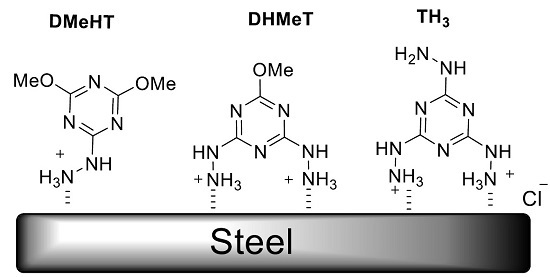
Hydrazino-methoxy-1,3,5-triazine Derivatives’ Excellent Corrosion Organic Inhibitors of Steel in Acidic Chloride Solution
Abstract
:1. Introduction
2. Results and Discusions
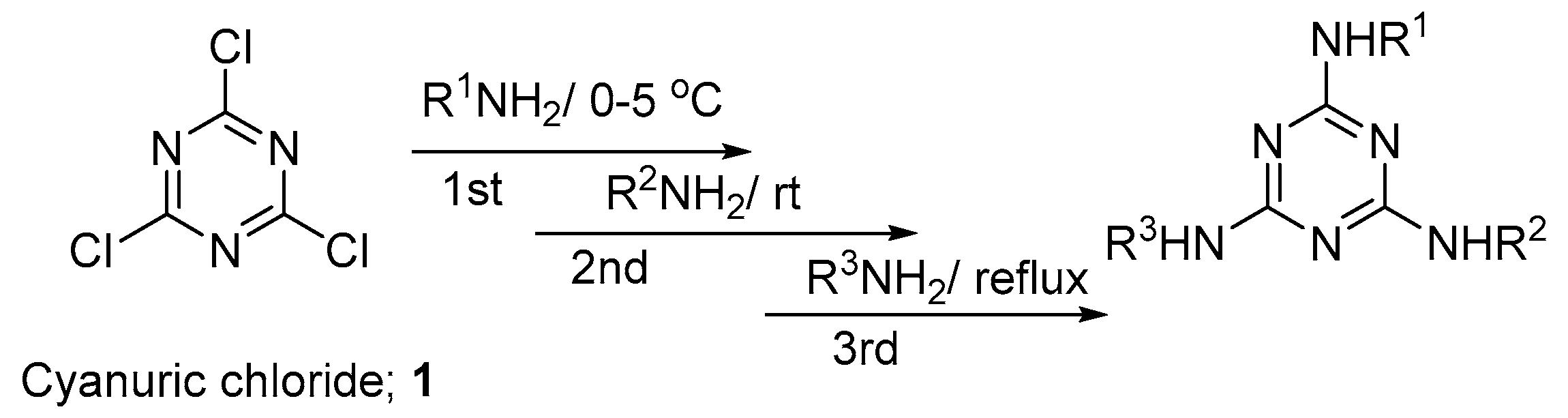
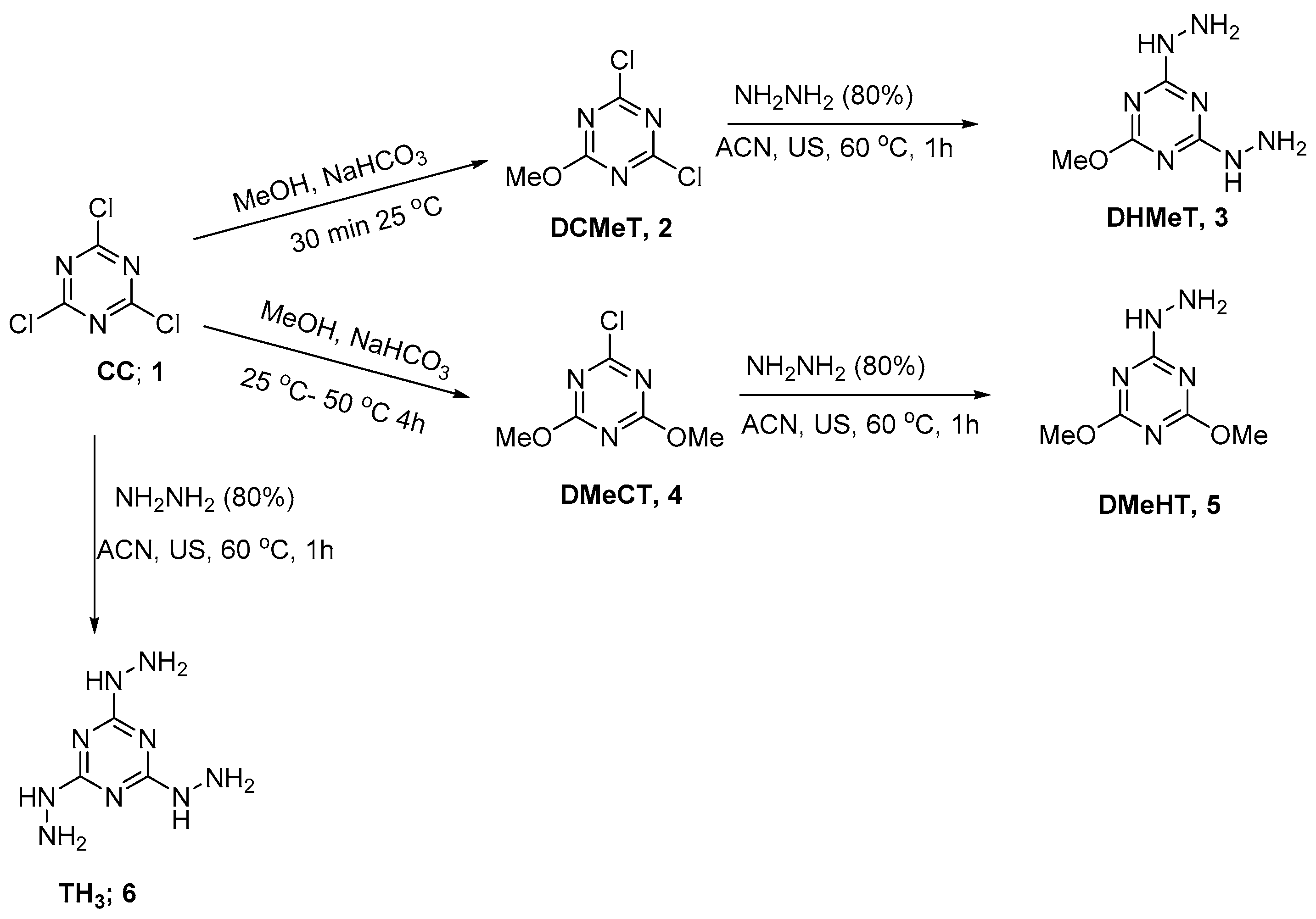
2.1. Synthesis of the Hydrazino-triazine Derivatives
2.2. Potentiodynamic Polarization Measurements
2.3. Electrochemical Impedance Spectroscopy (EIS)
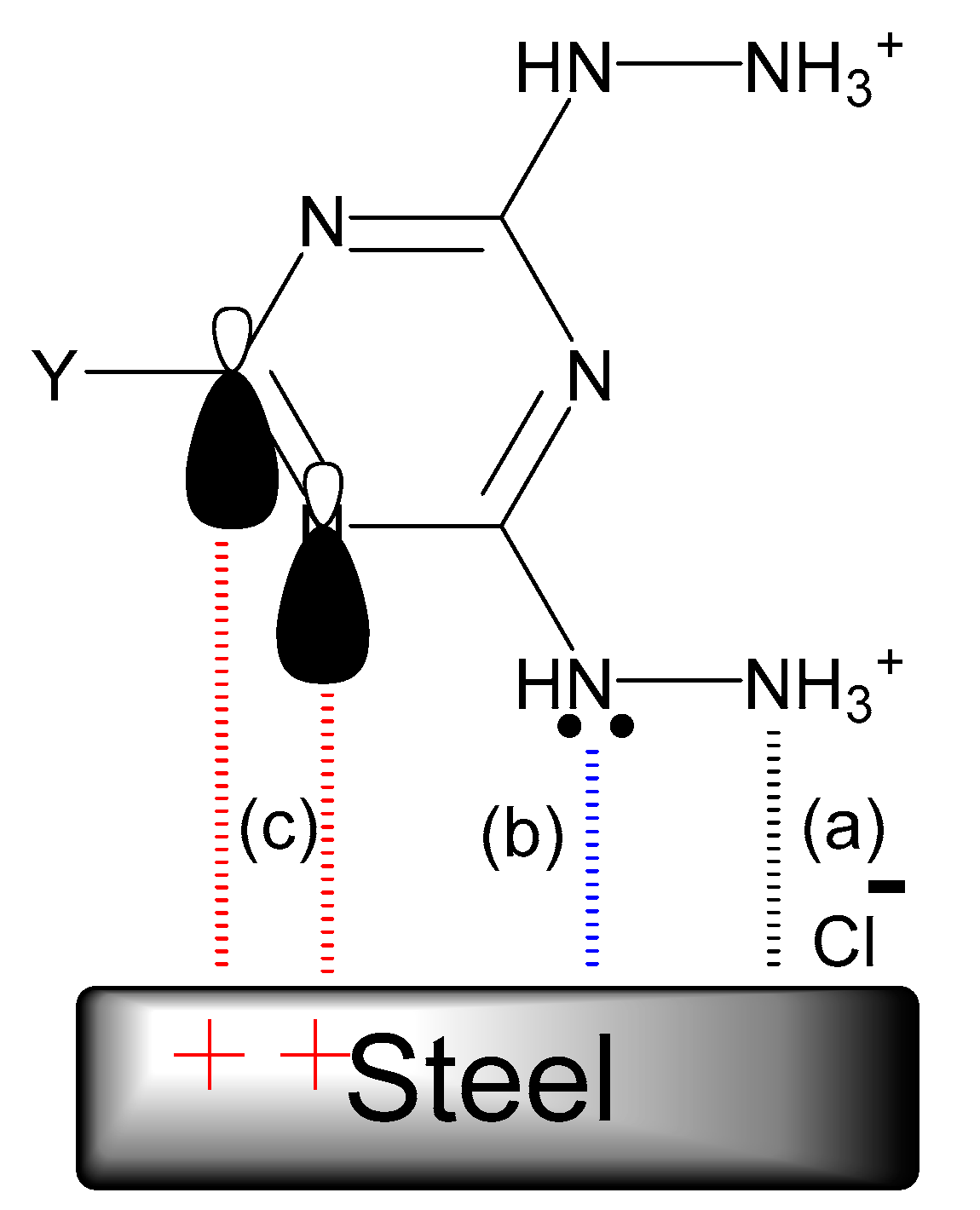
2.4. Adsorption Isotherm
3. Experimental Section
3.1. Materials and Methods
3.2. Electrochemical Measurements
3.3. Synthesis of 2,4-Dichloro-6-methoxy-1,3,5-triazine (DCMeT, 2)
3.4. Synthesis of 2-Chloro-4,6-dimethoxy-1,3,5-triazine (DMeCT, 4)
3.5. General Method for the Synthesis of Hydrazine-1,3,5-triazine Derivatives
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Singh, K.; Quraishi, M.A. Effect of Cefazolin on the corrosion of mild steel in HCl solution. Corros. Sci. 2010, 52, 152–160. [Google Scholar] [CrossRef]
- Shukla, S.K.; Quraishi, M.A.; Prakash, R. A self-doped conducting polymer “polyanthranilic acid”: An efficient corrosion inhibitor for mild steel in acidic solution. Corros. Sci. 2008, 50, 2867–2872. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Shukla, S.K. Poly(aniline-formaldehyde): A new and effective corrosion inhibitor for mild steel in hydrochloric acid. Mater. Chem. Phys. 2009, 113, 685–689. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Kadhum, H.; Alobaidy, A.H.M.; Mohamad, A.B.; Hoon, P.S. Novel Corrosion Inhibitor for Mild Steel in HCl. Materials 2014, 7, 662–672. [Google Scholar] [CrossRef]
- Tourabi, M.; Nohair, K.; Traisnel, M.; Jama, C.; Bentiss, F. Electrochemical and XPS studies of the corrosion inhibition of carbon steel in hydrochloric acid pickling solutions by 3,5-bis(2-thiehylmethyl)-4-amino-1,2,4-triazole. Corros. Sci. 2013, 75, 123–133. [Google Scholar] [CrossRef]
- Ouici, H.B.; Benali, O.; Harek, Y.; Larabi, L.; Hammouti, B.; Guendouzi, A. Inhibition of mild steel corrosion in 5% HCl solution by 5-(2-hydroxyphenyl)-1,2,4-triazole-3-thione. Res. Chem. Intermed. 2013, 39, 2777–2793. [Google Scholar] [CrossRef]
