In Vitro Antioxidant, Anti-Diabetes, Anti-Dementia, and Inflammation Inhibitory Effect of Trametes pubescens Fruiting Body Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antioxidant Activity Assay
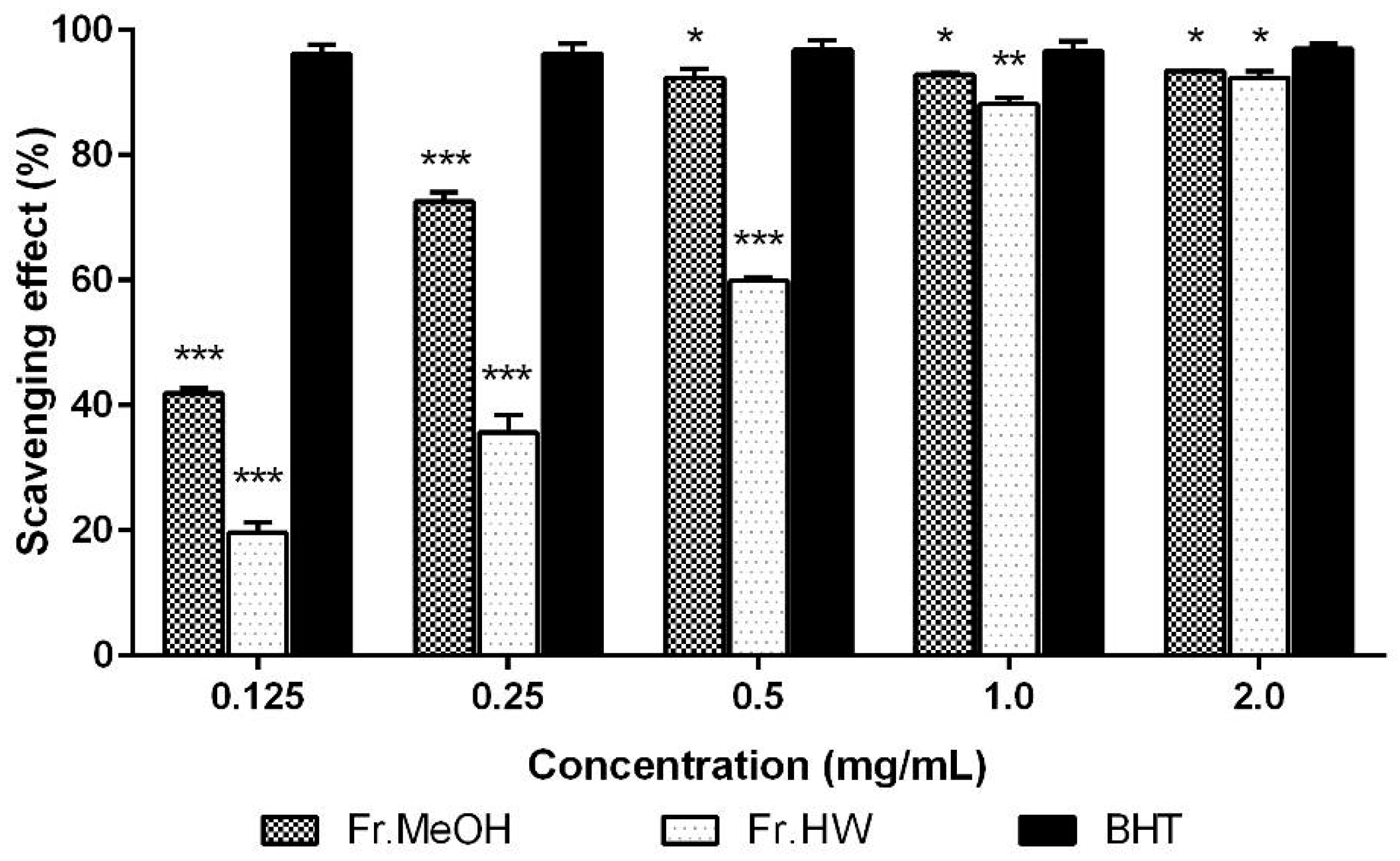
2.1.1. DPPH Radical Scavenging Activity
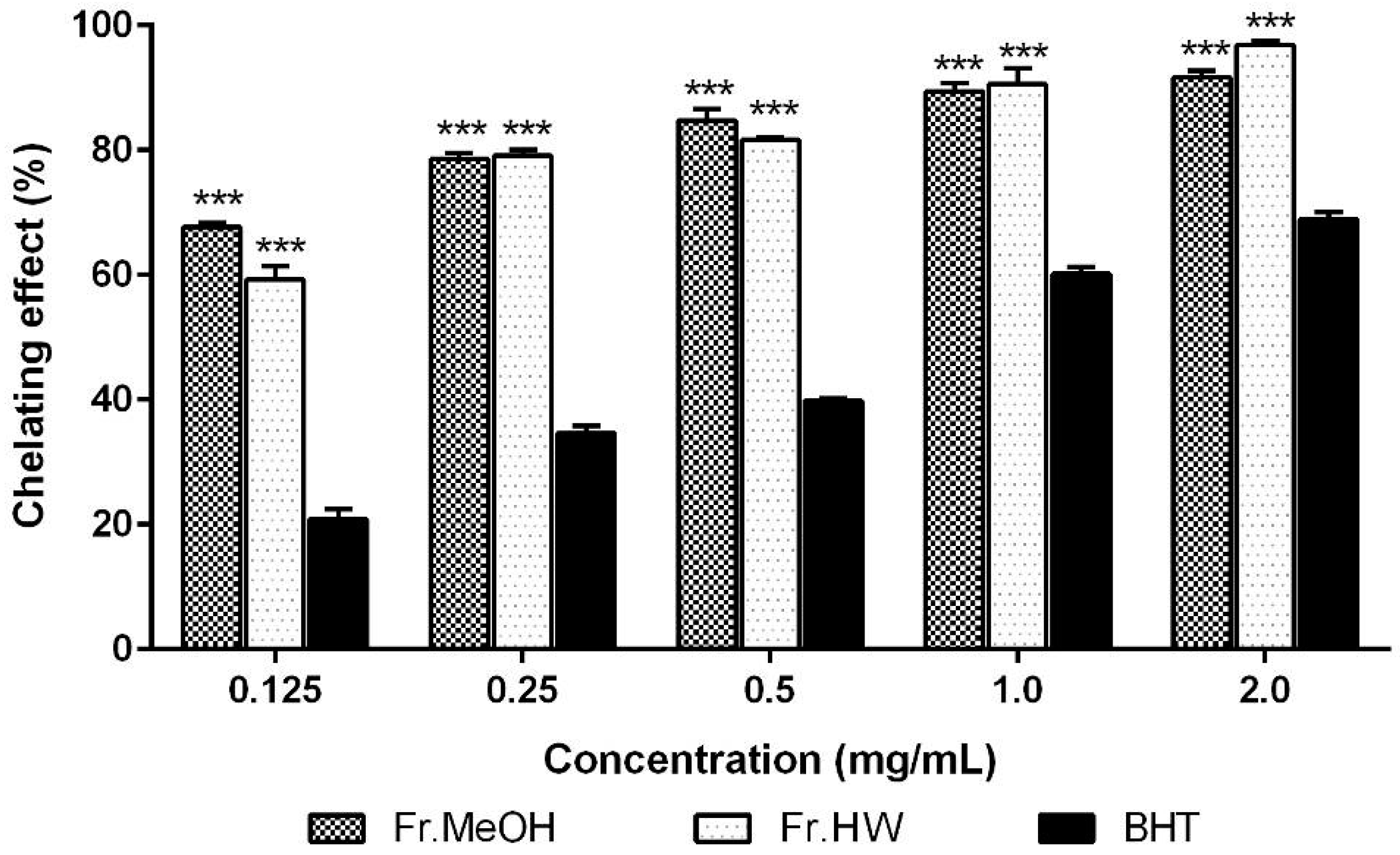
2.1.2. Metal Chelating Effects
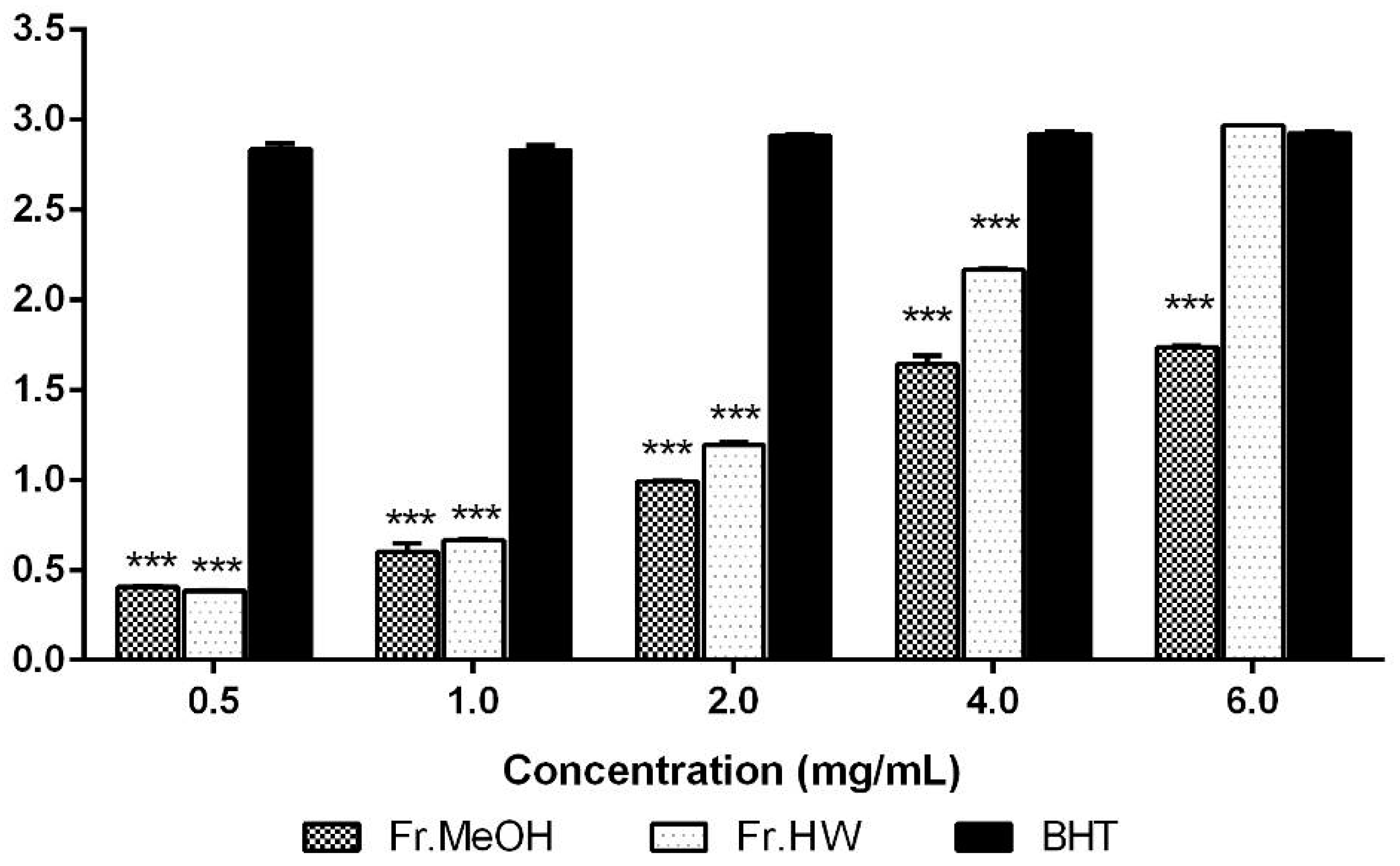
2.1.3. Reducing Power
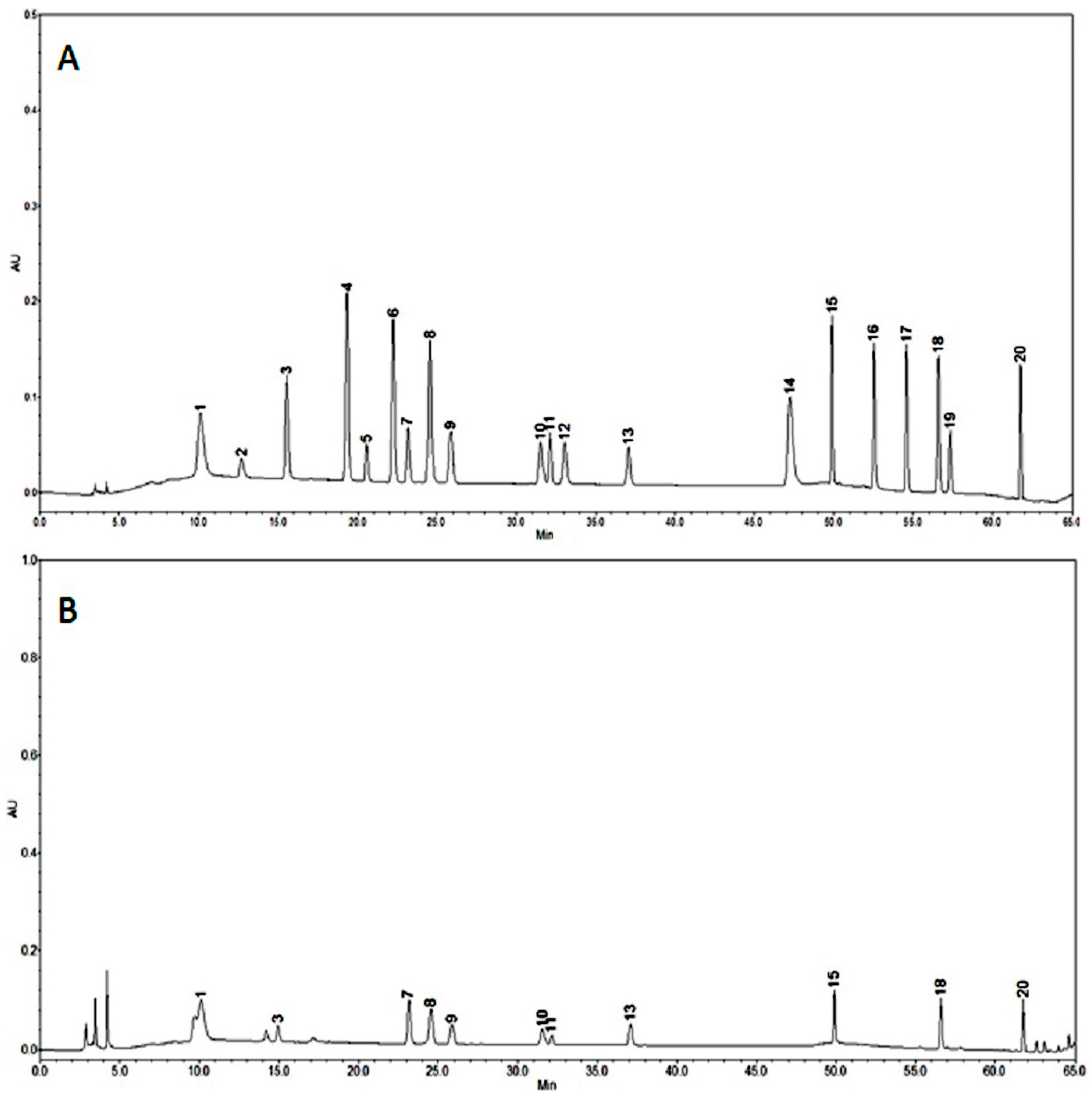
2.2. HPLC Analysis of Phenolic Compounds
2.3. In Vitro Anti-Diabetic Assay
2.3.1. α-Amylase Inhibitory Activity
2.3.2. α-Glucosidase Inhibitory Activity
2.4. Anti-Dementia Assay
2.4.1. Anti-Acetylcholinesterase Activity
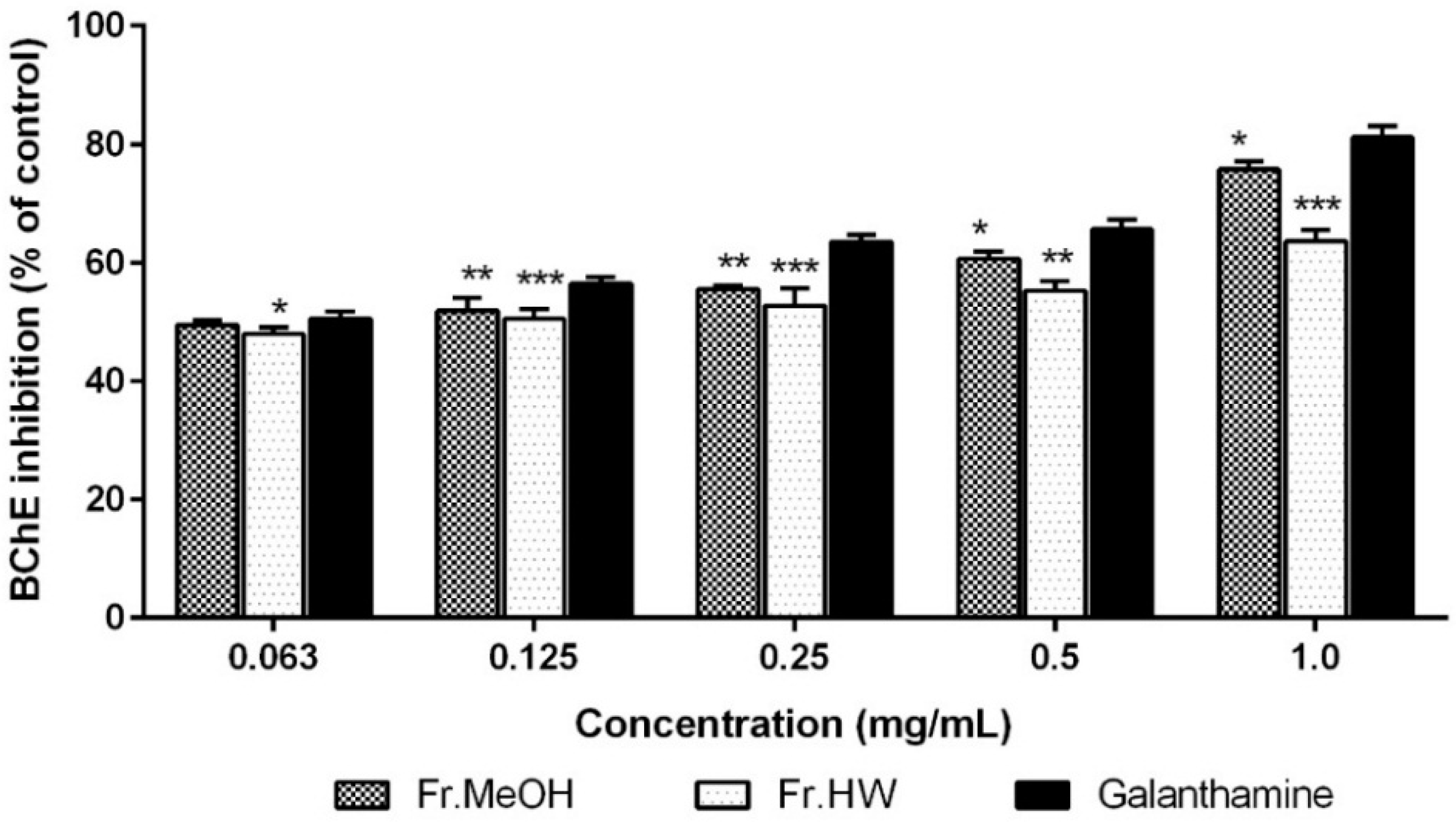
2.4.2. Anti-Butyrylcholinesterase Activity
