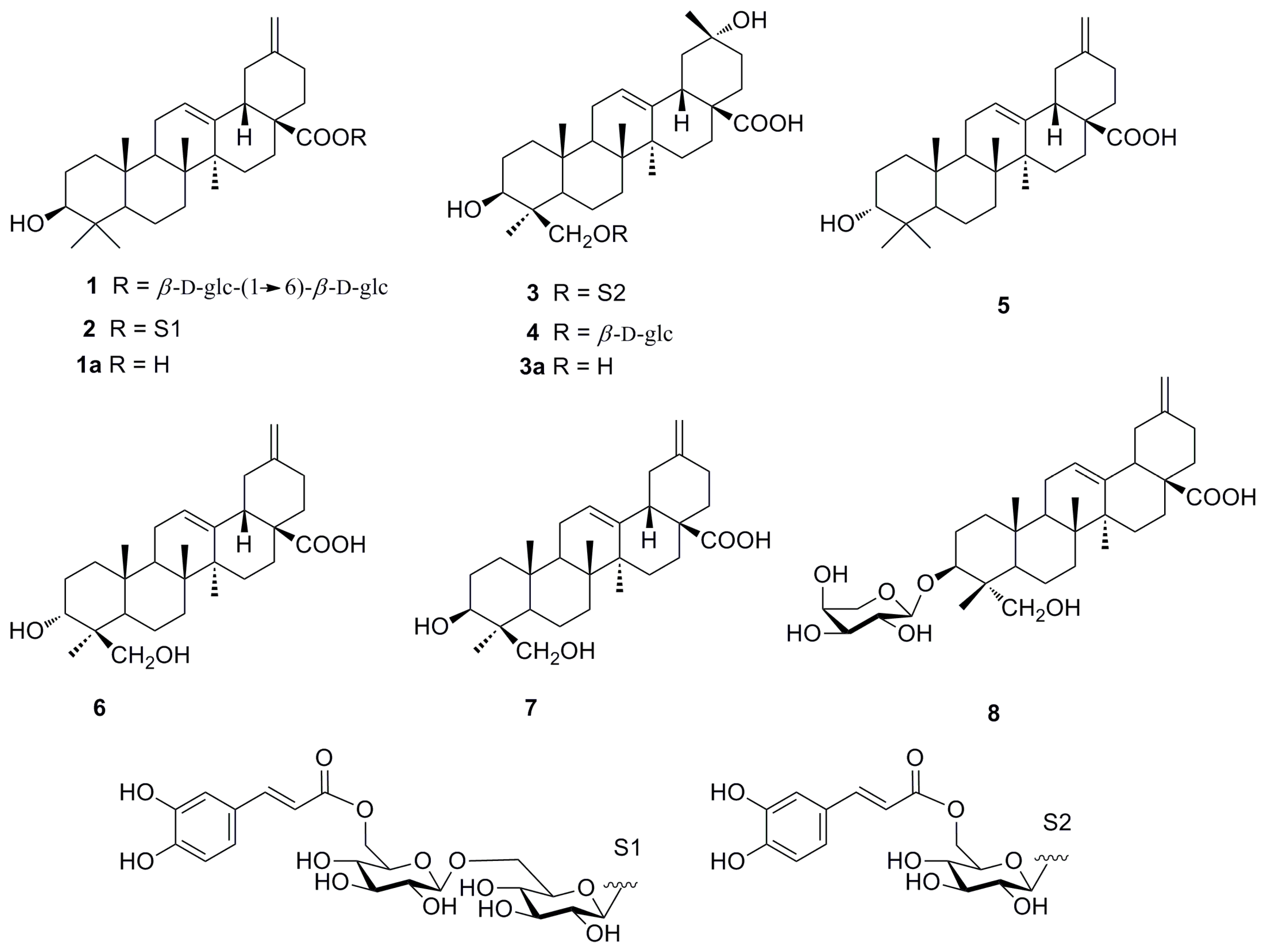
2.1. Identification of New Compounds
2.2. Cytotoxicity Assay
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectroscopic Data of 1–3
3.5. Chemical Hydrolysis of 1–3
3.6. Bioassay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Flora of China Editorial Committee. Flora of China; Science Press: Beijing, China, 2001; Volume 29, p. 13. [Google Scholar]
- Zhu, Z.; Chen, T.; Pi, H.F.; Ruan, H.L.; Wu, J.Z.; Zhang, P. A new triterpenoid saponin and other saponins from the roots of Holboellia coriacea. Chem. Nat. Compd. 2015, 51, 89–893. [Google Scholar] [CrossRef]
- Zhen, Q.A.; Yang, C.R. Chemotaxonomic study on the family of Lardizabalaceae. Chin. Bull. Bot. 2001, 18, 332–339. [Google Scholar]
- Ying, Q.; Liang, J.Y.; Feng, X. Research in nor-oleanane triterpenoids. Nat. Prod. Res. Dev. 2011, 23, 577–581. [Google Scholar]
- Wang, J.; Xu, Q.L.; Zheng, M.F.; Ren, H.; Lei, T.; Wu, P.; Zhou, Z.Y.; Wei, X.Y.; Tan, J.W. Bioactive 30-noroleanane triterpenes from the pericarps of Akebia trifoliata. Molecules 2014, 19, 4301–4312. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Oominami, H.; Yoshikawa, M. Bioactive saponins and glycosides. XVIII. Nortriterpene and triterpene oligoglycosides from the fresh leaves of Euptelea polyandra Sieb. et ZUCC.(2): Structures of eupteleasaponins VI, VI Acetate, VII, VIII, IX, X, XI, and XII. Chem. Pharm. Bull. 2001, 49, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, A.; Itokawa, H. Triterpenoids of Akebia quinata callus tissue. Phytochemistry 1986, 25, 1625–1628. [Google Scholar] [CrossRef]
- Ikuta, A.; Itokawa, H. A triterpene from Akebia quinata callus tissue. Phytochemistry 1988, 27, 3809–3810. [Google Scholar] [CrossRef]
- Ikuta, A.; Itokawa, H. Triterpenoids of Paeonia japonica callus tissue. Phytochemistry 1988, 27, 2813–2815. [Google Scholar] [CrossRef]
- Ikuta, A.; Itokawa, H. 30-Noroleanane saponins from callus tissues of Akebia quinata. Phytochemistry 1989, 28, 2663–2665. [Google Scholar] [CrossRef]
