Catalysts with Cerium in a Membrane Reactor for the Removal of Formaldehyde Pollutant from Water Effluents
Abstract
:1. Introduction
2. Results and Discussion
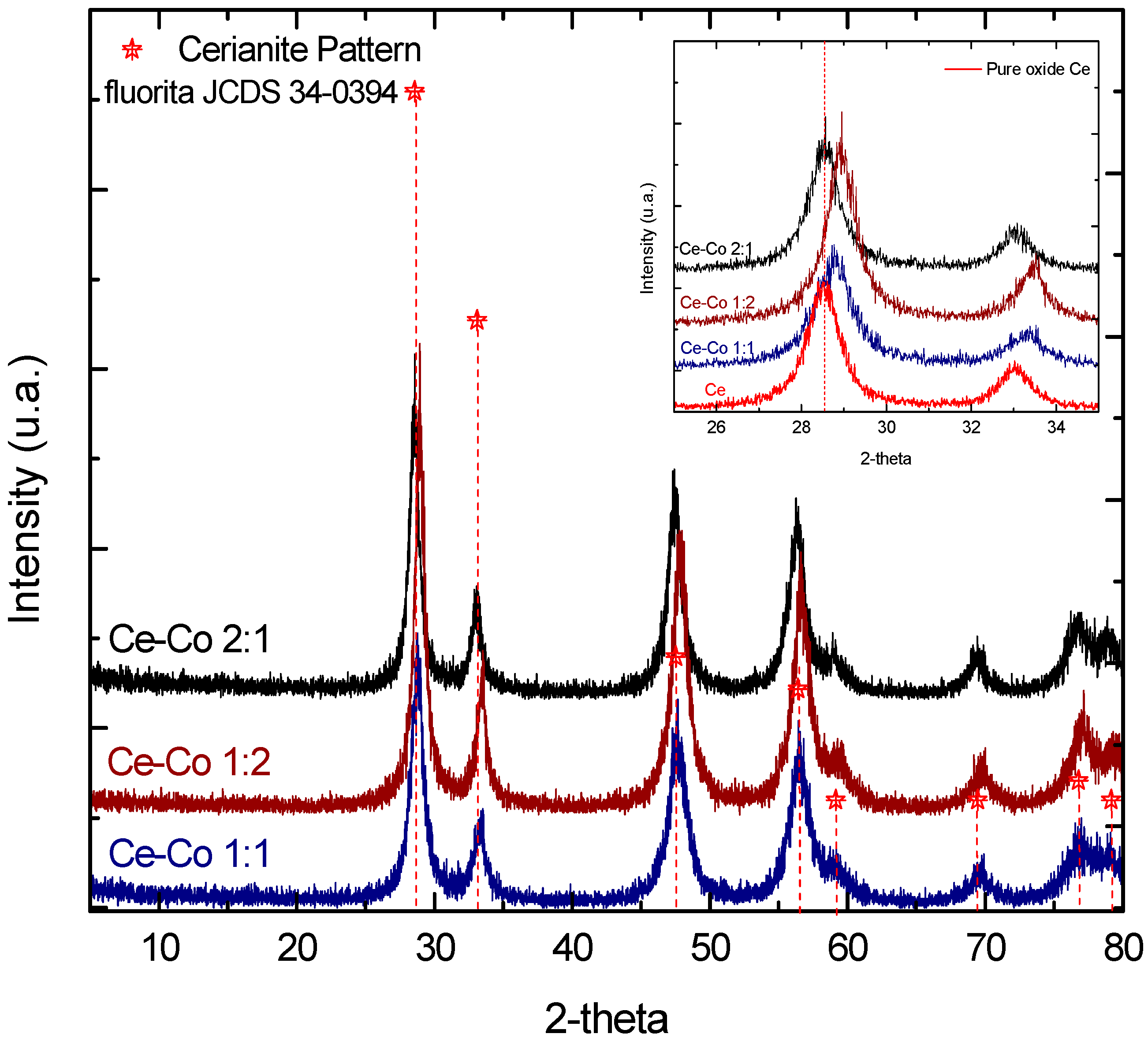
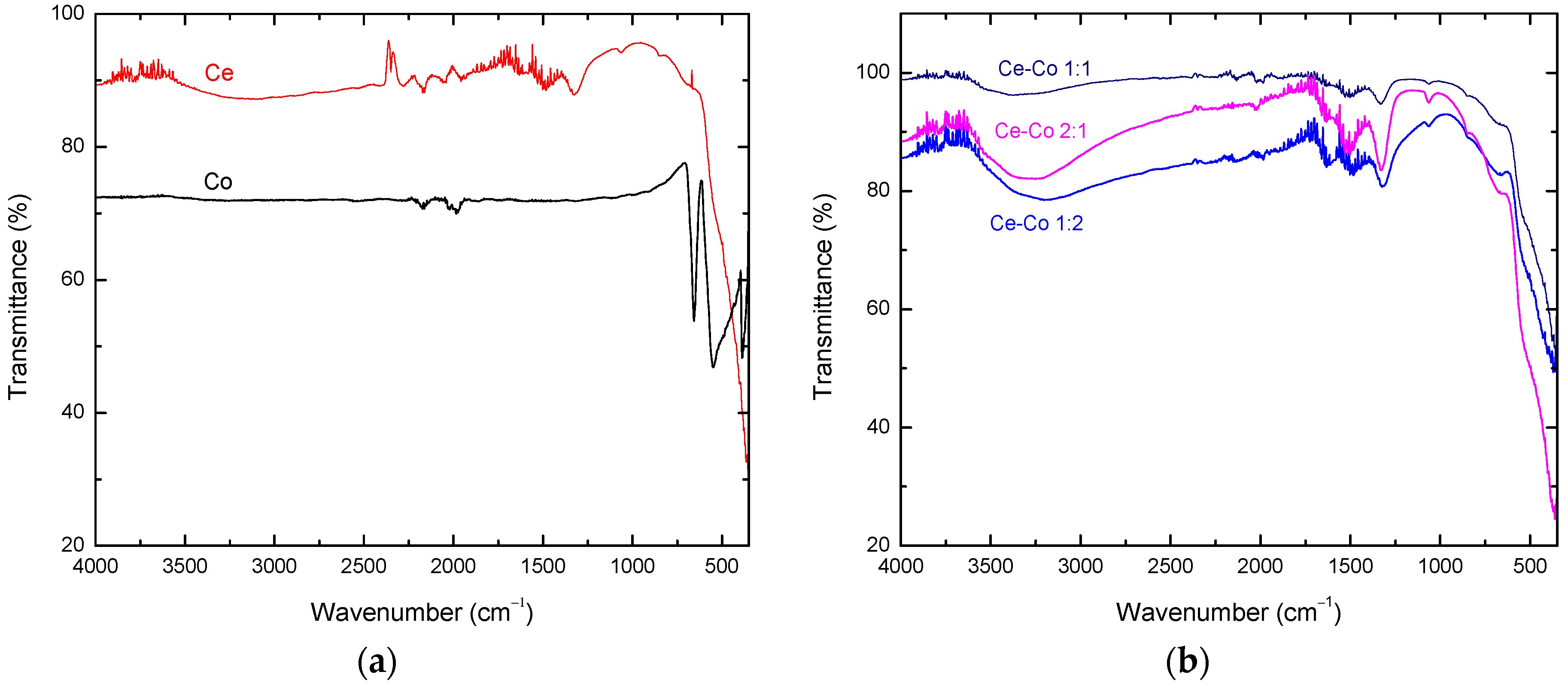
2.1. Bulk Catalyst Characterization
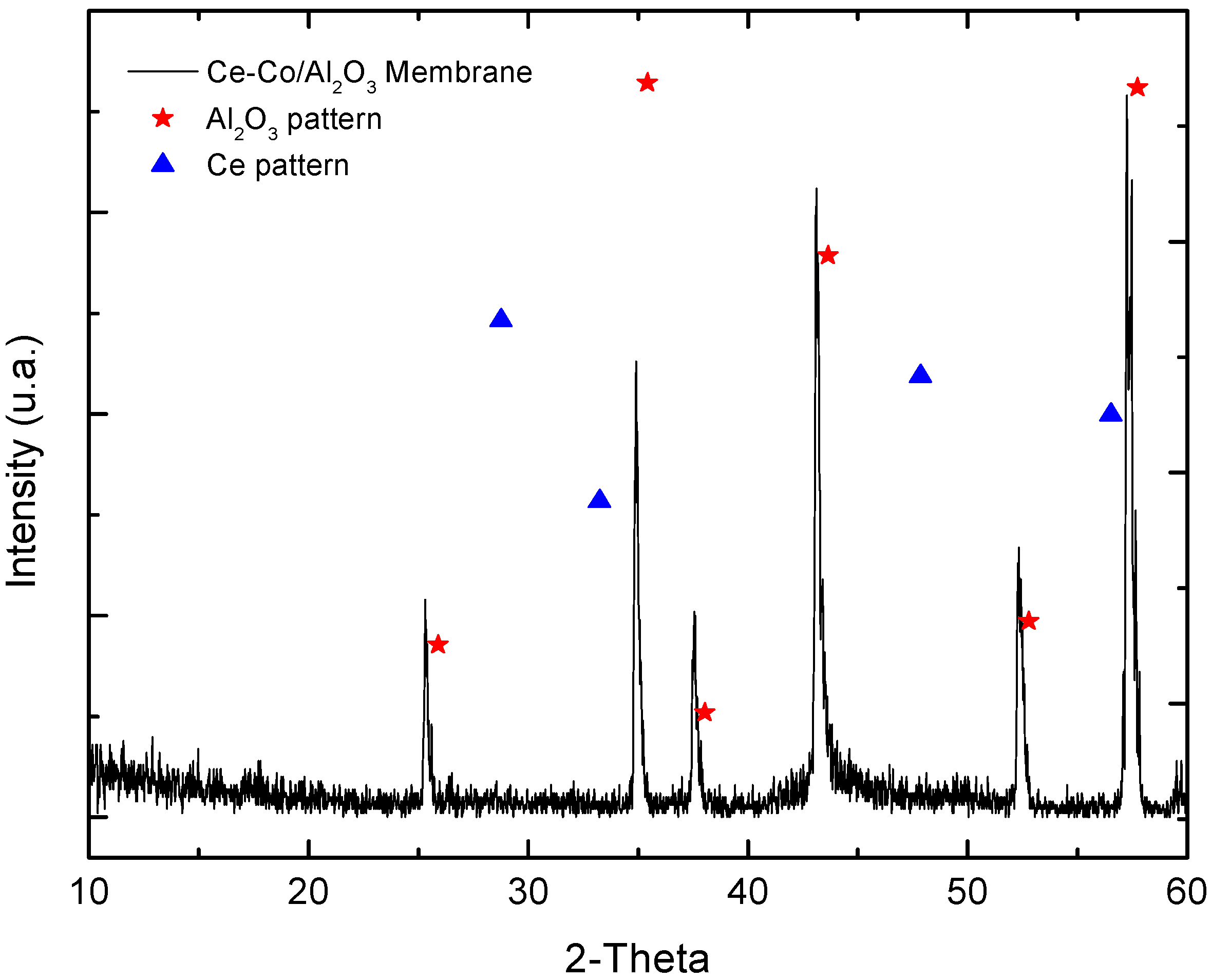
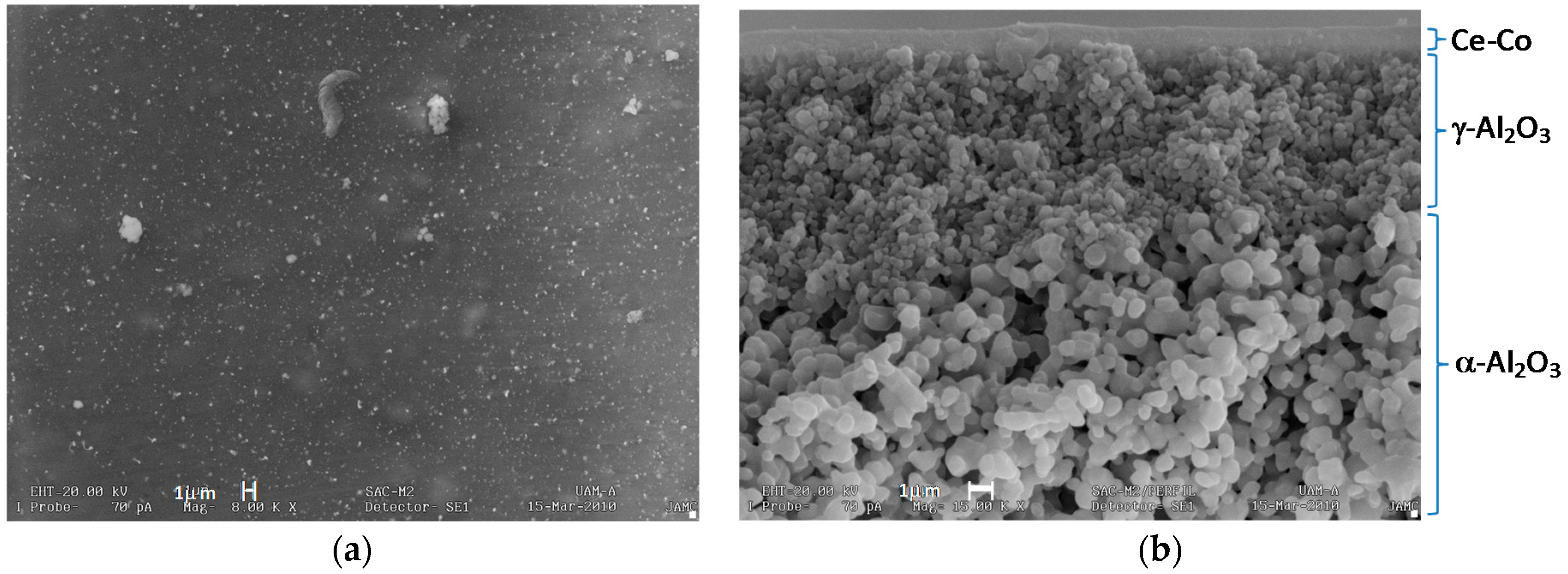
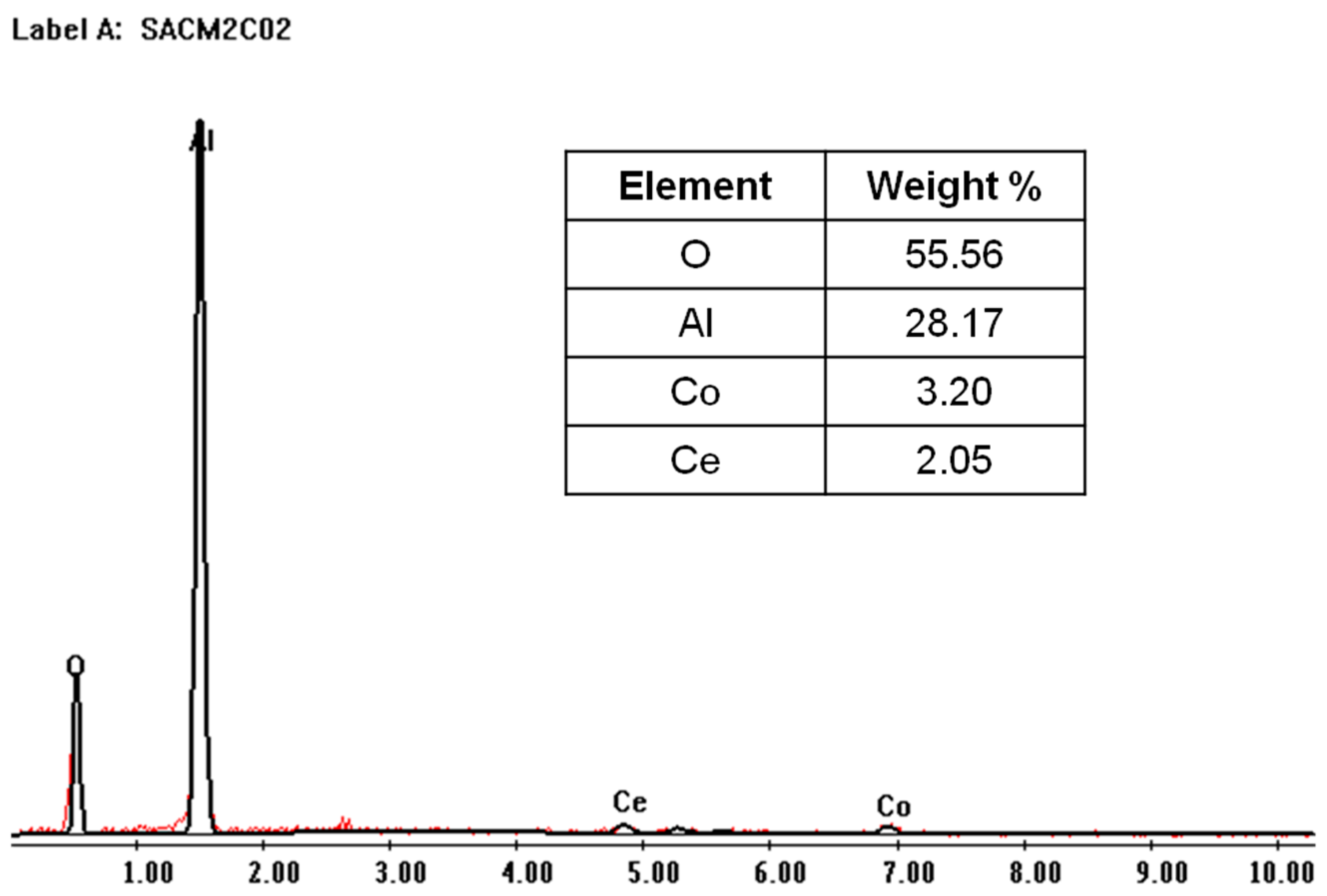
2.2. Catalytic Membrane Characterization
2.3. Catalytic Wet Oxidation Reaction
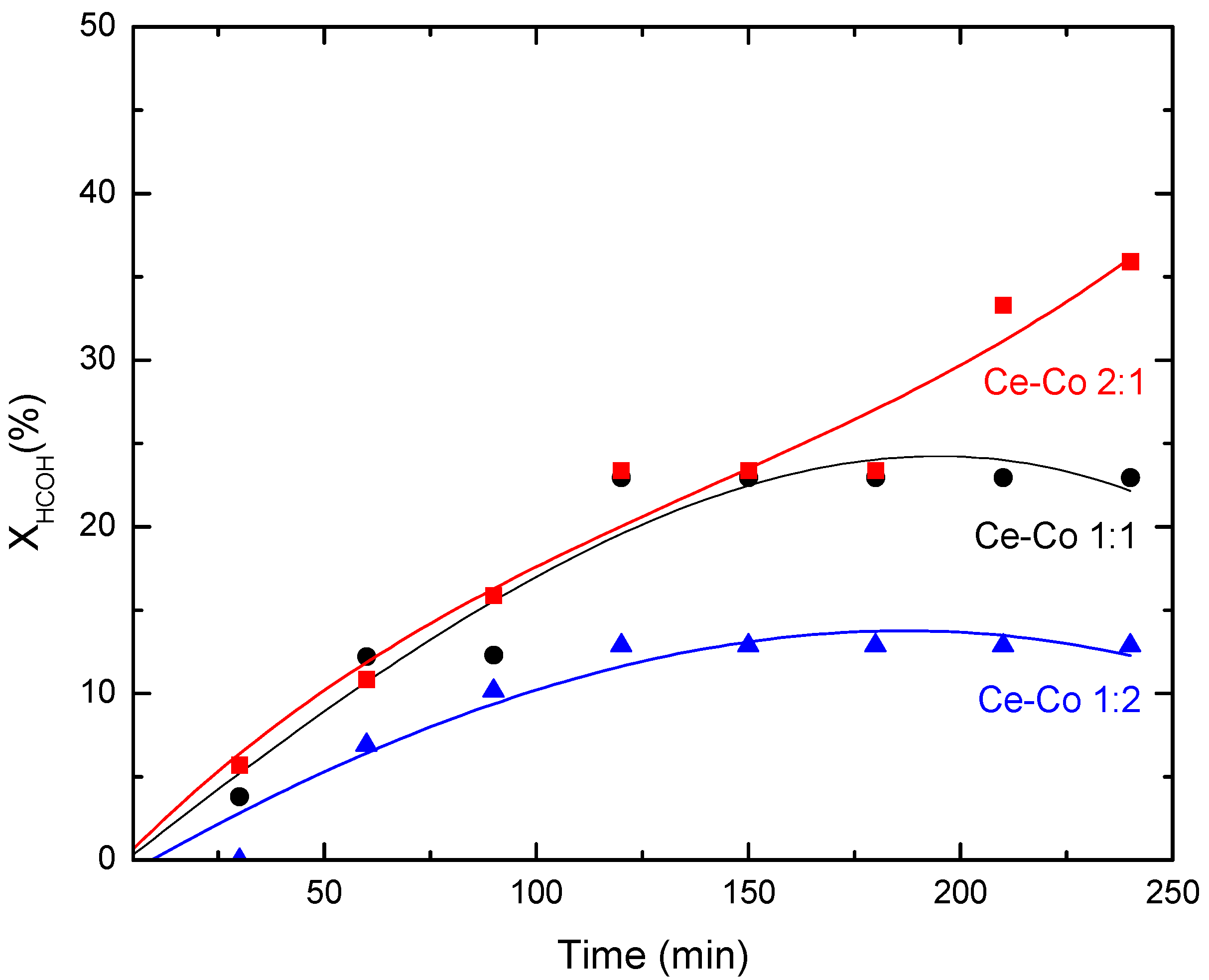
2.3.1. Commercial Autoclave Slurry Batch-Type Reactor
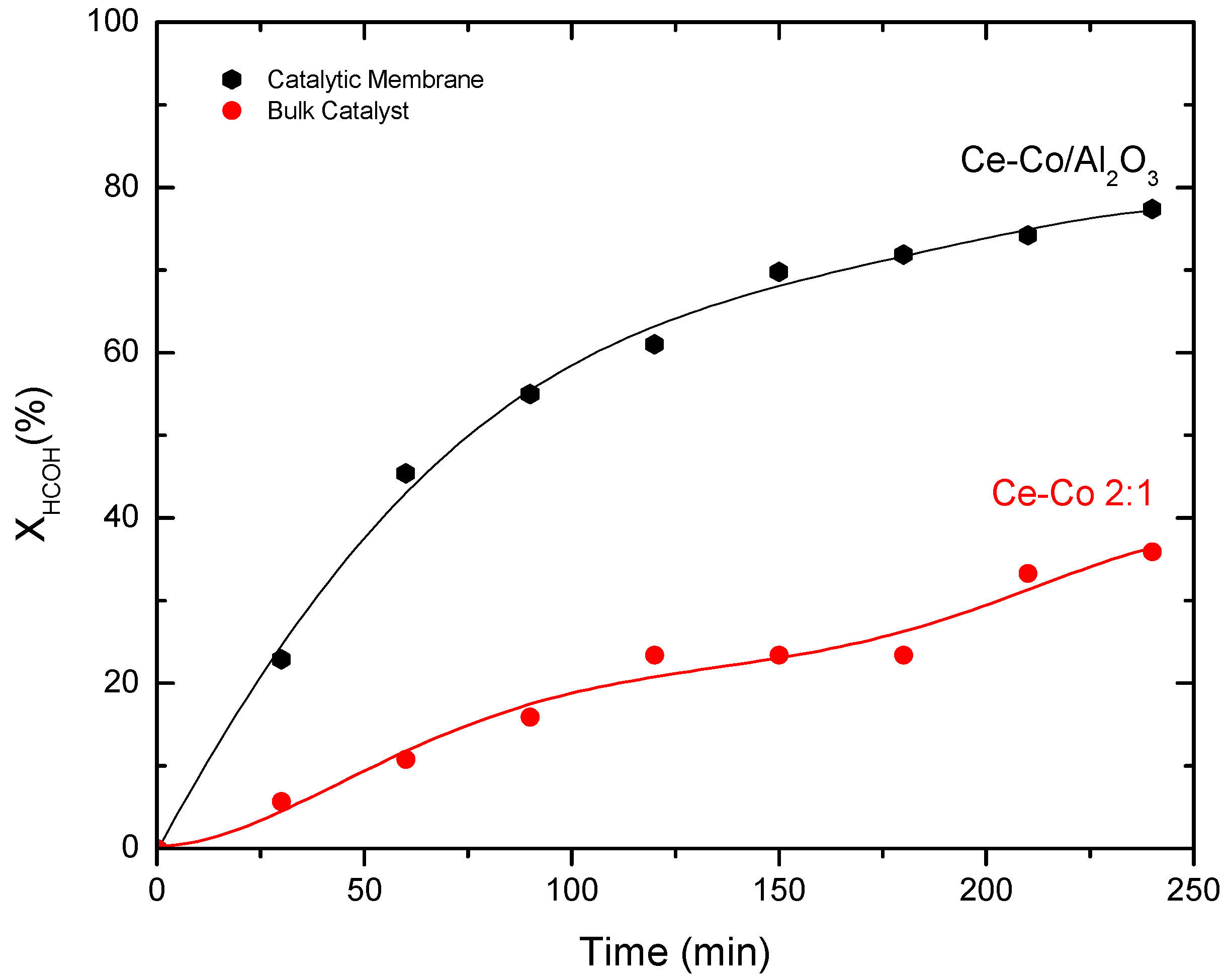
2.3.2. Catalytic Membrane Reactor, Contactor type
3. Discussion
4. Materials and Methods
5. Conclusions
5.1. Catalysts
5.2. Formaldehyde CWO
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CWO | Catalytic Wet Oxidation |
| CWAO | Catalytic Wet Air Oxidation |
| WAO | Wet Air Oxidation |
| CMR | Catalytic Membrane Reactors |
| OSC | Oxygen Storage/Transport Capacity |
| XRD | X-ray Diffraction |
| SEM/EDS | Scanning Electron Microscopy/Energy Dispersive Spectroscopy |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| GC | Gas Chromatography |
| FID | Flame Ionization Detector |
References
