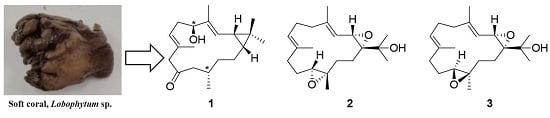
New Casbane and Cembrane Diterpenoids from an Okinawan Soft Coral, Lobophytum sp.
Abstract
:1. Introduction
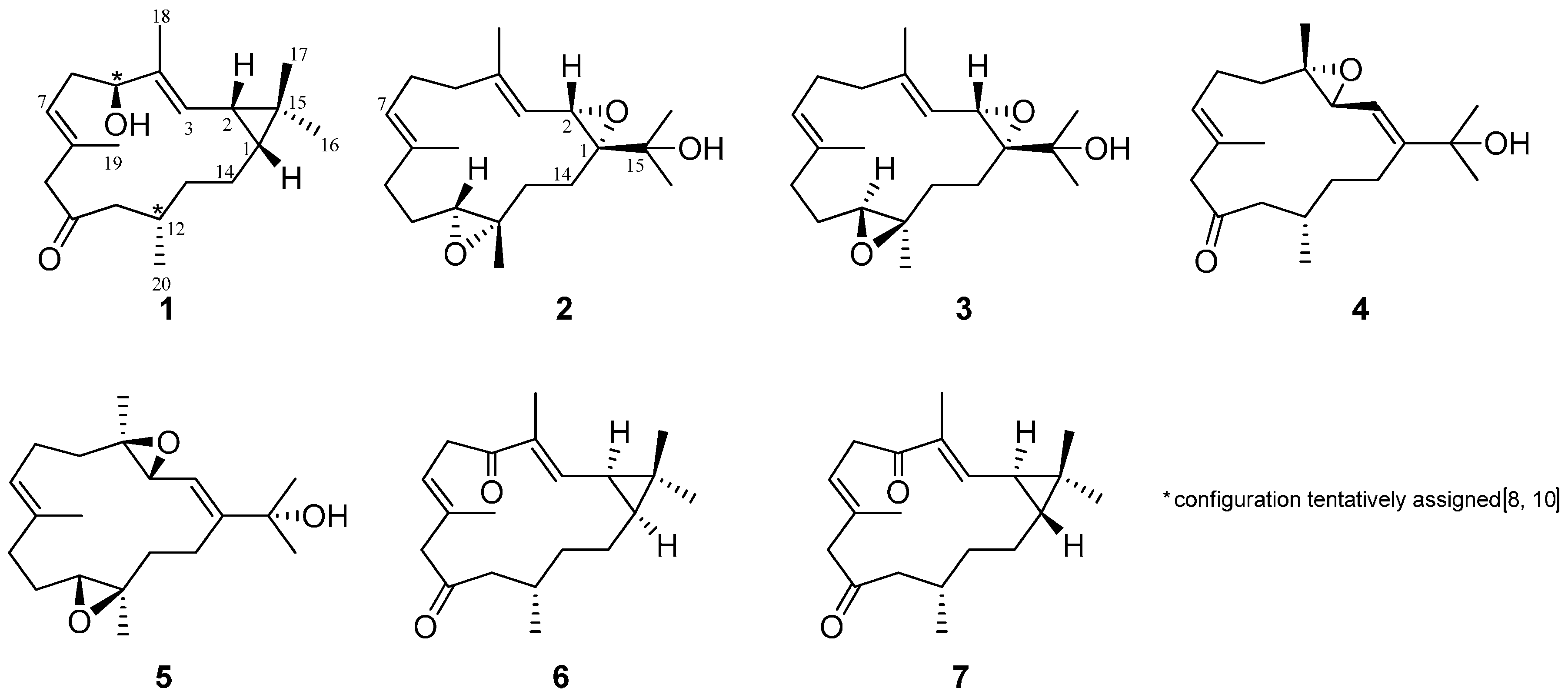
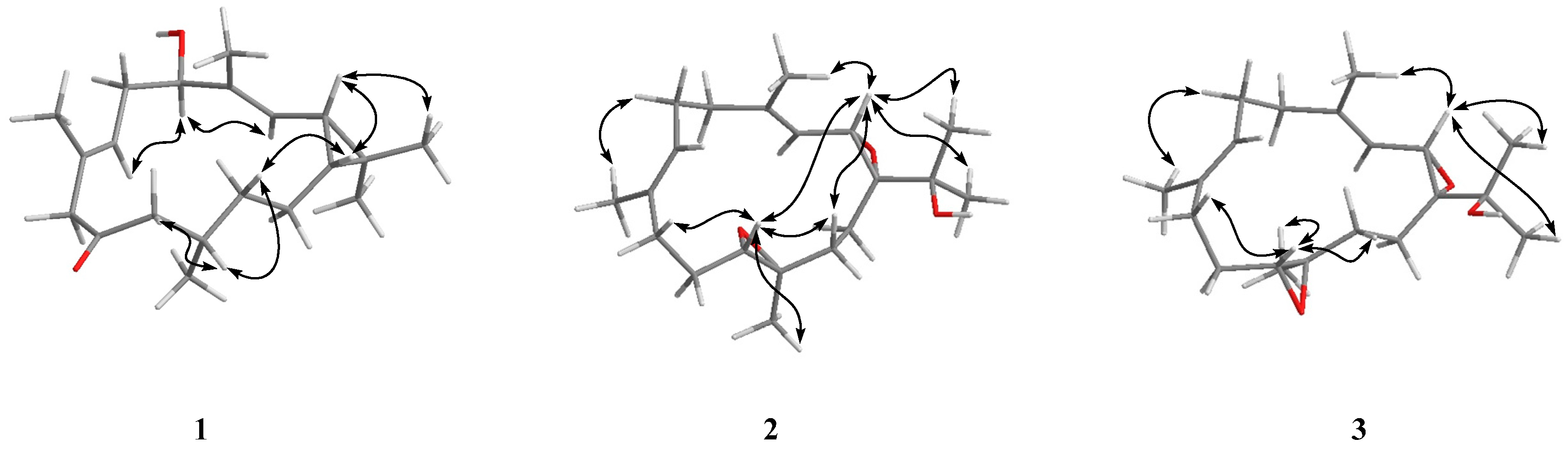
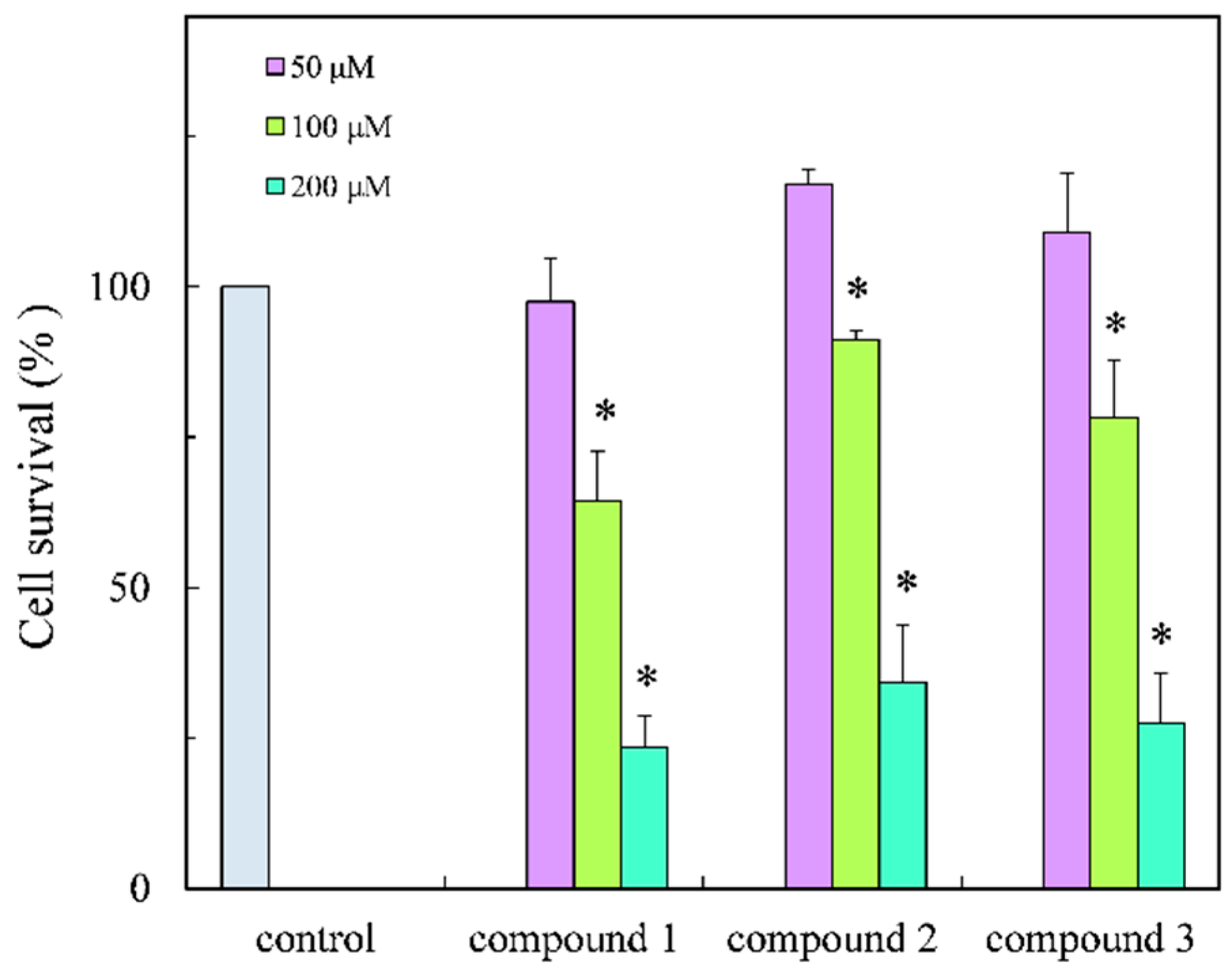
2. Results and Discussion
3. Experimental Section
3.1. General Procedures
3.2. Animal Materials
3.3. Extraction and Isolation
3.4. Molecular Mechanics Calculations
3.5. Anti-Bacterial Assay
3.6. Cell Culture
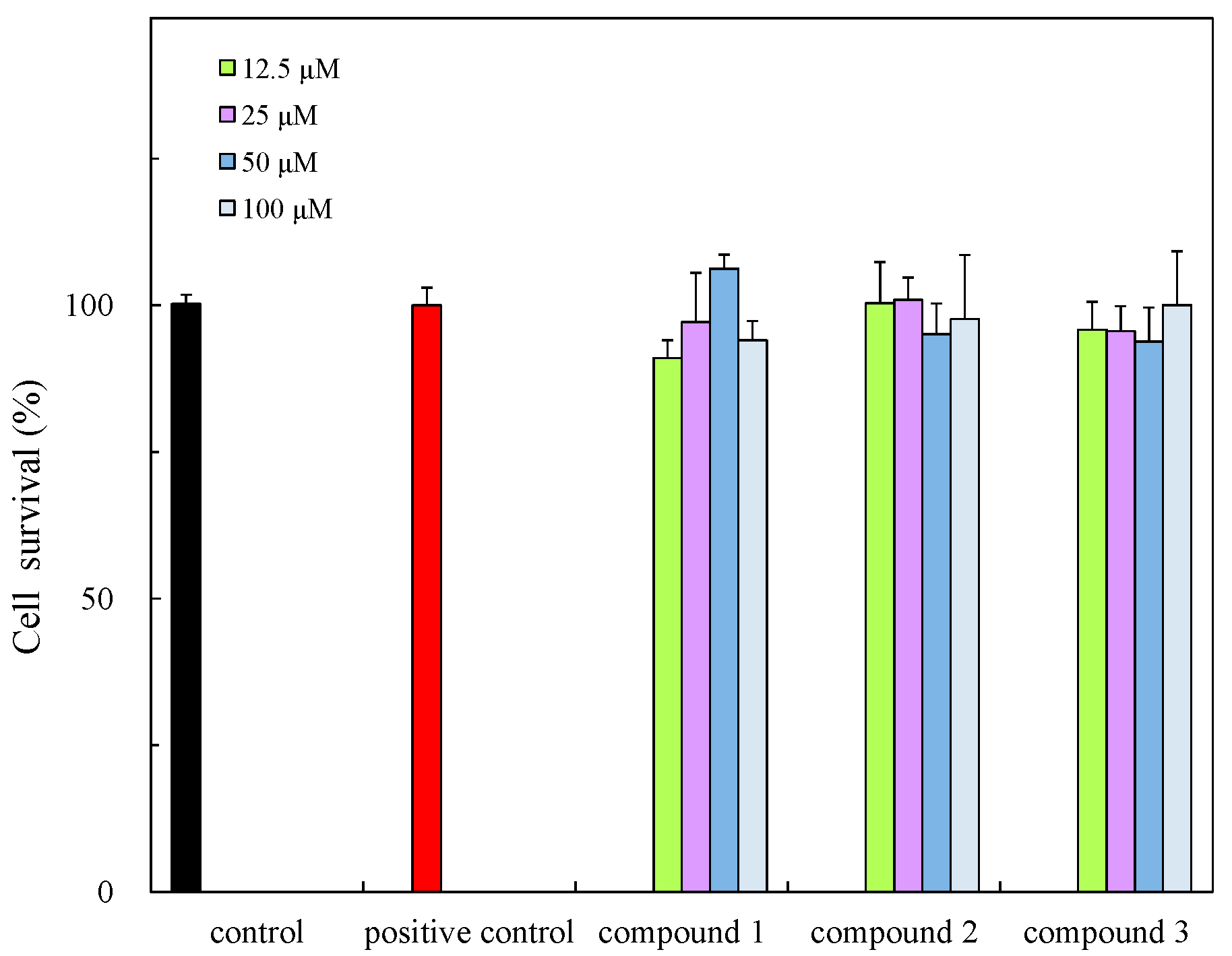
3.7. Cell Viability
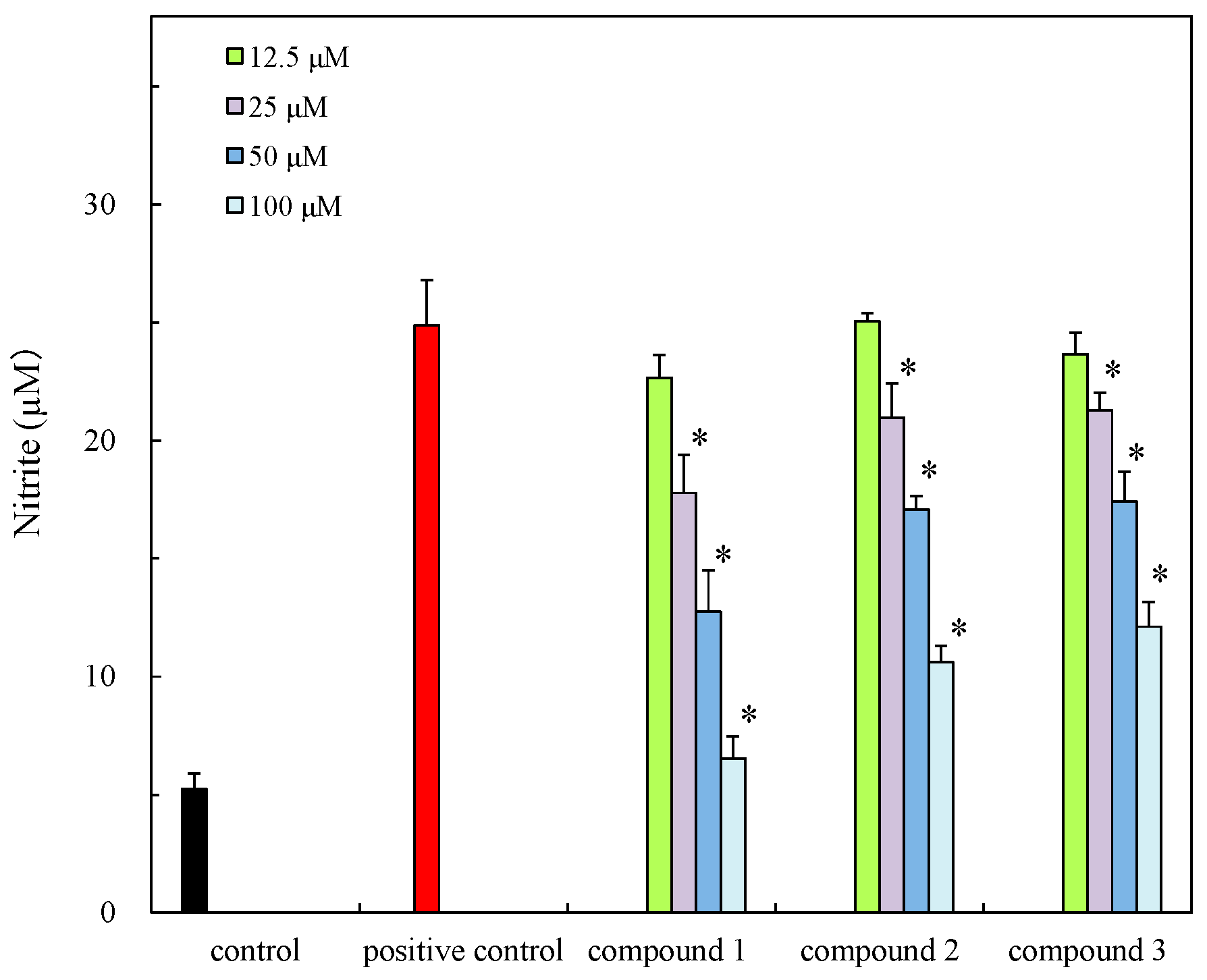
3.8. Anti-Inflammatory Effect on Nitrite Production on RAW 264.7 Macrophages
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burhan, R.Y.R.; Putri, E.M.M.; Zetra, Y.; Herdhiansyah, R.; Putra, M.Y. Terpenes from soft corals of the genus Lobophytum (Alcyoniidae): Chemistry and biological activities. J. Chem. Pharm. Res. 2014, 6, 585–595. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Northcote, P.N.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Maarisit, W.; Roy, S.R.; Taira, J.; Ueda, K. Cytotoxic alcyonolide congeners from an Okinawan soft coral Cespitularia sp. Heterocycles 2012, 85, 2465–2472. [Google Scholar]