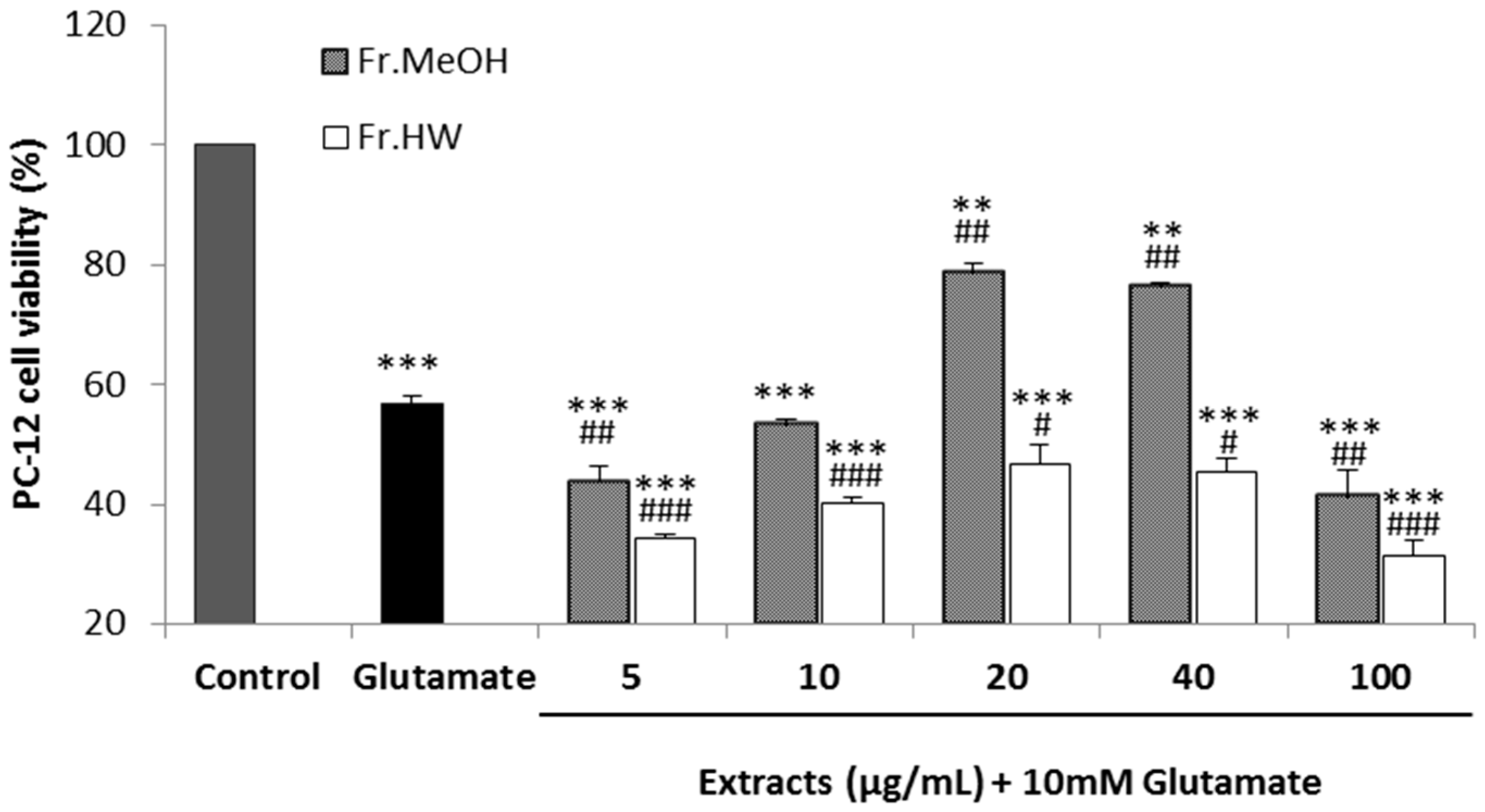
2.4.3. Glutamate-Induced Cytotoxicity
2.5. Inflammation Inhibitory Assay
2.5.1. Production of NO
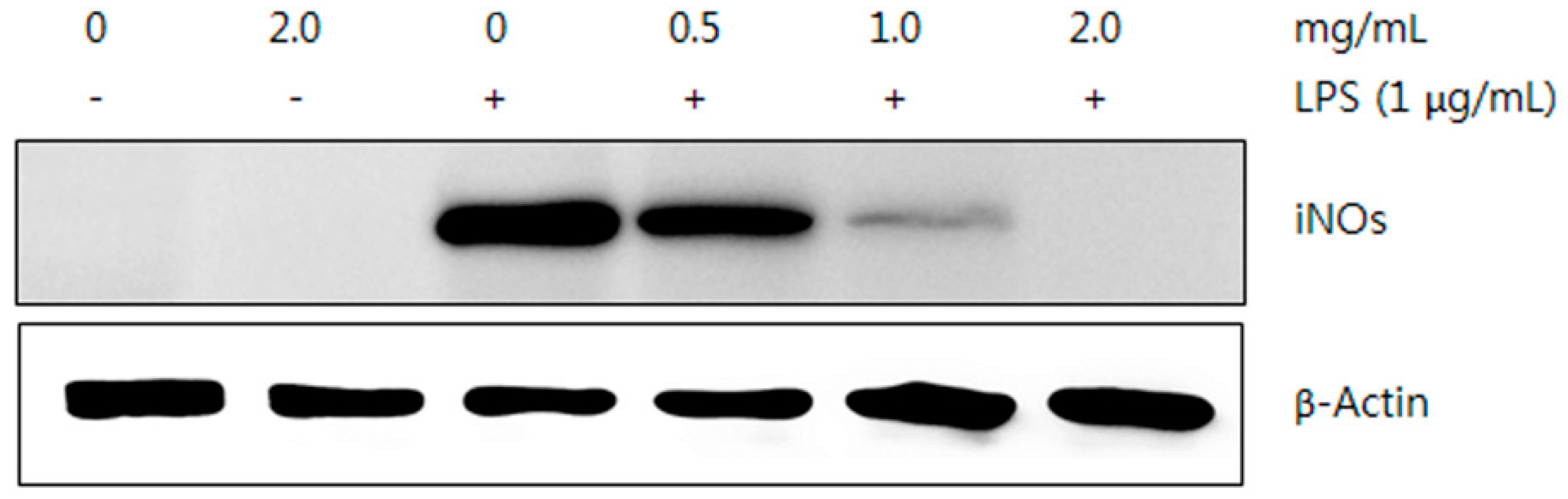
2.5.2. Western Blot Analysis
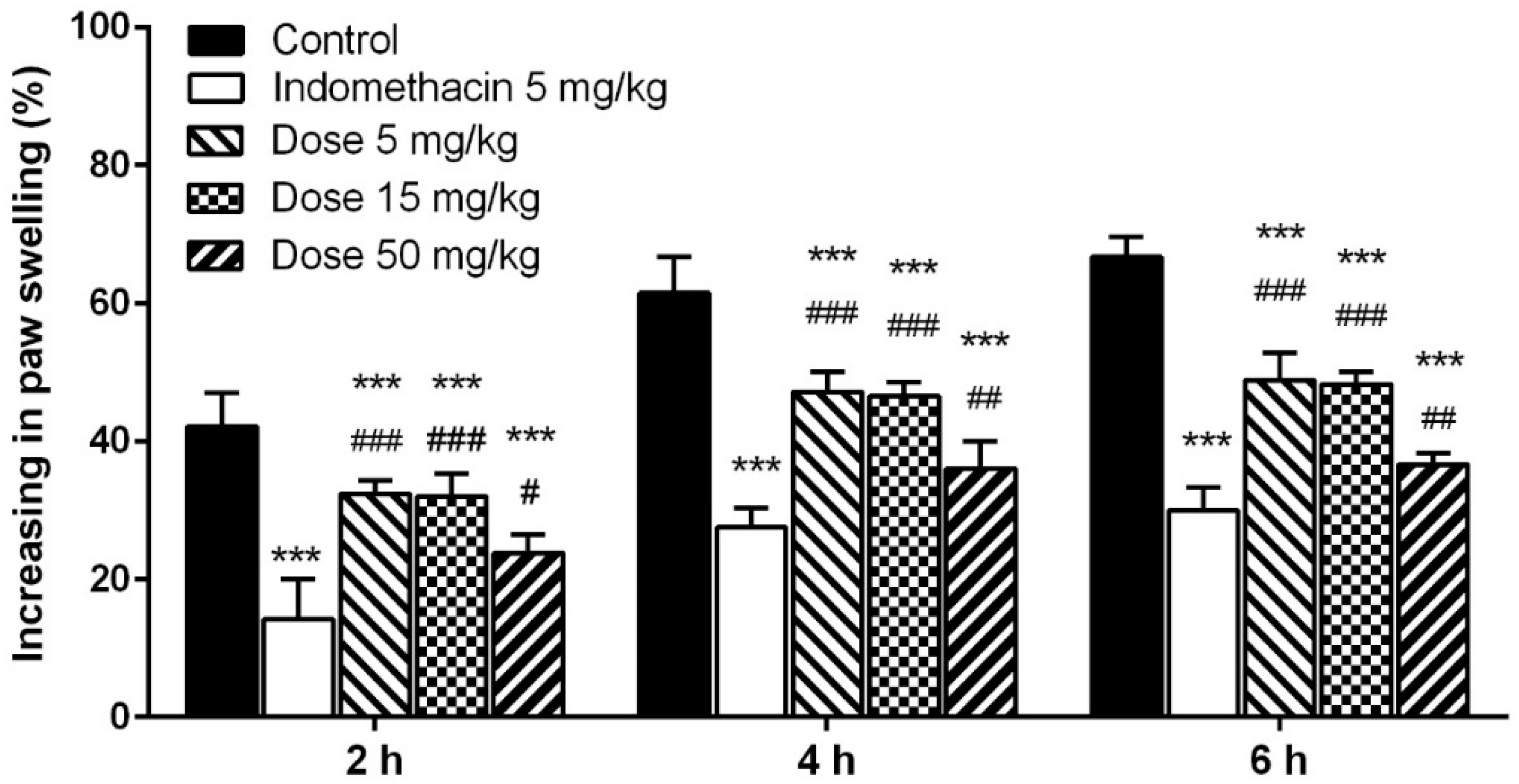
2.5.3. Carrageenan-Induced Paw Edema
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Experimental Animals
3.3. Mushroom Extract
3.4. Antioxidant Activity
3.4.1. DPPH Radical Scavenging
3.4.2. Chelating Effects on Ferrous Ions
3.4.3. Reducing Power Assay
3.5. Phenolic Compounds Analysis by HPLC
3.6. Anti-Diabetic Assay
3.6.1. α-Amylase Inhibition
3.6.2. α-Glucosidase Inhibition
3.7. Anti-Dementia Assay
3.7.1. Anti-Acetycholinesterase Activity
3.7.2. Anti-Butyrylcholinesterase Activity
