BiOBr/BiOI Photocatalyst Based on Fly Ash Cenospheres with Improved Photocatalytic Performance
Abstract
:1. Introduction
2. Results and Discussion
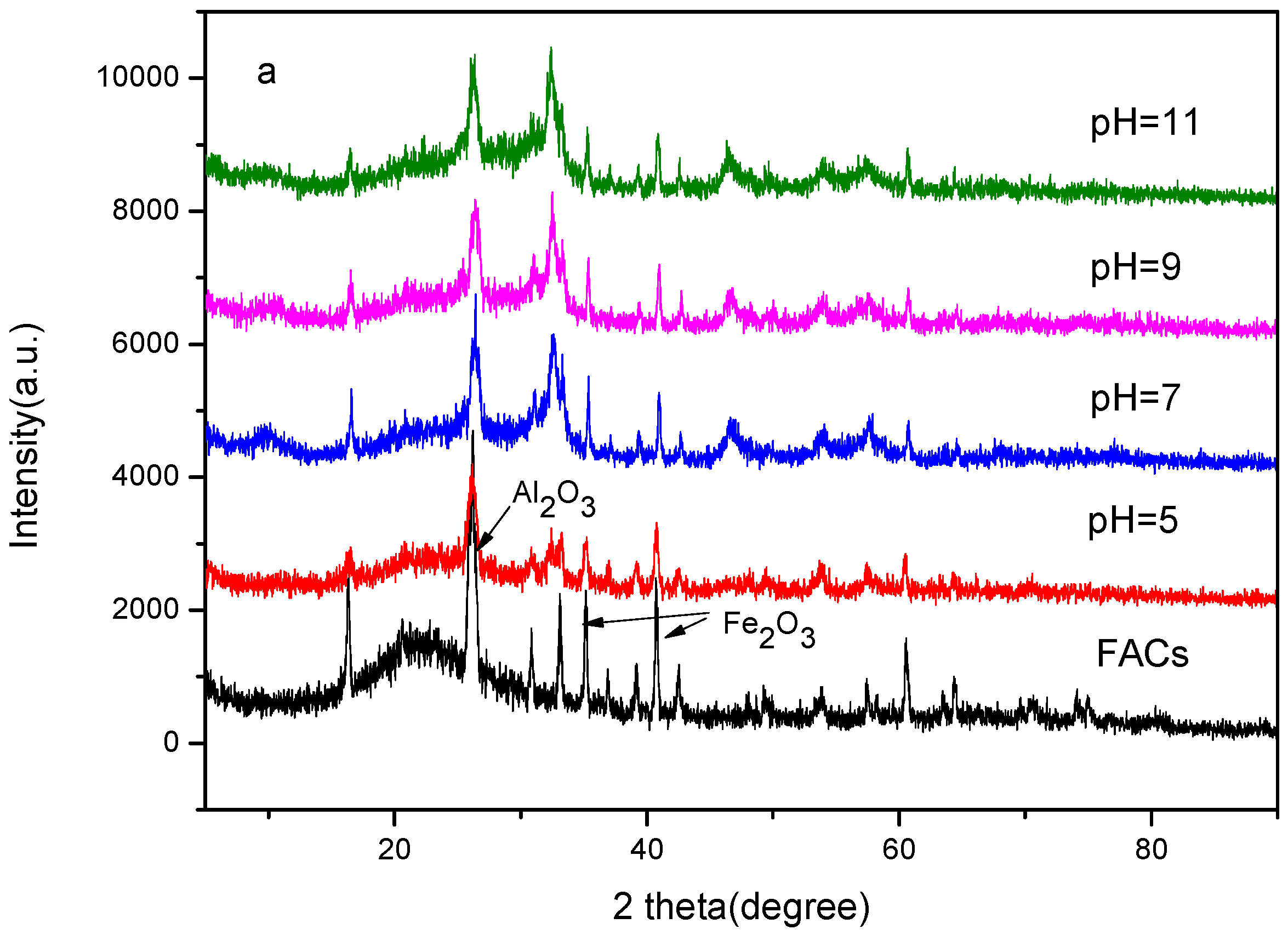
2.1. XRD Patterns
2.2. SEM and EDS Observation
2.3. DRS Analysis
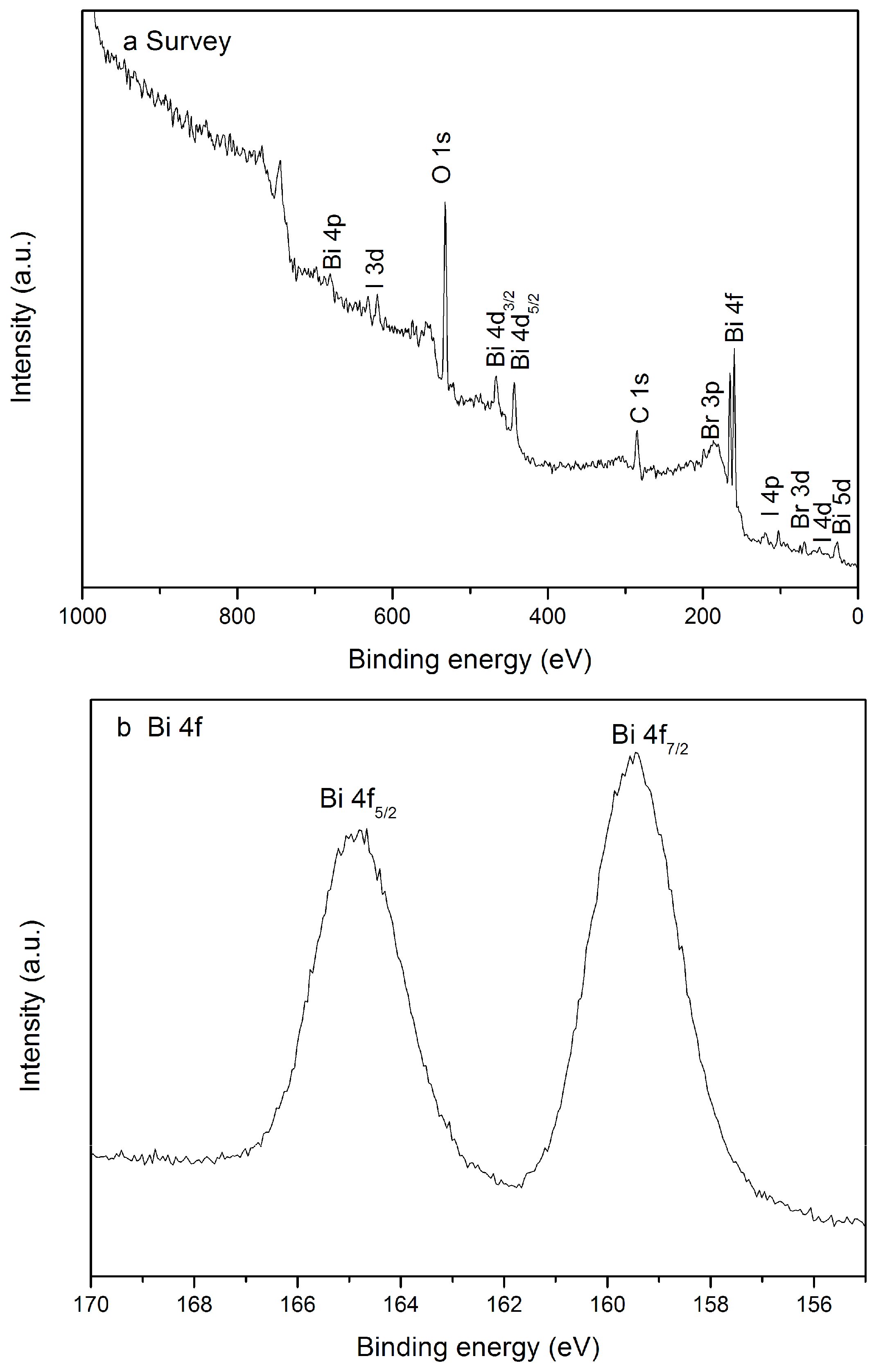
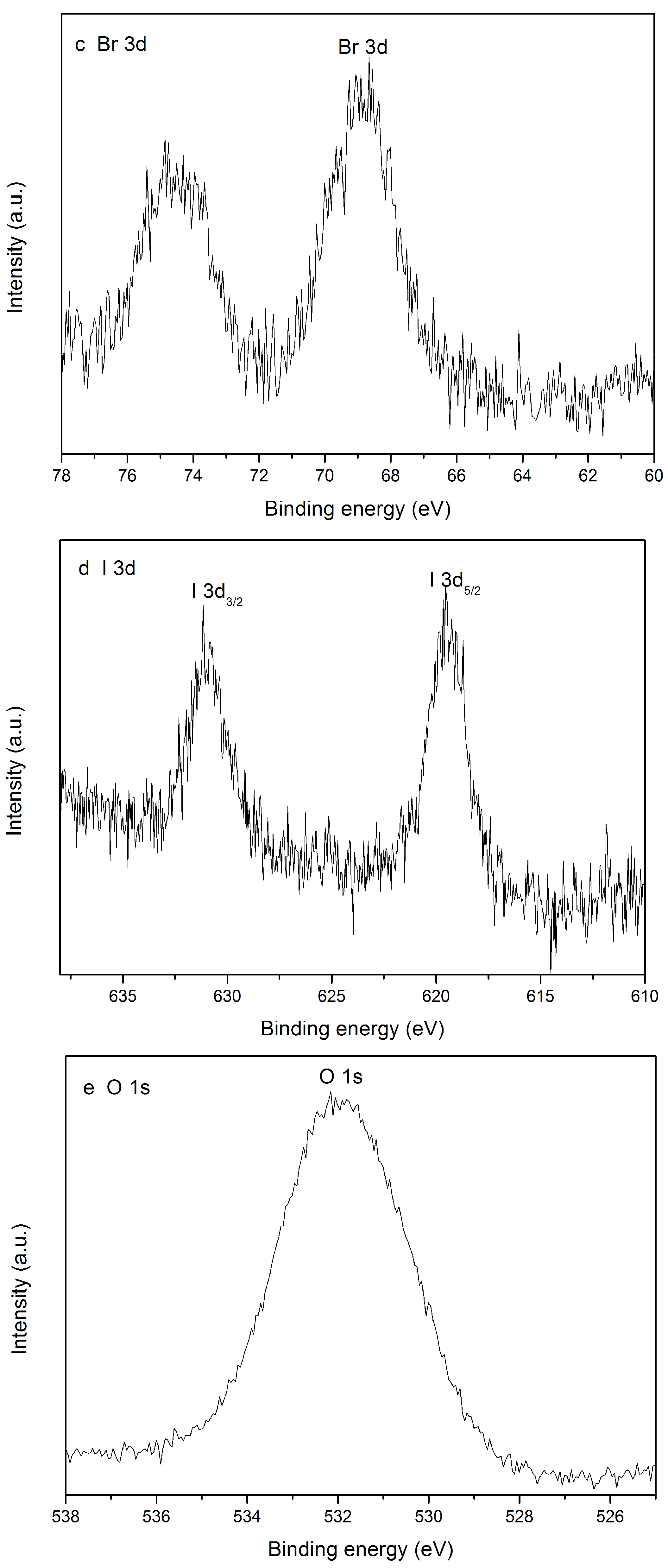
2.4. XPS Analysis
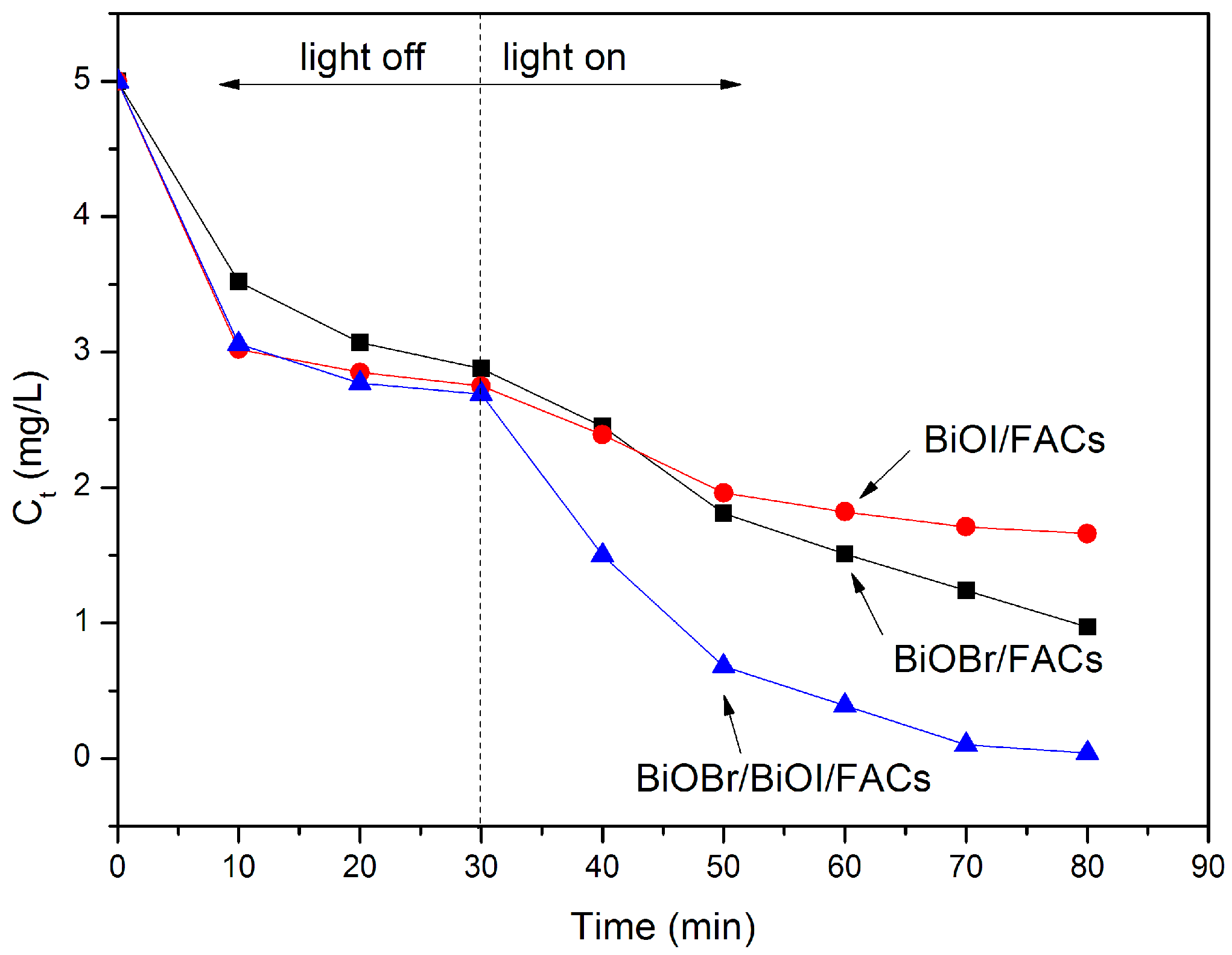
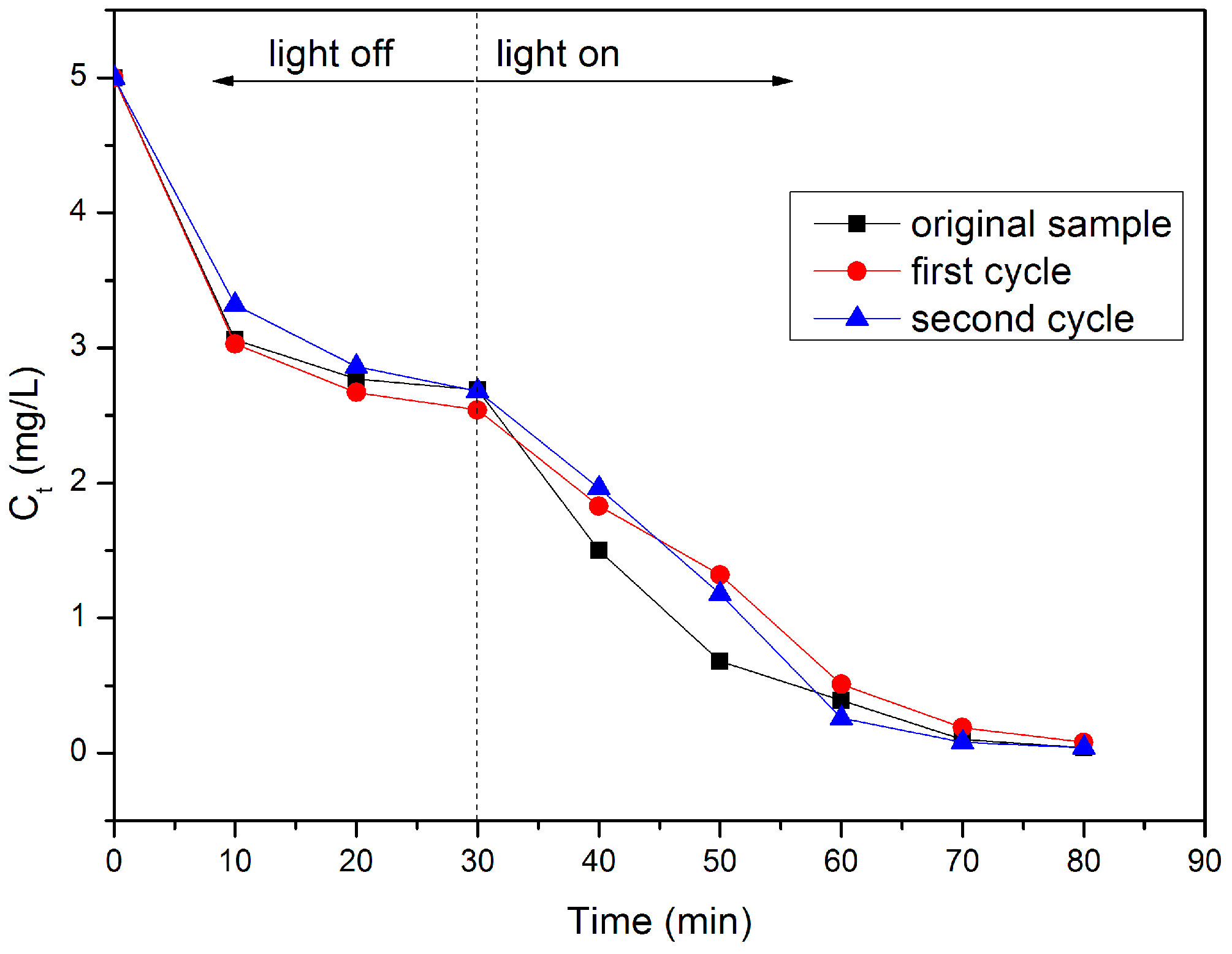
2.5. Photocatalytic Evaluation
3. Experimental Section
3.1. General Information
3.2. Photocatalyst Synthesis
3.3. Photocatalysis Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Paul, S.; Chetri, P.; Choudhury, A. Effect of manganese doping on the optical property and photocatalytic activity of nanocrystalline titania: Experimental and theoretical investigation. J. Alloys Compd. 2014, 583, 578–586. [Google Scholar] [CrossRef]
- Gao, Q.; Wu, X.; Fan, Y.; Zhou, X. Low temperature fabrication of nanoflower arrays of rutile TiO2 on mica particles with enhanced photocatalytic activity. J. Alloys Compd. 2013, 579, 322–329. [Google Scholar] [CrossRef]
- Jiang, W.J.; Joensa, J.A.; Dionysioub, D.D.; O’Shea, K.E. Optimization of photocatalytic performance of TiO2 coated glass microspheres using response surface methodology and the application for degradation of dimethyl phthalate. J. Photochem. Photobiol. A Chem. 2013, 262, 7–13. [Google Scholar] [CrossRef]