- Roy, P.K.; Maarisit, W.; Roy, M.C.; Taira, J.; Ueda, K. Five new diterpenoids from an Okinawan soft coral, Cespitularia sp. Mar. Drugs 2012, 10, 2741–2748. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Roy, M.C.; Taira, J.; Ueda, K. Structure and bioactivity of a trisnorditerpenoid and diterpenoid from an Okinawan soft coral, Cespitularia sp. Tetrahedron Lett. 2014, 55, 1421–1423. [Google Scholar] [CrossRef]
- Maarisit, W.; Yano, K.; Miyazato, T.; Roy, P.K.; Taira, J.; Ueda, K. Structures and bioactivities of xenicanes from an Okinawan soft coral Xenia sp. Heterocycles 2015, 91, 505–514. [Google Scholar]
- Ueda, K.; Hu, Y. Haterumalide B: A new cytotoxic macrolide from an Okinawan ascidian Lissoclinum sp. Tetrahedron Lett. 1999, 40, 6305–6308. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Tai, S.H.; Wen, Z.H.; Su, J.H.; Wu, Y.C.; Hu, W.P.; Sheu, J.H. A C-3 methylated isocembranoid and 10-oxocembranoids from a Formosan soft coral, Sinularia grandilobata. J. Nat. Prod. 2008, 71, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.S.; Duh, C.Y.; Chiang, M.Y.; Lin, C.N. Sinugibberol, a new cytotoxic cembranoid diterpene from the soft coral Sinularia gibberosa. J. Nat. Prod. 1995, 58, 1126–1130. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Carbone, M.; Vitale, R.M.; Amodeo, P.; Castelluccio, F.; Sicilia, G.; Mollo, E.; Nappo, M.; Cimino, G.; Guo, Y.W.; et al. Rare casbane diterpenoids from the Hainan soft coral Sinularia depressa. J. Nat. Prod. 2010, 73, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhao, M.; Ma, M.; Xu, Y.; Xiang, Z.; Kai, Y.; Dong, J.; Lei, X.; Huang, K.; Yan, P. New casbene diterpenoids from a South China Sea soft coral, Sinularia sp. Mar. Drugs 2013, 11, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.R.; West, C.A. Biosynthesis of cyclic diterpenes in extracts from seedlings of Ricinus communis L. I. Identification of diterpene hydrocarbons formed from mevalonate. Biochemistry 1970, 9, 70–79. [Google Scholar] [PubMed]
- Chio, Y.H.; Kim, J.; Pezzuto, J.M.; Kinghorn, A.D.; Farnsworth, N.R. Agrostistachin, a novel cytotoxic macrocyclic diterpene from Agrostistachys hookeri. Tetrahedron Lett. 1986, 48, 5795–5798. [Google Scholar] [CrossRef]
- Zhang, C.X.; Yan, S.J.; Zhang, G.W.; Lu, W.G.; Su, J.Y.; Zeng, L.M.; Gu, L.Q.; Yang, X.P.; Lian, Y.J. Cytotoxic sterols from the soft coral Nephthea erecta. J. Nat. Prod. 2005, 68, 1087–1089. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.H.; Sun, J.; Xu, R.S.; Qin, G.W. Casbane diterpenoids from Euphorbia ebracteolata. Phytochemistry 1998, 49, 149–151. [Google Scholar] [CrossRef]