- Levec, J.; Pintar, A. Catalytic wet-air oxidation processes: A review. Catal. Today 2007, 124, 172–184. [Google Scholar] [CrossRef]
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the removal of phenol from fluid streams: A short review of recent developments. J. Hazard. Mater. 2008, 160, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, F.J. Waste Disposal. U.S. Patent: 2,665,249, 5 January 1954. [Google Scholar]
- Bhargava, S.K.; Tardio, J.; Prasad, J.; Folger, K.; Akolekar, D.B.; Grocott, S.C. Wet Oxidation and Catalytic Wet Oxidation. Ind. Eng. Chem. Res. 2006, 45, 1221–1258. [Google Scholar] [CrossRef]
- Silva, A.M.T.; Castelo-Branco, I.M.; Quinta-Ferreira, R.M.; Levec, J. Catalytic studies in wet oxidation of effluents from formaldehyde industry. Chem. Eng. Sci. 2003, 58, 963–970. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, W.; Wang, J.; Chen, Z. Catalytic wet air oxidation of phenol over CeO2-TiO2 catalyst in the batch reactor and the packed-bed reactor. J. Hazard. Mater. 2008, 153, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Pintar, A.; Batista, J.; Tišler, T. Catalytic wet-air oxidation of aqueous solutions of formic acid, acetic acid and phenol in a continuous-flow trickle-bed reactor over Ru/TiO2 catalysts. Appl. Catal. B Environ. 2008, 84, 30–41. [Google Scholar] [CrossRef]