- Gülcemal, D.; Masullo, M.; Senol, S.G.; Alankuş-Çalişkan, Ö.; Piacente, S. Saponins from Cephalaria aristata C. Koch. Rec. Nat. Prod. 2014, 8, 228–233. [Google Scholar]
- Flamini, G.; Braca, A.; Cioni, P.L.; Morelli, I.; Tomè, F. Three new flavonoids and other constituents from Lonicera implexa. J. Nat. Prod. 1997, 60, 449–452. [Google Scholar] [CrossRef]
- Fu, H.; Koike, K.; Zheng, Q.; Mitsunaga, K.; Jia, Z.; Nikaido, T.; Lin, W.; Guo, D.; Zhang, L. Fargosides A-E, triterpenoid saponins from Holboellia fargesii. Chem. Pharm. Bull. 2001, 49, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.B.; Huang, R.; Zhou, Z.S.; Li, Y.Z. New sesquiterpenoids from Ambrosia artemisiifolia L. Molecules 2015, 20, 4450–4459. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.H.; Tan, N.H.; Zeng, G.Z.; Zhang, Y.M. Two new cinnamyl isovalerate derivatives from Sabina gaussenii. Molecules 2016, 21, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 6–8 are available from the authors.
| No. | δc | δH (mult., J in Hz) | No. | δc | δH (mult., J in Hz) |
|---|---|---|---|---|---|
| Ag-1 | 38.5 CH2 | 0.98 (m); 1.52 (m) | 23 | 28.2 CH3 | 1.22 (s) |
| 2 | 27.5 CH2 | 1.79–1.82 (m) | 24 | 16.0 CH3 | 1.02 (s) |
| 3 | 77.6 CH | 3.44 (dd, 9.9, 5.6) | 25 | 15.1 CH3 | 0.94 (s) |
| 4 | 38.8 qC | 26 | 17.0 CH3 | 1.12 (s) | |
| 5 | 55.3 CH | 0.84 (d, 11.2) | 27 | 25.5 CH3 | 1.20 (s) |
| 6 | 18.3 CH2 | 1.36 (m); 1.51 (m) | 28 | 175.3 qC | |
| 7 | 32.6 CH2 | 1.35 (m); 1.47 (m) | 29 | 106.7 CH2 | 4.65 (s); 4.71 (s) |
| 8 | 39.4 qC | ||||
| 9 | 47.6 CH | 1.63 (dd, 9.7, 7.4) | Glc-1′ | 95.2 CH | 6.21 (d, 8.0) |
| 10 | 36.8 qC | 2′ | 73.3 CH | 4.12 (m) | |
| 11 | 23.2 CH2 | 1.90 (m) | 3′ | 78.1 CH | 4.19 (m) |
| 12 | 122.9 CH | 5.45 (br s) | 4′ | 70.4 CH | 4.33 (m) |
| 13 | 143.0 qC | 5′ | 77.3 CH | 4.07 (m) | |
| 14 | 41.5 qC | 6′ | 69.0 CH2 | 4.35 (dd, 12.1, 4.9) | |
| 15 | 27.7 CH2 | 1.18 (m); 2.34 (m) | 4.69 (br d, 12.1) | ||
| 16 | 23.0 CH2 | 2.06 (m); 2.14 (m) | Glc-1′′ | 104.8 CH | 5.00 (d, 7.7) |
| 17 | 46.8 qC | 2′′ | 74.6 CH | 4.00 (t, 7.7) | |
| 18 | 47.0 CH | 3.13 (dd, 4.7, 13.4) | 3′′ | 77.8 CH | 4.21 (m) |
| 19 | 41.2 CH2 | 2.20 (m); 2.59 (t, 13.4) | 4′′ | 71.0 CH | 4.19 (m) |