- John, S.; Joseph, A. Electro analytical, surface morphological and theoretical studies on the corrosion inhibition behavior of different 1,2,4-triazole precursors on mild steel in 1 M hydrochloric acid. Mater. Chem. Phys. 2012, 133, 1083–1091. [Google Scholar] [CrossRef]
- Ansari, K.R.; Yadav, D.K.; Ebenso, E.E.; Quraishi, M.A. Novel and effective pyridyl substituted 1,2,4-triazole as corrosion inhibitor for mild steel in acid solution. Int. J. Electrochem. Sci. 2012, 7, 4780–4799. [Google Scholar]
- Mert, B.D.; Mert, M.E.; Kardaş, G.; Yazıcı, B. Experimental and theoretical investigation of 3-amino-1,2,4-triazole-5-thiol as a corrosion inhibitor for carbon steel in HCl medium. Corros. Sci. 2011, 53, 4265–4272. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, S.; Quan, H.; Huang, Z.; Xu, S. Synthesis and corrosion inhibition performance of alkyl triazole derivatives. Res. Chem. Intermed. 2013. [Google Scholar] [CrossRef]
- Deng, Q.; Ding, N.-N.; Wei, X.-L.; Cai, L.; He, X.-P.; Long, Y.-T.; Chen, G.-R.; Chen, K. Identification of diverse 1,2,3-triazole-connected benzyl glycoside-serine/threonine conjugates as potent corrosion inhibitors for mild steel in HCl. Corros. Sci. 2012, 64, 64–73. [Google Scholar] [CrossRef]
- Deng, Q.; Shi, H.-W.; Ding, N.-N.; Chen, B.-Q.; He, X.-P.; Liu, G.; Tang, Y.; Long, Y.-T.; Chen, G.-R. Novel triazolyl bis-amino acid derivatives readily synthesized via click chemistry as potential corrosion inhibitors for mild steel in HCl. Corros. Sci. 2012, 57, 220–227. [Google Scholar] [CrossRef]
- Zhang, H.-L.; He, X.-P.; Deng, Q.; Long, Y.-T.; Chen, G.-R.; Chen, K. Research on the structuresurface adsorptive activity relationships of triazolyl glycolipid derivatives for mild steel in HCl. Carbohydr. Res. 2012, 354, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Malki Alaoui, L.; Hammouti, B.; Bellaouchou, A.; Benbachir, A.; Guenbour, A.; Kertit, S. Corrosion inhibition and adsorption properties of 3-amino-1,2,3-triazole on mild steel in H3PO4. Pharm. Chem. 2011, 3, 353–360. [Google Scholar]