- Wang, S.K.; Duh, C.Y.; Wu, Y.C.; Wang, Y.; Cheng, M.C.; Soong, K.; Fang, L.S. Studies on Formosan soft corals, II. Cytotoxic cembranolides from the soft coral Lobophytum michaelae. J. Nat. Prod. 1992, 55, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Duh, C.Y. New cytotoxic cembranolides from the soft coral Lobophytum michaelae. Mar. Drugs 2012, 10, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.T.; Wang, S.K.; Soong, K.; Duh, C.Y. New cytotoxic cembranolides from the soft coral Lobophytum michaelae. Chem. Pharm. Bull. 2007, 55, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Coval, S.J.; Patton, R.W.; Petrin, J.M.; James, L.; Rothofsky, M.L.; Lin, S.; Patel, M.; Reed, J.K.; McPhil, A.T.; Bishop, W.R. A cembranolide diterpene farnesyl protein transferase inhibitor from the marine soft corals Lobophytum cristagalli. Bioorg. Med. Chem. Lett. 1696, 6, 909–912. [Google Scholar] [CrossRef]
- Higuchi, R.; Miyamoto, T.; Yamada, K.; Komori, T. Cytotoxic and ichthyotoxic compounds from marine Opisthobranchia and soft coral. Toxicon 1998, 36, 1703–1705. [Google Scholar] [CrossRef]
- Matthée, G.F.; König, G.M.; Wright, A.D. Three new diterpenes from the marine soft coral Lobophytum crassum. J. Nat. Prod. 1998, 61, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Duh, C.Y.; Wang, S.K.; Huang, B.T.; Dai, C.F. Cytotoxic cembrenolide diterpenes from the Formosan soft coral Lobophytum crassum. J. Nat. Prod. 2000, 63, 884–885. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Wang, S.K.; Duh, C.Y. Secocrassumol, a seco-cembranoid from the Dongsha a toll soft coral Lobophytum crissum. Mar. Drugs 2014, 12, 6028–6037. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.T.; Wang, S.K.; Cheng, S.Y.; Duh, C.Y. Lobocrasol, a new diterpenoid from the soft coral Lobophytum crassum. Org. Lett. 2009, 14, 3012–3014. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Wen, Z.H.; Chiou, S.F.; Hsu, C.H.; Wang, S.K.; Dai, C.F.; Chiang, M.Y.; Duh, C.Y. Durumolides A–E, anti-inflammatory and antibacterial cembranolides from the soft coral Lobophytum durum. Tetrahedron 2008, 64, 9698–9704. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Wen, Z.H.; Wang, S.K.; Chiou, S.F.; Hsu, C.H.; Dai, C.F.; Duh, C.Y. Anti-inflammatory cembranolides from the soft coral Lobophytum durum. Bioorg. Med. Chem. 2009, 17, 3763–3769. [Google Scholar] [CrossRef] [PubMed]
- Breitmaier, E.; Voelter, W. Carbon-13 NMR Spectroscopy; VCH: New York, NY, USA, 1987; pp. 192–196. [Google Scholar]
- Ericsson, H. The paper disc method for determination of bacterial sensitivity to antibiotics studies on the accuracy of the technique. Scand. J. Clin. Lab. Invest 1960, 12, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Taira, J.; Tsuchida, E.; Uehara, M.; Kinjyo, Y.; Roy, P.K.; Ueda, K. Dual biological functions of the apoptotic activity and anti-inflammatory effect by alcyonolide congeners from the Okinawan soft coral, Cespitularia sp. Bioorg. Med. Chem. Lett. 2015, 25, 4496–4499. [Google Scholar] [CrossRef] [PubMed]
- Taira, J.; Uehara, M. Anti-inflammatory effect of fucoxanthinol as bioavailable marine carotenoid on LPS-stimulated RAW 264.7 macrophages through iNOS suppression and nitrogen radical-scavenging. World J. Pharm. Sci. 2015, 3, 1747–1754. [Google Scholar]
- Taira, J.; Nanbu, H.; Ueda, K. Nitric oxide-scavenging compounds in Agrimonia pilosa Ledeb on LPS-induced RAW 264.7 macrophages. Food Chem. 2009, 115, 1221–1227. [Google Scholar] [CrossRef]
- Allinger, N.L. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977, 99, 8127–8134. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–7 are available from the authors.

| C No. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH (mult., J/Hz) | δC | δH (mult., J/Hz) | δC | δH (mult., J/Hz) | δC | |
| 1 | 0.65 (ddd, 3.1, 9.0, 11.2) | 31.4 (CH) | 68.2 (C) | 68.0 (C) | ||
| 2 | 1.22 (dd, 9.5, 9.0) | 25.3 (CH) | 3.75 (d, 4.2) | 57.5 (CH) | 3.80 (d, 5.8) | 57.9 (CH) |
| 3 | 5.09 (brd, 9.5) | 126.0 (CH) | 5.08 (brd, 4.2) | 118.8 (CH) | 4.79 (brd, 5.8) | 121.0 (CH) |