| 20 | 147.9 qC | 5′′ | 77.9 CH | 3.88 (m) | |
| 21 | 29.5 CH2 | 2.09 (m); 2.20 (m) | 6′′ | 62.1 CH2 | 4.48 (br d, 10.5) |
| 22 | 37.1 CH2 | 1.74 (m); 2.03 (m) | 4.32 (m) |
| No. | δc | δH (mult., J in Hz) | No. | δc | δH (mult., J in Hz) |
|---|---|---|---|---|---|
| Ag-1 | 38.4 CH2 | 0.96 (m); 1.51 (m) | 27 | 25.5 CH3 | 1.17 (s) |
| 2 | 27.5 CH2 | 1.79 (m); 1.82 (m) | 28 | 175.2 qC | |
| 3 | 77.6 CH | 3.42 (dd, 11.0, 4.9) | 29 | 106.8 CH2 | 4.63 (s); 4.68 (s) |
| 4 | 38.8 qC | Glc-1′ | 95.2 CH | 6.21 (d, 8.2) | |
| 5 | 55.3 CH | 0.82 (d, 11.9) | 2′ | 73.3 CH | 4.09 (m) |
| 6 | 18.2 CH2 | 1.36 (m); 1.51 (m) | 3′ | 78.1 CH | 4.18 (t, 9.0) |
| 7 | 32.6 CH2 | 1.33 (m); 1.45 (m) | 4′ | 70.4 CH | 4.31 (t, 9.0 ) |
| 8 | 39.4 qC | 5′ | 77.3 CH | 4.05 (m) | |
| 9 | 47.5 CH | 1.60 (dd, 10.9, 6.9) | 6′ | 69.0 CH2 | 4.36 (dd, 11.4, 4.8) |
| 10 | 36.8 qC | 4.73 (br d, 11.4) | |||
| 11 | 23.2 CH2 | 1.86 (m); 1.89 (m) | Glc-1′′ | 104.7 CH | 5.02 (d, 7.6) |
| 12 | 122.9 CH | 5.40 (t, 3.6) | 2′′ | 74.9 CH | 4.02 (m) |
| 13 | 142.9 qC | 3′′ | 78.1 CH | 4.18 (t, 9.0) | |
| 14 | 41.5 qC | 4′′ | 70.8 CH | 4.11 (m) | |
| 15 | 27.7 CH2 | 2.31 (m) | 5′′ | 74.5 CH | 4.01 (m) |
| 16 | 23.0 CH2 | 2.02 (m); 2.12 (m) | 6′′ | 64.0 CH2 | 4.88 (dd, 11.8, 5.5) |
| 17 | 46.8 qC | 5.03 (dd, 11.8, 1.6) | |||
| 18 | 47.0 CH | 3.10 (dd, 13.4, 5.1) | Caff-1′′′ | 126.4 qC | |
| 19 | 41.1 CH2 | 2.15 (m); 2.55 (t, 13.4) | 2′′′ | 115.3 CH | 7.54 (br s) |
| 20 | 147.8 qC | 3′′′ | 147.0 qC | ||
| 21 | 29.5 CH2 | 2.08 (m); 2.17 (m) | 4′′′ | 149.8 qC | |
| 22 | 37.1 CH2 | 1.75 (m); 2.03 (m) | 5′′′ | 116.1 CH | 7.17 (d, 8.2 ) |
| 23 | 28.2 CH3 | 1.20 (s) | 6′′′ | 121.6 CH | 7.09 (dd, 8.2, 1.9) |
| 24 | 16.0 CH3 | 1.01 (s) | 7′′′ | 145.4 CH | 7.91 (d, 15.8) |
| 25 | 15.1 CH3 | 0.91 (s) | 8′′′ | 114.4 CH | 6.61 (d, 15.8) |
| 26 | 17.0 CH3 | 1.09 (s) | 9′′′ | 167.1 qC |
| No. | δc | δH (mult., J in Hz) | No. | δc | δH (mult., J in Hz) |
|---|---|---|---|---|---|
| Ag-1 | 38.9 CH2 | 0.92 (m); 1.45 (m) | 24 | 73.1 CH2 | 4.25 (d, 10.0); 4.36 (d, 10.0) |
| 2 | 28.3 CH2 | 1.86 (m); 1.93 (m) | 25 | 15.6 CH3 | 0.84 (s) |