- González-Olvera, R.; Espinoza-Vázquez, A.; Negrón-Silva, G.E.; Palomar-Pardavé, M.E.; Romero-Romo, M.A.; Santillan, R. Multicomponent click synthesis of new 1,2,3-triazole derivatives of pyrimidine nucleobases: Promising acidic corrosion inhibitors for steel. Molecules 2013, 18, 15064–15079. [Google Scholar] [CrossRef] [PubMed]
- González-Olvera, R.; Román-Rodríguez, V.; Negrón-Silva, G.E.; Espinoza-Vázquez, A.; Rodríguez-Gómez, F.J.; Santillan, R. Multicomponent synthesis and evaluation of new 1,2,3-triazole derivatives of dihydropyrimidinones as acidic corrosion inhibitors for steel. Molecules 2016, 21, 250. [Google Scholar] [CrossRef] [PubMed]
- Klenke, B.; Stewart, M.; Barrett, M.P.; Brun, R.; Gilbert, I.H. Synthesis and biological evaluation of s-triazine substituted polyamines as potential new anti-trypanosomal drugs. J. Med. Chem. 2001, 44, 3440–3452. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-P.; Chen, H.-L.; Zheng, Z. Ferrocene-based chiral phosphine-triazines: A new family of highly efficient P,N ligands for asymmetric catalysis. Adv. Synth. Catal. 2005, 347, 541–548. [Google Scholar] [CrossRef]
- Patel, H.S.; Patel, V.C. Polyimides containing s-triazine ring. Eur. Polym. J. 2001, 37, 2263–2271. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Batten, S.R.; Hamit, H.; Hoskins, B.F.; Robson, R.; Wellsian, A. Three-dimensional racemate: Eight interpenetrating, enantiomorphic (10,3)-a nets, four right- and four left-handed. Chem. Commun. 1996, 11, 1313–1314. [Google Scholar] [CrossRef]
- Zerkowski, J.A.; Seto, C.T.; Whitesides, G.M. Solid-state structures of rosette and crinkled tape motifs derived from the cyanuric acid melamine lattice. J. Am. Chem. Soc. 1992, 114, 5473–5475. [Google Scholar] [CrossRef]
- Mooibroek, T.J.; Gamez, P. The s-triazine ring, a remarkable unit to generate supramolecular interactions. Inorg. Chim. Acta 2007, 360, 381–404. [Google Scholar] [CrossRef]
- Liu, F.G.; Du, M.; Zhang, J.; Qiu, M. Electrochemical behavior of Q235 steel in saltwater saturated with carbon dioxide based on new imidazoline derivative inhibitor. Corros. Sci. 2009, 51, 102–109. [Google Scholar] [CrossRef]