3.7.3. Glutamate-Induced Cytotoxicity
3.8. Anti-Inflammatory Activities
3.8.1. Inhibitory Effect on NO Production
3.8.2. Western Blot Analysis
3.8.3. Carrageenan-Induced Paw Edema
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Forbes-Hernández, T.Y.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J.L.; Alvarez-Suarez, J.M.; Battino, M. The effects of bioactive compounds from plant foods on mitochondrial function: A focus on apoptotic mechanisms. Food Chem. Toxicol. 2014, 68, 154–182. [Google Scholar] [CrossRef] [PubMed]
- Panicka, K.S.; Anderson, R.A. Effect of polyphenols on oxidative stress and mitochondrial dysfunction in neuronal death and brain edema in cerebral ischemia. Int. J. Mol. Sci. 2011, 12, 8181–8207. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Avarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Gonzàlez-Paramàs, A.M.; Santos-Buelga, C.; Quiles, J.L.; Bompadre, S.; Mezzetti, B.; et al. Polyphenol-rich strawberry extract protects human dermal fibroblasts against hydrogen peroxide oxidative damage and improves mitochondrial functionality. Molecules 2014, 19, 7798–7816. [Google Scholar] [CrossRef] [PubMed]
- Jeena, G.S.; Punecha, H.; Prakash, O.; Chandra, M.; Kushwaha, K.P.S. Study on in vitro antioxidant potential of some cultivated Pleurotus species (Oyster mushroom). Indian J. Nat. Resour. 2014, 5, 56–61. [Google Scholar]
- Yildiz, O.; Can, Z.; Laghari, A.Q.; Sahin, H.; Malkoc, M. Wild edible mushrooms as a natural source of phenolics and antioxidants. J. Food Biochem. 2015, 39, 148–154. [Google Scholar] [CrossRef]