- Pintar, A.; Levec, J. Catalytic Liquid-Phase Oxidation of Phenol Aqueous Solutions. A Kinetic Investigation. Ind. Eng. Chem. Res. 1994, 33, 3070–3077. [Google Scholar] [CrossRef]
- Miachon, S.; Perez, V.; Crehan, G.; Torp, E.; Ræder, H.; Bredesen, R.; Dalmon, J.A. Comparison of a contactor catalytic membrane reactor with a conventional reactor: Example of wet air oxidation. Catal. Today 2003, 82, 75–81. [Google Scholar] [CrossRef]
- Berčič, G.; Pintar, A.; Levec, J. Positioning of the reaction zone for gas–liquid reactions in catalytic membrane reactor by coupling results of mass transport and chemical reaction study. Catal. Today 2005, 105, 589–597. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Pina, M.P.; Torres, M.; Cauqui, M.A.; Herguido, J. Catalytic wet oxidation of phenol using membrane reactors: A comparative study with slurry-type reactors. Catal. Today 2010, 149, 326–333. [Google Scholar] [CrossRef]
- Trovarelli, A.; Boaro, M.; Rocchini, E.; de Leitenburg, C.; Dolcetti, G. Some recent developments in the characterization of ceria-based catalysts. J. Alloys Compd. 2001, 323, 584–591. [Google Scholar] [CrossRef]