- Xuehui, P.; Baorong, H.; Weihua, L.; Faqian, L.; Zhigang, Y. 2,3,5-Triphenyl-2H-tetrazolium Chloride and 2,4,6-Tri(2-pyridyl)-s-triazine on the corrosion of mild steel in HCI. Chin. J. Chem. Eng. 2007, 15, 909–915. [Google Scholar]
- Shukla1, S.K.; Singh, A.K.; Quraishi, M.A. Triazines: Efficient corrosion inhibitors for mild steel in hydrochloric acid solution. Int. J. Electrochem. Sci. 2012, 7, 3371–3389. [Google Scholar]
- Yoo, S.-H.; Kim, Y.-W.; Chung, K.; Kim, N.-K.; Kim, J.-S. Corrosion inhibition properties of triazine derivatives containing carboxylic acid and amine groups in 1.0 M HCl solution. Ind. Eng. Chem. Res. 2013, 52, 10880–10889. [Google Scholar] [CrossRef]
- El-Faham, A.; Dahlous, K.A.; AL Othman, Z.A.; Al-Lohedan, H.A.; El-Mahdy, G.A. Sym-Trisubstituted 1,3,5-Triazine Derivatives as Promising Organic Corrosion Inhibitors for Steel in Acidic Solution. Molecules 2016, 21, 436. [Google Scholar] [CrossRef] [PubMed]
- de Hoog, P.; Gamez, P.; Driessen, W.L.; Reedijk, J. New polydentate and polynucleating N-donor ligands from amines and 2,4,6-trichloro-1,3,5-triazine. Tetrahedron Lett. 2002, 43, 6783–6786. [Google Scholar] [CrossRef]
- Blotny, G. Recent applications of 2,4,6-trichloro-1,3,5-triazine and its derivatives in organic synthesis. Tetrahedron 2006, 62, 9507–9522. [Google Scholar] [CrossRef]
- Kunishima, M.; Hioki, K.; Wada, A.; Kobayashi, H.; Tani, S. Approach to green chemistry of DMT-MM: Recovery and recycle of coproduct to chloromethane-free DMT-MM. Tetrahedron Lett. 2002, 43, 3323–3326. [Google Scholar] [CrossRef]
- Mikhaylichenko, S.N.; Patel, S.M.; Dalili, S.; Chesnyuk, A.A.; Zaplishny, V.N. Synthesis and structure of new 1,3,5-triazine-pyrazole derivatives. Tetrahedron Lett. 2009, 50, 2505–2508. [Google Scholar] [CrossRef]
- Bakharev, V.V.; Gidaspov, A.A.; Parfenov, V.E.; Ul’yankina, I.V.; Zavodskaya, A.V.; Selezneva, E.V.; Suponitskii, K.Y.; Sheremetev, A.B. Synthesis of 4-amino-6-chloro-1,3,5-triazin-2(1H)-ones. Russ. Chem. Bull. Int. Ed. 2012, 61, 99–112. [Google Scholar] [CrossRef]