- Zheng, S.; Jiang, W.J.; Cai, Y.; Dionysiou, D.D.; O’She, K.E. Adsorption and photocatalytic degradation of aromatic organoarsenic compounds in TiO2 suspension. Catal. Today 2014, 224, 83–88. [Google Scholar] [CrossRef]
- Chang, X.F.; Huang, J.; Cheng, C.; Sui, Q.; Sha, W.; Ji, G.B.; Deng, S.B.; Yu, G. BiOX (X = Cl, Br, I) photocatalysts prepared using NaBiO3 as the Bi source: Characterization and catalytic performance. Catal. Commun. 2010, 11, 460–464. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.Y.; Son, W.J.; Lu, J.B.; Huang, B.B.; Dai, Y.; Whangbo, M.H. Composition dependence of the photocatalytic activities of BiOCl1-xBrx solid solutions under visible light. Chem. Eur. J. 2011, 17, 9342–9349. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.F.; Wang, F.M.; Xin, F.; Zhang, B.Q. Simple solvothermal routes to synthesize 3D BiOBrxI1-x microspheres and their visible-light-induced photocatalytic properties. Ind. Eng. Chem. Res. 2011, 50, 6688–6694. [Google Scholar] [CrossRef]
- Liu, Z.S.; Ran, H.S.; Wu, B.T.; Feng, P.Z.; Zhu, Y.B. Synthesis and characterization of BiOI/BiOBr heterostructure films with enhanced visible light photocatalytic activity. Colloids Surf. A Physicochem. Eng. Aspects 2014, 452, 109–114. [Google Scholar] [CrossRef]