- Chen, X.; Carabineiro, S.A.C.; Bastos, S.S.T.; Tavares, P.B.; Orfao, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Catalytic oxidation of ethyl acetate on cerium-containing mixed oxides. Appl. Catal. A Gen. 2014, 472, 101–112. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, C.; He, H.; Teraoka, Y. Catalytic decomposition of N2O over CeO2 promoted Co3O4 spinel catalyst. Appl. Catal. B Environ. 2007, 75, 167–174. [Google Scholar] [CrossRef]
- Hammes, M.; Stöwe, K.; Maier, W.F. Cobalt based emission control catalysts with high resistance towards halide poisoning. Appl. Catal. B Environ. 2012, 117, 397–405. [Google Scholar] [CrossRef]
- Matheswaran, M.; Balaji, S.; Chung, S.J.; Moon, I.S. Studies on cerium oxidation in catalytic ozonation process: A novel approach for organic mineralization. Catal. Commun. 2007, 8, 1497–1501. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Monteiro, D.C.M.; Órfão, J.J.M.; Pereira, M.F.R. Cerium, manganese and cobalt oxides as catalysts for the ozonation of selected organic compounds. Chemosphere 2009, 74, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Hung, I.M.; Wang, H.P.; Lai, W.H.; Fung, K.Z.; Hon, M.H. Preparation of mesoporous cerium oxide templated by tri-block copolymer for solid oxide fuel cell. Electrochim. Acta 2004, 50, 745–748. [Google Scholar] [CrossRef]