- Kebede, B.; Retta, N.; Raju, V.J.T.; Chebude, Y. Synthesis and characterization of 2,4,6-tris(hydrazino)-s-triazine and its metal complexes. Trans. Metal Chem. 2006, 31, 19–26. [Google Scholar] [CrossRef]
- Naseer, M.M.; Wang, D.-X.; Zhao, L.; Huang, Z.-T.; Wang, M.-X. Synthesis and functionalization of heteroatom-bridged bicyclocalixaromatics, large molecular triangular prisms with electron-rich and -deficient aromatic interiors. J. Org. Chem. 2011, 76, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- El-Mahdy, G.A.; Atta, A.M.; Al-lohedan, H.A.; Ezzat, A.O. Synthesis of Water Soluble Hyperbranched Poly (amine-ester) as Corrosion Inhibitors for Steel. Int. J. Electrochem. Sci. 2014, 9, 7925–7934. [Google Scholar]
- Tamil Selvi, S.; Raman, V.; Rajendran, N. Corrosion inhibition of mild steel by benzotriazole derivatives in acidic medium. J. Appl. Electrochem. 2003, 33, 1175–1182. [Google Scholar] [CrossRef]
- Ferreira, E.S.; Giacomelli, C.; Giacomelli, F.C.; Spinelli, A. Evaluation of the inhibitor effect of l-ascorbic acid on the corrosion of mild steel. Mater. Chem. Phys. 2004, 83, 129–134. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Singh, A.; Singh, V.K.; Yadav, D.K.; Singh, A.K. Green approach to corrosion inhibition of mild steel in hydrochloric acid and sulphuric acid solutions by the extract of Murraya koenigii leaves. Mater. Chem. Phys. 2010, 122, 114–122. [Google Scholar] [CrossRef]
- Riggs, O.L. Corrosion Inhibitors; Nathan, C.C., Ed.; NACE: Houston, TX, USA, 1973; p. 109. [Google Scholar]
- Qu, Q.; Hao, Z.; Li, L.; Bai, W.; Liu, Y.; Ding, Z. Synthesis and evaluation of tris- hydroxymethyl-(2-hydroxybenzylidenamino)-methane as a corrosion inhibitor for cold rolled steel in hydrochloric acid. Corros. Sci. 2009, 51, 569–574. [Google Scholar] [CrossRef]
- Bentiss, F.; Lebrini, M.; Lagrenée, M. Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2,5-bis (n-thienyl)-1,3,4 thiadiazoles/hydro chloric acid system. Corros. Sci. 2005, 47, 2915–2931. [Google Scholar] [CrossRef]