| 3 | 79.3 CH | 3.50 (dd, 4.1, 11.7) | 26 | 17.0 CH3 | 0.91 (s) |
| 4 | 43.0 qC | 27 | 25.8 CH3 | 1.17 (s) | |
| 5 | 56.5 CH | 0.88 (br d, 11.7) | 28 | 180.1 qC | |
| 6 | 19.2 CH2 | 1.49 (m); 1.63 (m) | 29 | 25.5 CH3 | 1.55 (s) |
| 7 | 33.3 CH2 | 1.19 (m); 1.35 (m) | |||
| 8 | 39.5 qC | Glc-1′ | 105.6 CH | 4.93 (d, 7.7) | |
| 9 | 47.9 CH | 1.56 (m) | 2′ | 74.9 CH | 4.02 (dd, 8.3, 7.7) |
| 10 | 37.0 qC | 3′ | 78.2 CH | 4.21 (dd, 8.4, 8.3) | |
| 11 | 23.7 CH2 | 1.82 (m) | 4′ | 71.2 CH | 4.11 (dd, 9.6, 8.4) |
| 12 | 122.3 CH | 5.49 (br s) | 5′ | 75.3 CH | 4.10 (m) |
| 13 | 144.2 qC | 6′ | 64.3 CH2 | 4.95 (dd, 11.4, 4.7) | |
| 14 | 41.8 qC | 5.03 (br d, 10.1) | |||
| 15 | 28.1 CH2 | 1.11 (m); 2.12 (m) | Caff-1′′ | 126.6 qC | |
| 16 | 23.7 CH2 | 1.99 (m); 2.20 (m) | 2′′ | 115.7 CH | 7.49 (d, 1.6) |
| 17 | 46.6 qC | 3′′ | 147.5 qC | ||
| 18 | 44.2 CH | 3.32 (dd, 14.3, 3.9) | 4′′ | 150.0 qC | |
| 19 | 47.9 CH2 | 1.88 (m); 2.40 (t, 13.5) | 5′′ | 116.6 CH | 7.18 (d, 8.2) |
| 20 | 69.7 qC | 6′′ | 121.9 CH | 7.05 (dd, 8.2, 1.6) | |
| 21 | 36.0 CH2 | 1.08 (m); 2.02 (m) | 7′′ | 145.8 CH | 7.88 (d, 15.8) |
| 22 | 34.9 CH2 | 2.03 (m) | 8′′ | 114.6 CH | 6.54 (d, 15.8) |
| 23 | 23.3 CH3 | 1.51 (s, 3H) | 9′′ | 167.4 qC |
| Compounds | HepG2 | HCT-116 | SGC-7901 |
|---|---|---|---|
| 1 | >100 | >100 | 87.0 |
| 2 | >100 | >100 | >100 |
| 3 | >100 | >100 | >100 |
| 4 | >100 | >100 | >100 |
| 5 | 15.2 | 9.1 | 41.0 |
| Adriamycin | 0.46 | 3.3 | 5.1 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; Li, Y.; Li, G.; He, H.; Li, Z.; Li, Y. New 30-Noroleanane Triterpenoid Saponins from Holboellia coriacea Diels. Molecules 2016, 21, 734. https://doi.org/10.3390/molecules21060734
Ding W, Li Y, Li G, He H, Li Z, Li Y. New 30-Noroleanane Triterpenoid Saponins from Holboellia coriacea Diels. Molecules. 2016; 21(6):734. https://doi.org/10.3390/molecules21060734
Chicago/Turabian StyleDing, Wenbing, Ye Li, Guanhua Li, Hualiang He, Zhiwen Li, and Youzhi Li. 2016. "New 30-Noroleanane Triterpenoid Saponins from Holboellia coriacea Diels" Molecules 21, no. 6: 734. https://doi.org/10.3390/molecules21060734