| 4 | 137.1 (C) | 141.9 (C) | 140.0 (C) | |||
| 5 | 4.09 (dd, 4.4, 11.0) | 79.2 (CH) | 2.27 (m) | 38.9 (CH2) | 2.11 (m) | 38.9 (CH2) |
| 6 | 2.44 (m) | 33.0 (CH2) | 2.24 (m) | 24.5 (CH2) | 2.31 (m) | 24.8 (CH2) |
| 2.34 (m) | ||||||
| 7 | 4.90 (dd, 6.9, 6.9) | 124.1 (CH) | 5.15 (t, 6.1) | 126.2 (CH) | 5.10 (t, 5.2) | 125.8 (CH) |
| 8 | 131.3 (C) | 133.7 (C) | 134.0 (C) | |||
| 9 | 3.15 (d, 14.7) | 51.9 (CH2) | 2.23 (m) | 36.8 (CH2) | 2.18 (m) | 36.8 (CH2) |
| 2.82 (d, 14.7) | 2.04 (m) | |||||
| 10 | 210.6 (C) | 2.25 (m) | 24.3 (CH2) | 2.29 (m) | 23.7 (CH2) | |
| 1.96 (m) | 2.02 (m) | |||||
| 11 | 2.22 (d, 7.0) | 52.4 (CH2) | 2.71 (dd, 3.4, 9.1) | 61.6 (CH) | 2.59 (dd, 3.3, 10.6) | 61.9 (CH) |
| 12 | 1.88 (m) | 31.6 (CH) | 61.2 (C) | 61.4 (C) | ||
| 13 | 1.15 (m) | 37.2 (CH2) | 1.99 (m) | 35.2 (CH2) | 2.27 (m) | 35.1 (CH2) |
| 1.96 (m) | ||||||
| 14 | 1.59 (m) | 23.8 (CH2) | 1.86 (m) | 25.4 (CH2) | 2.09 (m) | 24.3 (CH2) |
| 0.75 (m) | 1.34 (m) | |||||
| 15 | 21.0 (C) | 70.1 (C) | 70.8 (C) | |||
| 16 | 1.01 (s) | 15.7 (CH3) | 1.25 (s) | 26.2 (CH3) | 1.29(s) | 25.4 (CH3) |
| 17 | 1.05 (s) | 29.1 (CH3) | 1.31 (s) | 26.7 (CH3) | 1.28 (s) | 26.5 (CH3) |
| 18 | 1.64 (s) | 10.3 (CH3) | 1.70 (s) | 17.7 (CH3) | 1.70 (s) | 17.0 (CH3) |