- Li, R.; Fan, C.M.; Zhang, X.C.; Wang, Y.W.; Wang, Y.F.; Zhang, H. Preparation of BiOBr thin films with micro-nano-structure and their photocatalytic applications. Thin Solid Films 2014, 562, 506–512. [Google Scholar] [CrossRef]
- Ye, L.Q.; Chen, J.N.; Tian, L.H.; Liu, J.Y.; Peng, T.Y.; Deng, K.J.; Zan, L. BiOI thin film via chemical vapor transport: Photocatalytic activity, durability, selectivity and mechanism. Appl. Catal. B: Environ. 2013, 130–131, 1–7. [Google Scholar] [CrossRef]
- Liu, Z.S.; Wu, B.T.; Niu, J.N.; Huang, X.; Zhu, Y.B. Solvothermal synthesis of BiOBr thin film and its photocatalytic performance. Appl. Surf. Sci. 2014, 288, 369–372. [Google Scholar] [CrossRef]
- Ao, Y.; Tang, H.; Wang, P.; Wang, C.; Hou, J.; Qian, J. Synthesis, characterization and photocatalytic activity of BiOBr-AC composite photocatalyst. Compos. Part B 2014, 59, 96–100. [Google Scholar] [CrossRef]
- Huo, P.W.; Lu, Z.Y.; Liu, X.L.; Wu, D.; Liu, X.L.; Pan, J.M.; Gao, X.; Guo, W.L.; Li, H.M.; Yan, Y.S. Preparation photocatalyst of selected photodegradation antibiotics by molecularimprinting technology onto TiO2/fly-ash cenospheres. Chem. Eng. J. 2012, 189–190, 75–83. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, H.; Wang, B.; Li, C.; Zhai, J.P.; Li, Q. Fly ash cenospheres supported visible-light-driven BiVO4 photocatalyst: Synthesis, characterization and photocatalytic application. Chem. Eng. J. 2013, 22, 737–746. [Google Scholar] [CrossRef]
- Lin, L.; Huang, M.H.; Long, L.P.; Sun, Z.; Zheng, W.; Chen, D.H. Fabrication of a three-dimensional BiOBr/BiOI photocatalyst with enhanced visible light photocatalytic performance. Ceram. Int. 2014, 40, 11493–11501. [Google Scholar] [CrossRef]
- Lin, L.; Huang, M.H.; Long, L.P.; Chen, D.H. Novel photocatalysts of fly ash cenospheres supported BiOBr hierarchical microspheres with high photocatalytic performance. J. Alloys Compd. 2014, 615, 929–932. [Google Scholar] [CrossRef]
- Lei, Y.Q.; Wang, G.H.; Guo, P.R.; Song, H.C. The Ag-BiOBrxI1−x composite photocatalyst: Preparation, characterization and their novel pollutants removal property. Appl. Surf. Sci. 2013, 279, 374–379. [Google Scholar] [CrossRef]
- Huo, P.W.; Lu, Z.Y.; Liu, X.L.; Liu, X.L.; Gao, X.; Pan, J.M.; Wu, D.; Ying, J.; Li, H.M.; Yan, Y.S. Preparation molecular/ions imprinted photocatalysts of La3+@POPD/TiO2/fly-ashcenospheres: Preferential photodegradation of TCs antibiotics. Chem. Eng. J. 2012, 198–199, 73–80. [Google Scholar] [CrossRef]