- Qu, Q.; Jiang, S.; Bai, W.; Li, L. Effect of ethylenediamine tetraacetic acid disodium on the corrosion of cold rolled steel in the presence of benzotriazole in hydrochloric acid. Electrochim. Acta 2007, 52, 6811–6820. [Google Scholar] [CrossRef]
- Ramesh, S.; Rajeswari, S. Corrosion inhibition of mild steel in neutral aqueous solution by new triazole derivatives. Electrochim. Acta 2004, 49, 811–820. [Google Scholar] [CrossRef]
- Chaieb, E.; Bouyanzer, A.; Hammouti, B.; Benkaddour, M. Inhibition of the corrosion of steel in 1 M HCl by eugenol derivatives. Appl. Surf. Sci. 2005, 246, 199–206. [Google Scholar] [CrossRef]
- Dadgarnezhad, A.; Sheikhshoaie, I.; Baghaei, F. Corrosion inhibitory effects of a new synthetic symmetrical Schiff-base on carbon steel in acid media. Anti-Corros. Meth. Mater. 2004, 51, 266–271. [Google Scholar] [CrossRef]
- Espinoza-Vázquez, A.; Negrón-Silva, G.E.; González-Olvera, R.; Angeles-Beltrán, D.; Herrera-Hernández, H.; Romero-Romo, M.; Palomar-Pardavé, M. Mild steel corrosion inhibition in HCl by di-alkyl and di-1,2,3-triazole derivatives of uracil and thymine. Mater. Chem. Phys. 2014, 145, 407–417. [Google Scholar] [CrossRef]
- Khodyrev, Y.P.; Batyeva, E.S.; Badeeva, E.K.; Platova, E.V.; Tiwari, L.; Sinyashin, O.G. The inhibition action of ammonium salts of O,O′-dialkyldithiophosphoric acid on carbon dioxide corrosion of mild steel. Corros. Sci. 2011, 53, 976–983. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Li, Y.; Wang, F.H. Corrosion inhibition and adsorption behavior of methionine on mild steel in sulfuric acid and synergistic effect of iodide ion. J. Colloid Interface Sci. 2007, 310, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Okafor, P.C.; Zheng, Y. Synergistic inhibition behaviour of methylbenzyl quaternary imidazoline derivative and iodide ions on mild steel in H2SO4 solutions. Corros. Sci. 2009, 51, 850–859. [Google Scholar] [CrossRef]
- Obot, I.; Obi-Egbedi, N. Inhibition of aluminium corrosion in hydrochloric acid using nizoral and the effect of iodide ion addition. J. Chem. 2010, 7, 837–843. [Google Scholar] [CrossRef]
- Flory, P.J. Thermodynamics of high polymer solutions. J. Chemi. Phys. 1942, 10, 51–61. [Google Scholar] [CrossRef]