| 19 | 1.74 (s) | 17.8 (CH3) | 1.64 (s) | 15.3 (CH3) | 1.64 (s) | 14.9 (CH3) |
| 20 | 0.91 (d, 6.6) | 20.4 (CH3) | 1.24 (s) | 17.0 (CH3) | 1.25 (s) | 16.0 (CH3) |
| Compound | Antibacterial Activity a | Cytotoxicity (IC50, µM) | Anti-Inflammatory Effect (IC50, µM) | ||
|---|---|---|---|---|---|
| S. aureus | S. enterica | E. coli | HCT116 cells | RAW 256.7 cells | |
| 1 | 10 | N.A b | 10 | 135.57 | 41.21 |
| 2 | 9 | 12 | 10 | 177.11 | 64.96 |
| 3 | 9 | 10 | 10 | 153.11 | 74.76 |
| 4 | 10 | N.A b | 12 | N.T c | N.T c |
| 5 | 10 | N.A b | 15 | N.T c | N.T c |
| Streptomycin sulfate | 15 | N.T c | 13 | N.T c | N.T c |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, P.K.; Ashimine, R.; Miyazato, H.; Taira, J.; Ueda, K. New Casbane and Cembrane Diterpenoids from an Okinawan Soft Coral, Lobophytum sp. Molecules 2016, 21, 679. https://doi.org/10.3390/molecules21050679
Roy PK, Ashimine R, Miyazato H, Taira J, Ueda K. New Casbane and Cembrane Diterpenoids from an Okinawan Soft Coral, Lobophytum sp. Molecules. 2016; 21(5):679. https://doi.org/10.3390/molecules21050679
Chicago/Turabian StyleRoy, Prodip K., Runa Ashimine, Haruna Miyazato, Junsei Taira, and Katsuhiro Ueda. 2016. "New Casbane and Cembrane Diterpenoids from an Okinawan Soft Coral, Lobophytum sp." Molecules 21, no. 5: 679. https://doi.org/10.3390/molecules21050679