- Li, J.; Lu, G.; Wang, Y.; Guo, Y.; Guo, Y. A high activity photocatalyst of hierarchical 3D flowerlike ZnO microspheres: Synthesis, characterization and catalytic activity. J. Colloid Interf Sci. 2012, 377, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Q.; Gu, X.; Chen, F.; Zhang, J.L. Preparation of Nano BiOCl Microsphere and Its Fabrication Machanism. Chin. J. Catal. 2011, 32, 129–134. [Google Scholar] [CrossRef]
- Butler, M.A. Photoelectrolysis and physical properties of the semiconducting electrode WO2. J. Appl Phys. 1977, 48, 1914–1920. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, S.W.; Lu, Q.F.; Xu, F.X.; Sun, H.Y. Fabrication of electrospun Bi2WO6 microbelts with enhanced visible photocatalytic degradation activity. J. Alloys Compd. 2013, 578, 12–16. [Google Scholar] [CrossRef]
- Huang, W.L.; Zhu, Q. DFT calculations on the electronic structures of BiOX (X = F, Cl, Br, I) photocatalysts with and without semicore Bi 5d states. J. Comput. Chem. 2009, 30, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.L.; Ye, H.F.; Li, X.; Cao, J.; Chen, S.F. Facile anion-exchange synthesis of BiOI/BiOBr composite with enhanced photoelectrochemical and photocatalytic properties. Ceram. Intern. 2014, 40, 9743–9750. [Google Scholar] [CrossRef]