- Toro, A.R.; Quispe, A.; León, I. Preparación y caracterización de electrodos de espinela de cobalto dopados con niquel. REVCIUNI 2005, 9, 36–45. [Google Scholar]
- Ratnasamy, P.; Srinivas, D.; Satyanarayana, C.V.V.; Manikandan, P.; Senthil Kumaran, R.S.; Sachin, M.; Shetti, V.N. Influence of the support on the preferential oxidation of CO in hydrogen-rich steam reformates over the CuO–CeO2–ZrO2 system. J. Catal. 2004, 221, 455–465. [Google Scholar] [CrossRef]
- Garrido Pedrosa, A.M.; Souza, M.J.B.; Melo, D.M.A.; Araujo, A.S.; Zinner, L.B.; Fernandes, J.D.G.; Martinelli, A.E. Systems involving cobalt and cerium oxides: Characterization and catalytic behavior in the C6–C7 n-alkanes combustion. Solid State Sci. 2003, 5, 725–728. [Google Scholar] [CrossRef]
- Holmgren, A.; Andersson, B.; Duprez, D. Interactions of CO with Pt/ceria catalysts. Appl. Catal. B Environ. 1999, 22, 215–230. [Google Scholar] [CrossRef]
- Feret, F.R.; Roy, D.; Boulanger, C. Determination of alpha and beta alumina in ceramic alumina by X-ray diffraction. Spectrochim. Acta 2000, 55, 1051–1061. [Google Scholar] [CrossRef]
- Tanner, B.K.; Hase, T.P.A.; Lafford, T.A.; Goorsky, M.S. Grazing incidence in-plane X-ray diffraction in the laboratory. JCPDS Adv. X-ray Anal. 2004, 47, 309–314. [Google Scholar] [CrossRef]