- Okafor, P.C.; Liu, C.B.; Liu, X.; Zheng, Y.G.; Wang, F.; Liu, C.Y. Corrosion inhibition and adsorption behavior of imidazoline salt on N80 carbon steel in CO2-saturated solutions and its synergism with thiourea. J. Solid State Electrochem. 2009, 14, 1367–1376. [Google Scholar] [CrossRef]
- Yadav, M.; Behera, D.; Kumar, S.; Sinha, R.R. Experimental and quantum chemical studies on the corrosion Inhibition performance of benzimidazole Derivatives for Mild Steel in HCl. Ind. Eng. Chem. Res. 2013, 52, 6318–6328. [Google Scholar] [CrossRef]
- Qin, T.T.; Li, J.; Luo, H.Q.; Li, M.; Li, N.B. Corrosion inhibition of copper by 2,5-dimercapto-1,3,4-thiadiazole monolayer in acidic solution. Corros. Sci. 2011, 53, 1072–1078. [Google Scholar] [CrossRef]
- Zarrouk, A.; Hammouti, B.; Dafali, A.; Bentiss, F. Inhibitive properties and adsorption of purpald as a corrosion inhibitor for copper in nitric acid medium. Ind. Eng. Chem. Res. 2013, 52, 2560–2568. [Google Scholar] [CrossRef]
- Küstü, C.; Emregül, K.C.; Atakol, O. Schiff bases of increasing complexity as mild steel corrosion inhibitors in 2 M HCl. Corros. Sci. 2007, 49, 2800–2814. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, W.; Yin, X.; Liu, Y.; Wan, R.; Wang, J. Phenylsubstituted amino thiadiazoles as corrosion inhibitors for copper in 0.5 M H2SO4. Mater. Chem. Phys. 2009, 116, 479–483. [Google Scholar] [CrossRef]
- Noor, E.A.; Al-Moubaraki, A.H. Thermodynamic study of metal corrosion and inhibitor adsorption processes in mild steel/1-methyl-4[4′(-X)-styryl pyridinium iodides/hydrochloric acid systems. Mater. Chem. Phys. 2008, 110, 145–154. [Google Scholar] [CrossRef]
- Obot, I.; Obi-Egbedi, N. Inhibitory effect and adsorption characteristics of 2, 3-diamino naphthalene at aluminum/hydrochloric acid interface: Experimental and theoretical study. Surf. Rev. Lett. 2008, 15, 903–910. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds TH3, DmeHT, and DHMeT are available from the authors.
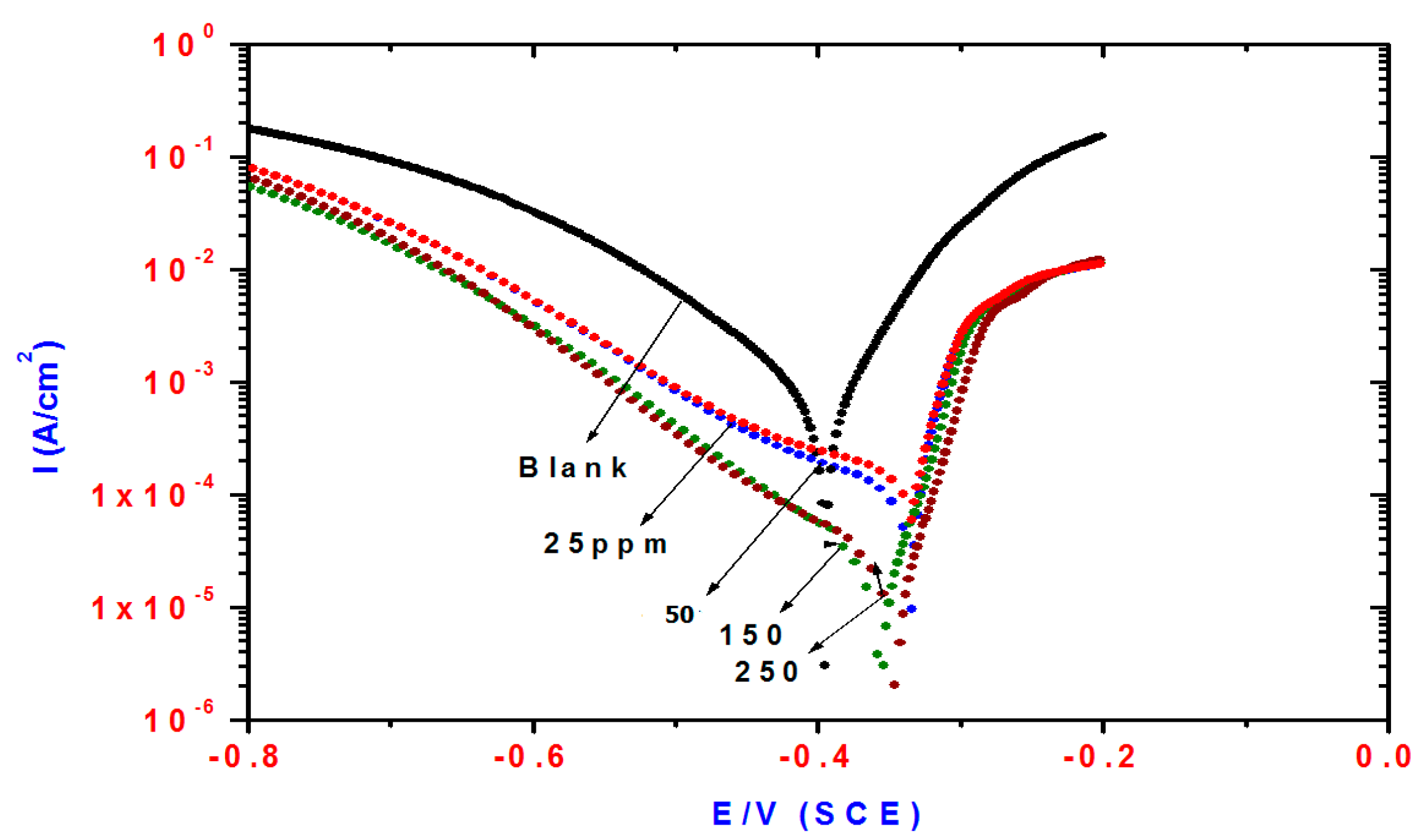
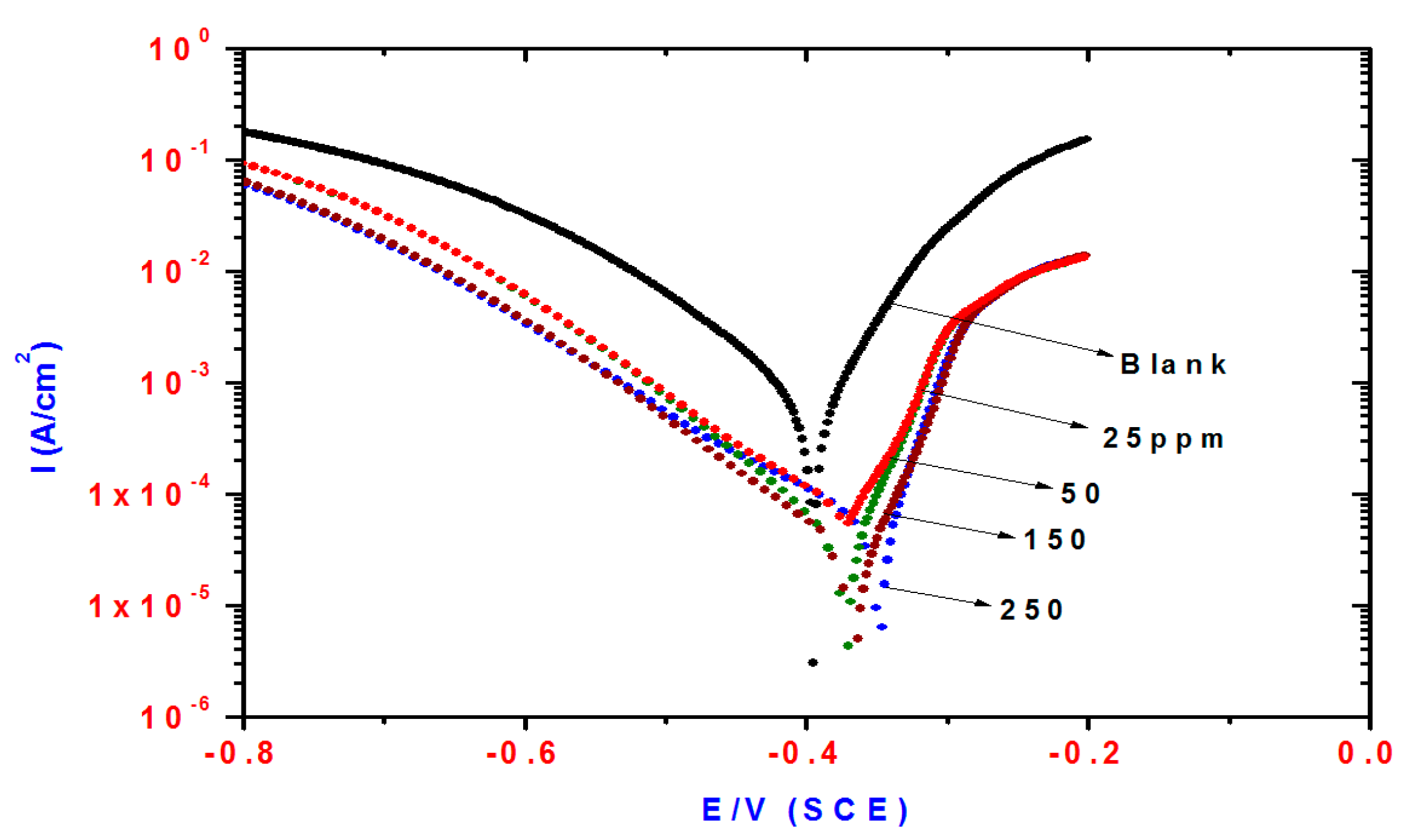