- Liu, H.; Su, Y.; Chen, Z.; Jin, Z.T.; Wang, Y. Graphene sheets grafted threedimensional BiOBr0.2I0.8 microspheres with excellent photocatalytic activity under visible light. J. Hazard. Mater. 2014, 266, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Sun, Y.J.; Fu, M.; Wu, Z.B.; Lee, S.C. Room temperature synthesis and highly enhanced visible light photocatalytic activity of porous BiOI/BiOCl composites nanoplates microflowers. J. Hazard. Mater. 2012, 219–220, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.N.; Zhang, J.; Miao, M.; Jin, Y. Solvothermal synthesis of flowerlike BiOBr microspheres with highly visible-light photocatalytic performances. Appl. Catal. B 2012, 111–112, 334–341. [Google Scholar] [CrossRef]
- Chen, L.; Huang, R.; Xiong, M.; Yuan, Q.; He, J.; Jia, J.; Yao, M.Y.; Luo, S.L.; Au, C.T.; Yin, S.F. Room-Temperature Synthesis of Flower-Like BiOX (X = Cl, Br, I) Hierarchical Structures and Their Visible-Light Photocatalytic Activity. Inorg. Chem. 2013, 52, 11118–11125. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Ouyang, S.X.; Bi, Y.P. Nano-photocatalytic Materials: Possibilities and Challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.




| Peak | Position BE (eV) | FWHM (eV) | R.S.F. | Area (CPS) | Atomic Conc% |
|---|---|---|---|---|---|