- Bouroushian, M.; Kosanovic, T. Characterization of Thin Films by Low Incidence X-ray Diffraction. Cryst. Struct. Theory Appl. 2012, 1, 35–39. [Google Scholar] [CrossRef]
- Silva, A.M.T.; Quinta-Ferreira, R.M.; Levec, J. Catalytic and Noncatalytic Wet Oxidation of Formaldehyde. A Novel Kinetic Model. Ind. Eng. Chem. Res. 2003, 42, 5099–5108. [Google Scholar] [CrossRef]
- Yang, X.; Shen, Y.; Yuan, Z.; Zhu, H. Ferric ions doped 5A molecular sieves for the oxidation of HCHO with low concentration in the air at moderate temperatures. J. Mol. Catal. A Chem. 2005, 237, 224–231. [Google Scholar]
- Gutiérrez-Arzaluz, M.; Torres-Rodríguez, M.; Mugica-Álvarez, V.; Aguilar-Pliego, J.; Romero-Romo, M. Wet Oxidation of Formaldehyde with Heterogeneous Catalytic Materials. Int. J. Environ. Sci. Dev. 2016, 7, 166–171. [Google Scholar] [CrossRef]
- Li, K.; Wang, H.; Wei, Y.; Liu, M. Catalytic performance of cerium iron complex oxides for partial oxidation of methane to synthesis gas. J. Rare Earths 2008, 26, 705–710. [Google Scholar] [CrossRef]
- Tang, X.; Chen, J.; Huang, X.; Xu, Y.; Shen, W. Pt/MnOx–CeO2 catalysts for the complete oxidation of formaldehyde at ambient temperature. Appl. Catal. B Environ. 2008, 81, 115–121. [Google Scholar] [CrossRef]