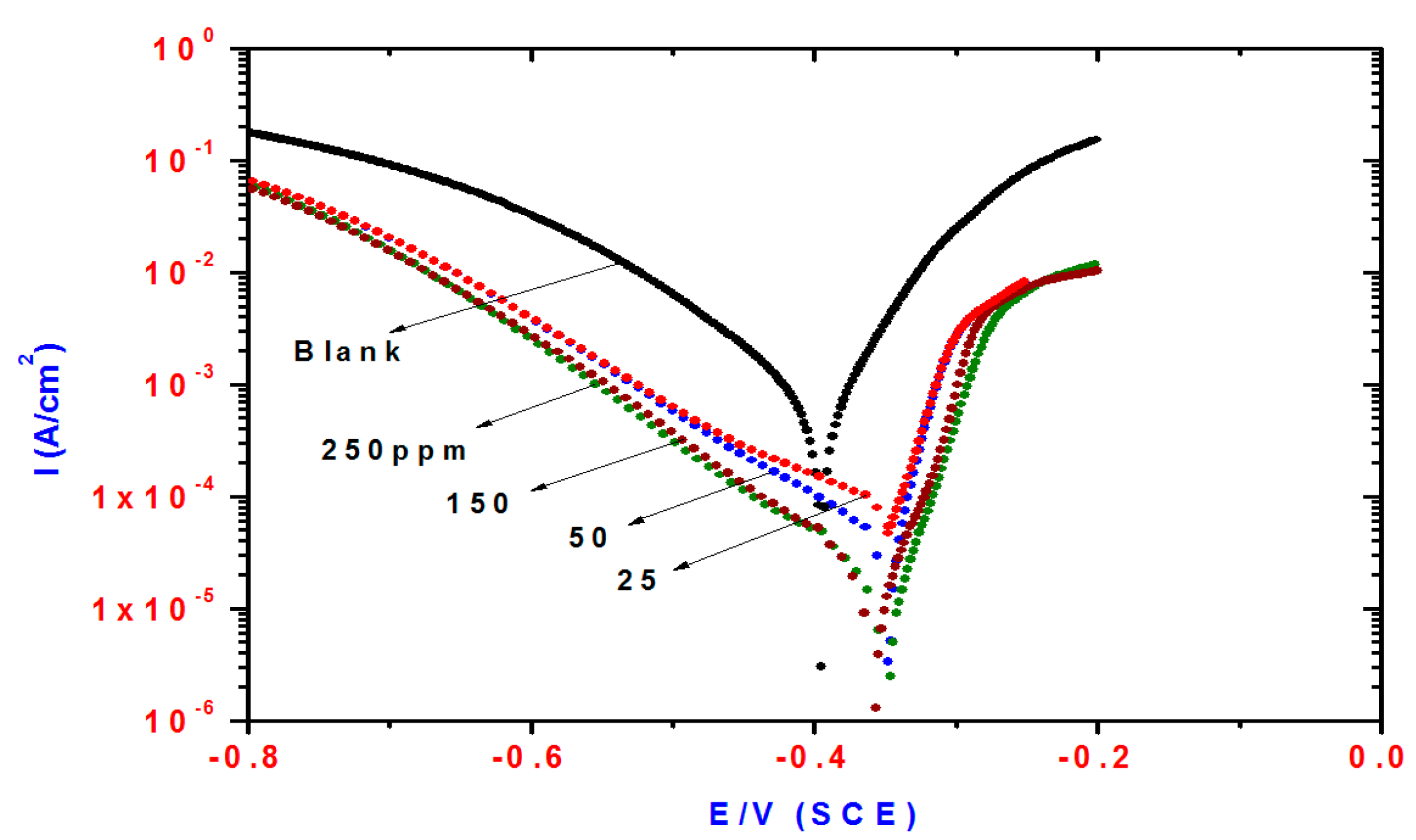
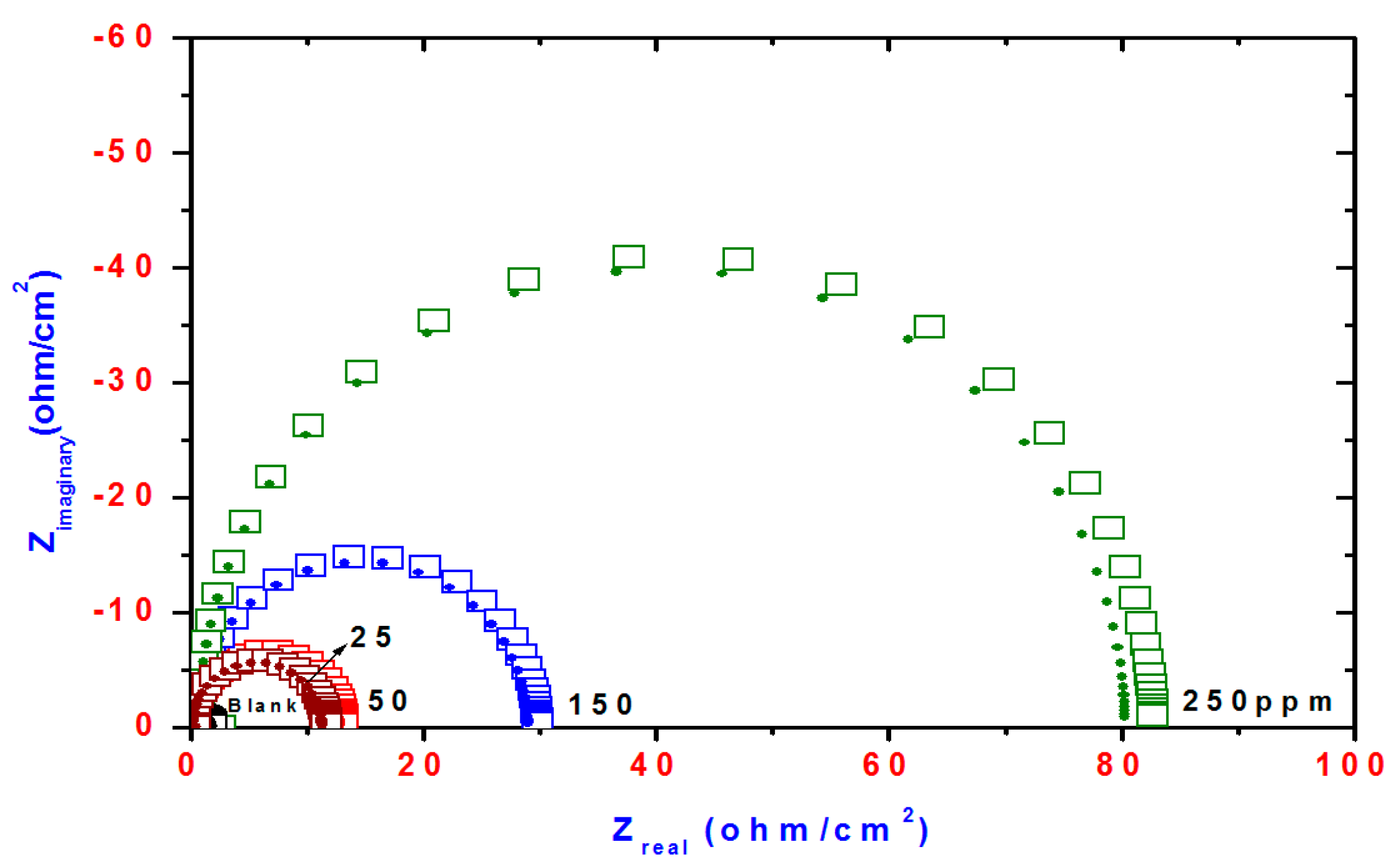
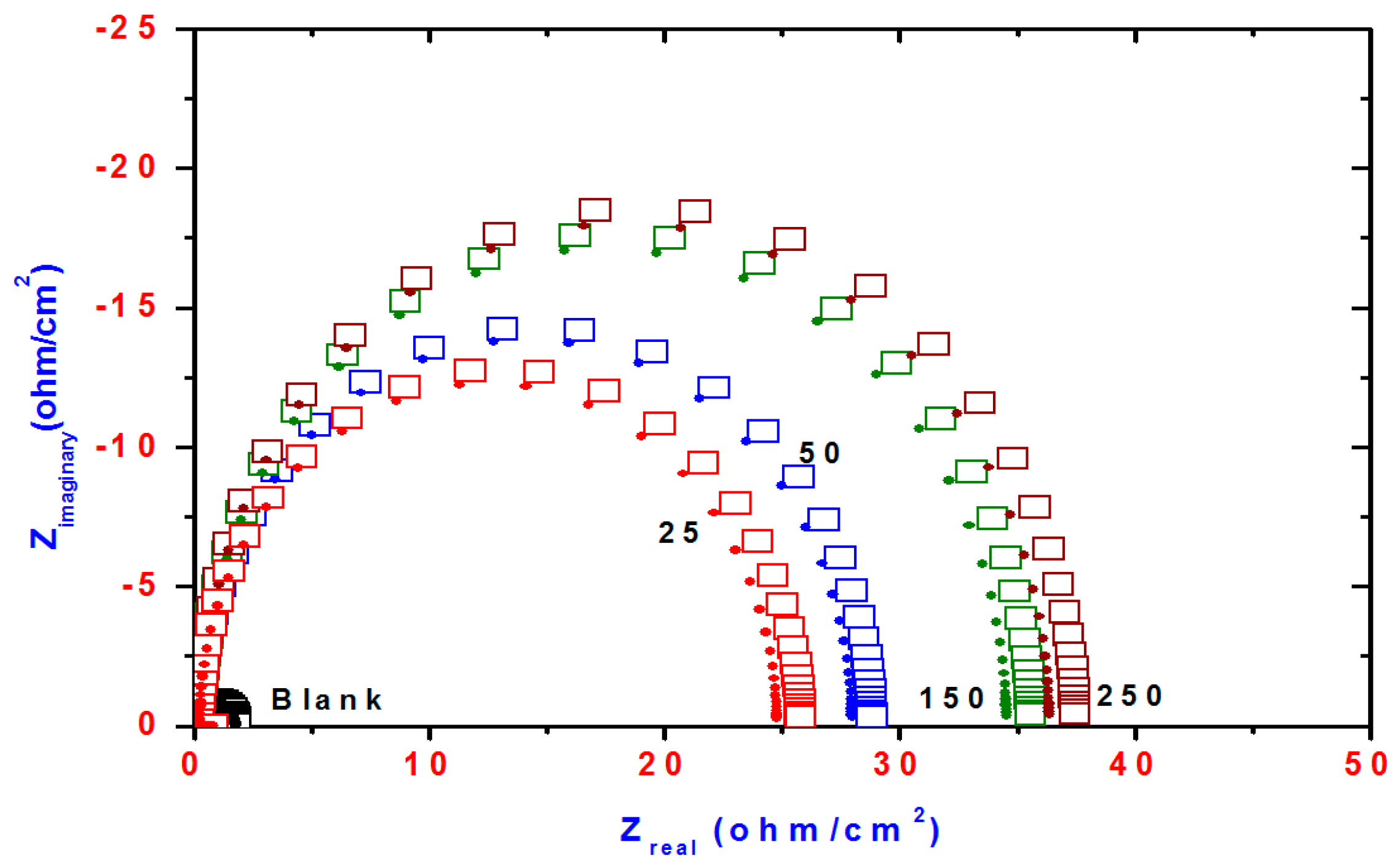
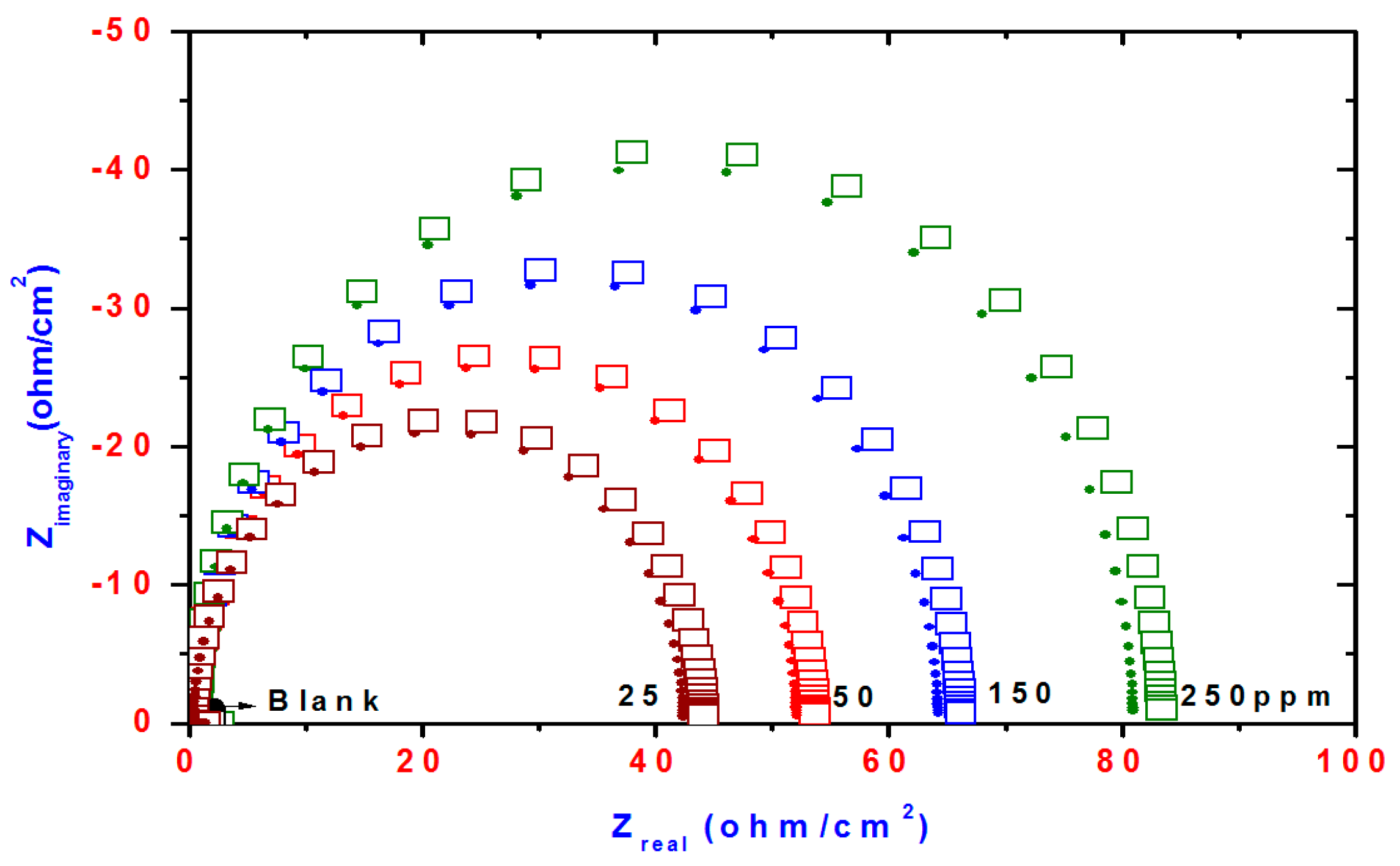
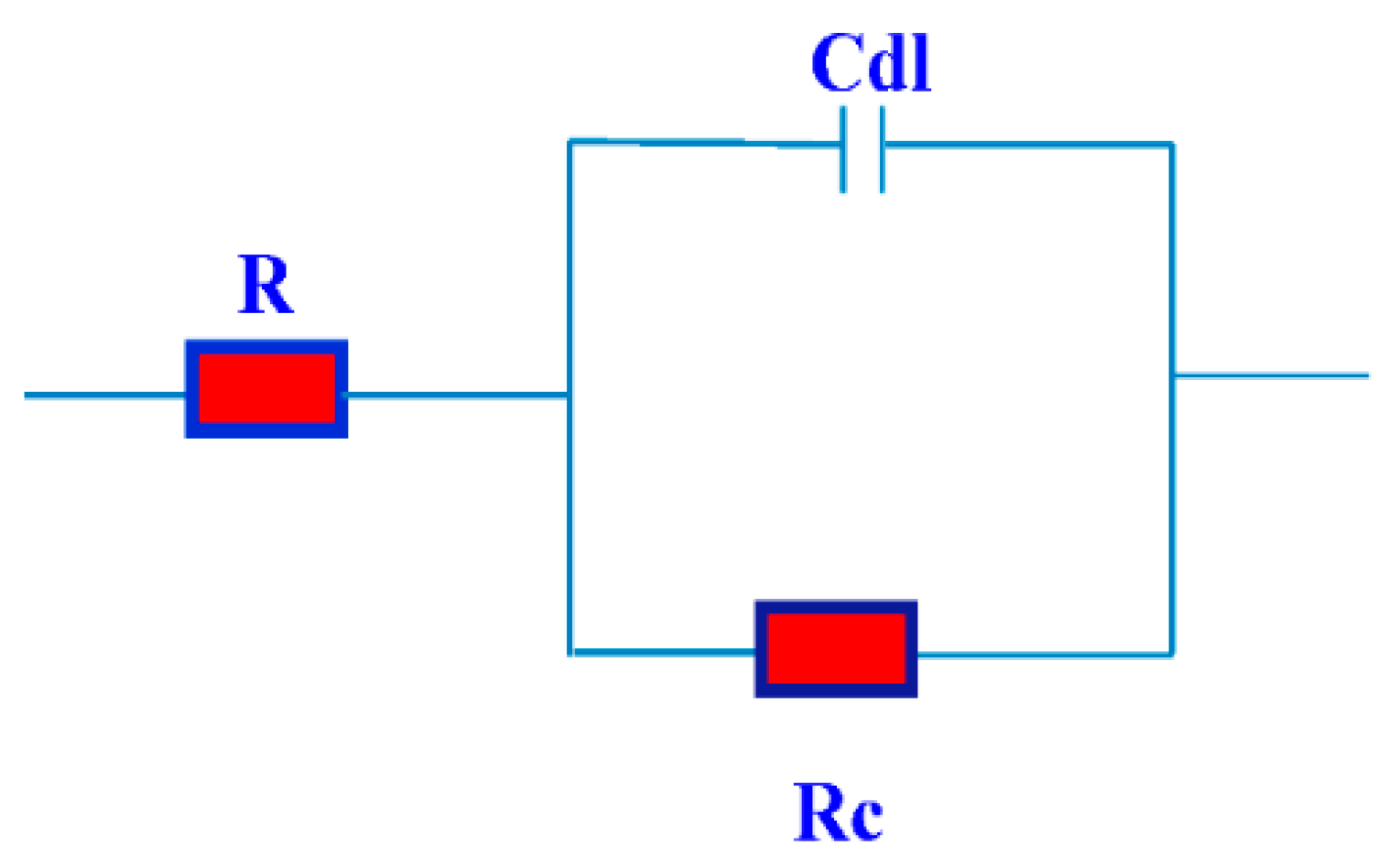
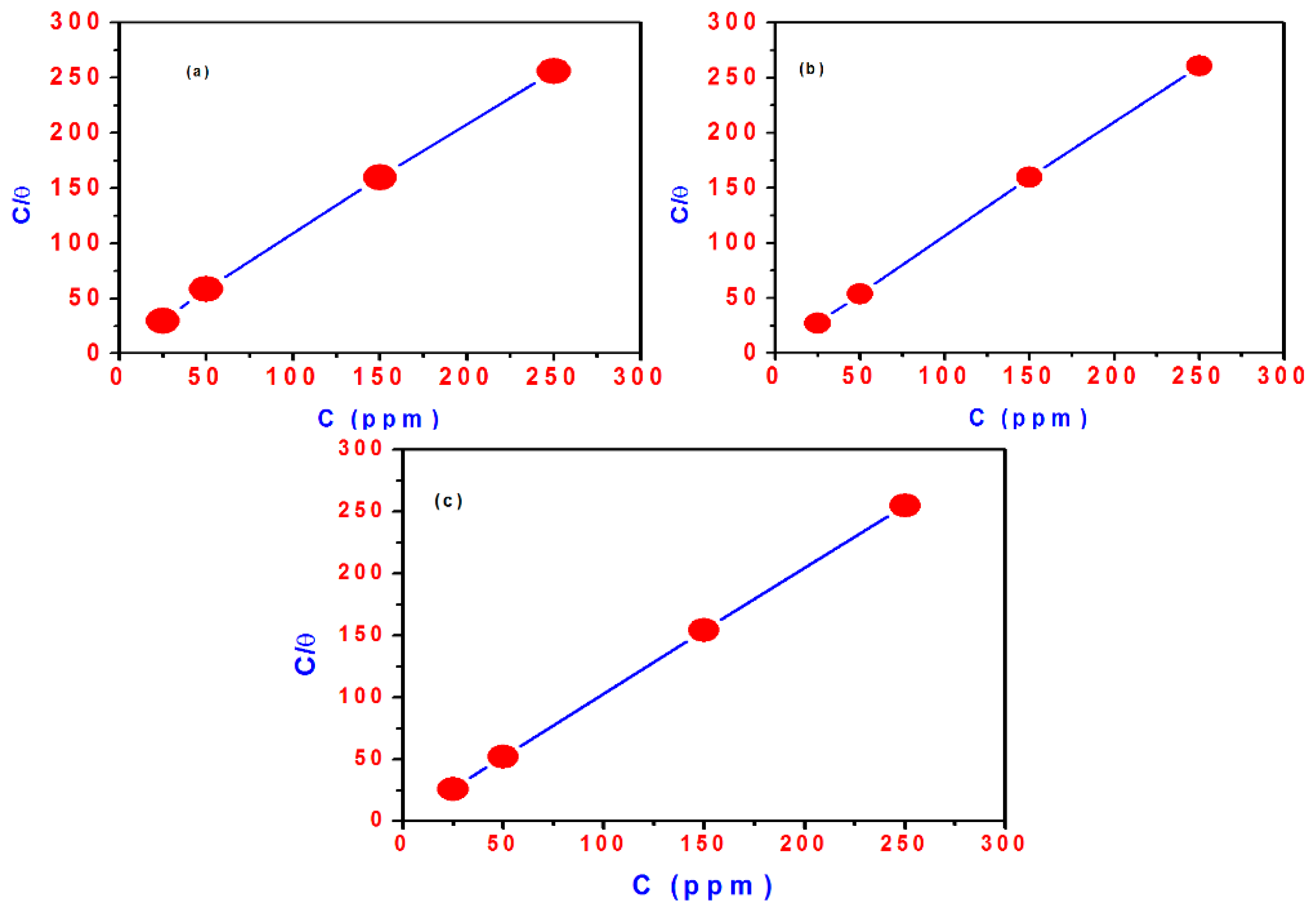
| Compd. | Polarization Method | EIS Method | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Conc. (ppm) | Ba (mV) | Bc (mV) | Ecorr (V) | icorr μA/cm2 | IE% | Rct Ohm | Cdl (μF/cm2) | IE% | |
| Blank | 69 | 120 | −0.3955 | 839 | _____ | 1.80 | 334 | ____ | |
| DHMeT; 3 | 25 | 53 | 119 | −0.3583 | 36 | 95.6 | 43.0 | 110 | 95.1 |
| 50 | 54 | 117 | −0.3534 | 32 | 96.1 | 54.0 | 100 | 96.6 | |
| 150 | 49 | 140 | −0.3489 | 22 | 97.3 | 66.5 | 95.0 | 97.2 | |
| 250 | 50 | 111 | −0.3587 | 19 | 98.0 | 84.0 | 93.0 | 97.8 | |
| DMeHT; 5 | 25 | 56 | 164 | −0.3398 | 59 | 92.9 | 33.0 | 118 | 92.8 |
| 50 | 55 | 160 | −0.3498 | 56 | 93.3 | 29.0 | 108 | 93.7 | |
| 150 | 58 | 111 | −0.3735 | 50 | 94.0 | 35.8 | 102 | 94.0 | |
| 250 | 53 | 118 | −0.3667 | 34 | 95.9 | 37.7 | 98 | 95.2 | |
| TH3; 6 | 25 | 62 | 230 | −0.3370 | 124 | 85.0 | 11.6 | 128 | 84.4 |
| 50 | 61 | 227 | -0.3380 | 120 | 85.6 | 13.2 | 122 | 86.0 | |
| 150 | 43 | 70 | -0.3616 | 51 | 93.9 | 30.0 | 114 | 94.0 | |
| 250 | 47 | 121 | -0.3480 | 19 | 97.7 | 83.0 | 94.0 | 97.8 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Faham, A.; Osman, S.M.; Al-Lohedan, H.A.; El-Mahdy, G.A. Hydrazino-methoxy-1,3,5-triazine Derivatives’ Excellent Corrosion Organic Inhibitors of Steel in Acidic Chloride Solution. Molecules 2016, 21, 714. https://doi.org/10.3390/molecules21060714
El-Faham A, Osman SM, Al-Lohedan HA, El-Mahdy GA. Hydrazino-methoxy-1,3,5-triazine Derivatives’ Excellent Corrosion Organic Inhibitors of Steel in Acidic Chloride Solution. Molecules. 2016; 21(6):714. https://doi.org/10.3390/molecules21060714
Chicago/Turabian StyleEl-Faham, Ayman, Sameh M. Osman, Hamad A. Al-Lohedan, and Gamal A. El-Mahdy. 2016. "Hydrazino-methoxy-1,3,5-triazine Derivatives’ Excellent Corrosion Organic Inhibitors of Steel in Acidic Chloride Solution" Molecules 21, no. 6: 714. https://doi.org/10.3390/molecules21060714