| O 1s | 532.16 | 3.355 | 2.85 | 47588.3 | 81.3 |
| Bi 4f | 159.41 | 2.045 | 24.9 | 58683.4 | 11.47 |
| Br 3d | 68.66 | 2.211 | 3.04 | 3378.6 | 5.41 |
| I 3d | 619.56 | 1.897 | 32.7 | 12216.4 | 1.82 |
| Element | Fly Ash Cenospheres (wt %) |
|---|---|
| Na | 1.46453 |
| Mg | 1.91019 |
| Al | 53.48339 |
| Si | 66.81443 |
| P | 0.15579 |
| S | 0.18619 |
| K | 0.24782 |
| Ca | 0.00320 |
| Ti | 0.37482 |
| Fe | 1.00285 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Huang, M.; Chen, D. BiOBr/BiOI Photocatalyst Based on Fly Ash Cenospheres with Improved Photocatalytic Performance. Molecules 2016, 21, 666. https://doi.org/10.3390/molecules21050666
Lin L, Huang M, Chen D. BiOBr/BiOI Photocatalyst Based on Fly Ash Cenospheres with Improved Photocatalytic Performance. Molecules. 2016; 21(5):666. https://doi.org/10.3390/molecules21050666
Chicago/Turabian StyleLin, Li, Manhong Huang, and Donghui Chen. 2016. "BiOBr/BiOI Photocatalyst Based on Fly Ash Cenospheres with Improved Photocatalytic Performance" Molecules 21, no. 5: 666. https://doi.org/10.3390/molecules21050666
APA StyleLin, L., Huang, M., & Chen, D. (2016). BiOBr/BiOI Photocatalyst Based on Fly Ash Cenospheres with Improved Photocatalytic Performance. Molecules, 21(5), 666. https://doi.org/10.3390/molecules21050666