- Islam, K.; Zuki, B.; Mustapha, M.; Mohd, Z.; Norshazlirah, S.; Eaqub, A. Characterisation of calcium carbonate and its polymorphs from cockle Shells. Powder Technol. 2011, 213, 188–191. [Google Scholar] [CrossRef]
- Rodriguez, J.; Shaw, S.; Benning, L. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Menahem, T.; Yitzhak, M. Controlled crystallization of calcium carbonate superstructures in macroemulsions. J. Cryst. Growth 2008, 310, 3552–3556. [Google Scholar] [CrossRef]
- Pintar, A.; Levec, J. Catalytic oxidation of organics in aqueous solutions: I. Kinetics of phenol oxidation. J. Catal. 1992, 135, 345–357. [Google Scholar] [CrossRef]
- Ángel-Cuevas, S.; Gutiérrez-Arzaluz, M.; Aguilar-Pliego, J.; Mugica-Alvarez, V.; Noreña-Franco, L.; Torres-Rodríguez, M. Remoción de formaldehido, de efluentes acuosos mediante oxidación húmeda catalítica. Av. Cienc. Ing. 2011, 2, 13–23. [Google Scholar]
- Chen, J.P.; Isa, K. Thermal Decomposition of Urea and Urea Derivatives by Simultaneous TG/(DTA)/MS. J. Mass Spectrom. Soc. Jpn. 1998, 46, 299–303. [Google Scholar] [CrossRef]
- Sample Availability: Membrane samples are not available and there are powder sample availability.


© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Arzaluz, M.; Noreña-Franco, L.; Ángel-Cuevas, S.; Mugica-Álvarez, V.; Torres-Rodríguez, M. Catalysts with Cerium in a Membrane Reactor for the Removal of Formaldehyde Pollutant from Water Effluents. Molecules 2016, 21, 668. https://doi.org/10.3390/molecules21060668
Gutiérrez-Arzaluz M, Noreña-Franco L, Ángel-Cuevas S, Mugica-Álvarez V, Torres-Rodríguez M. Catalysts with Cerium in a Membrane Reactor for the Removal of Formaldehyde Pollutant from Water Effluents. Molecules. 2016; 21(6):668. https://doi.org/10.3390/molecules21060668
Chicago/Turabian StyleGutiérrez-Arzaluz, Mirella, Luis Noreña-Franco, Saúl Ángel-Cuevas, Violeta Mugica-Álvarez, and Miguel Torres-Rodríguez. 2016. "Catalysts with Cerium in a Membrane Reactor for the Removal of Formaldehyde Pollutant from Water Effluents" Molecules 21, no. 6: 668. https://doi.org/10.3390/molecules21060668
APA StyleGutiérrez-Arzaluz, M., Noreña-Franco, L., Ángel-Cuevas, S., Mugica-Álvarez, V., & Torres-Rodríguez, M. (2016). Catalysts with Cerium in a Membrane Reactor for the Removal of Formaldehyde Pollutant from Water Effluents. Molecules, 21(6), 668. https://doi.org/10.3390/molecules21060668