- Patti, M.E.; Corvera, S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr. Rev. 2010, 31, 364–395. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Ferrannini, E.; Holman, R.R.; Sherwin, R.; Zinman, B. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy. Diabetes Care 2009, 32, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.A.; Wright, D.E.; Counsell, C.E.; Zajicek, J. Statistical analysis, trial design and duration in Alzheimer’s disease clinical trials: A review. Int. Psychogeriatr. 2012, 24, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [PubMed]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Moro, C.; Palacios, I.; Lozano, M.; D’Arrigo, M.; Guillamón, E.; Villares, A.; Martínez, J.A.; García-Lafuente, A. Anti-inflammatory activity of methanolic extracts from edible mushrooms in LPS activated RAW 264.7 macrophages. Food Chem. 2012, 130, 350–355. [Google Scholar] [CrossRef]
- Edwards, T. Inflammation, pain, and chronic disease: An integrative approach to treatment and prevention. Altern. Ther. Health Med. 2005, 11, 20–27. [Google Scholar] [PubMed]
- Lopez-Armada, M.; Riveiro-Naveira, R.R.; Vaamonde-Garcia, C.; Valcarcel-Ares, M.N. Mitochondrial dysfunction and the inflammatory response. Mitochondrion 2013, 13, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.K.S.; Lirk, K.; Tan, C.H.; Seymour, R.A. An evidence-based update on nonsteroidal anti-inflammatory drugs. Clin. Med. Res. 2007, 5, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Park, W.H.; Lee, H.D. Illustrated Book of Korean Medicinal Mushrooms; Kyo-Hak Publishing Co. Ltd.: Seoul, Korea, 2003. [Google Scholar]
- Liew, G.M.; Khong, H.Y.; Kutoi, C.J. phytochemical screening, antimicrobial and antioxidant activities of selected fungi from Mount Singai, Sarawak, Malaysia. Int. J. Res. Stud. Biosci. 2015, 3, 191–197. [Google Scholar]
- Si, J.; Cui, B.K. Study of the physiological characteristics of the medicinal mushroom Trametes pubescens (higher basidiomycetes) during the laccase-producing process. Int. J. Med. Mushrooms 2013, 15, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Sumathy, R.; Ajesh, T.P.; Kumuthakalavalli, P. Dpph free scavenging activity and total phenolic content of three species of oyster mushrooms. Indian J. Appl. Res. 2013, 3, 1–3. [Google Scholar] [CrossRef]
- Mau, J.L.; Lin, H.C.; Song, S.F. Antioxidant properties of several specialty mushrooms. Food Res. Int. 2002, 35, 519–526. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Tepe, B.; Semiz, D.K.; Solak, M.H. Evaluation of metal concentration and antioxidant activity of three edible mushrooms from Mugla, Turkey. Food Chem. Toxicol. 2010, 48, 1230–1233. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Yang, M.J.; Hung, L.T.; Lin, L.C. Antioxidant properties of methanol extract of a new commercial gelatinous mushrooms (white variety of Auricularia fuscosuccinea) of Taiwan. Afr. J. Biotechnol. 2013, 12, 6210–6221. [Google Scholar]
- Singh, N.; Rajini, P.S. Free radical scavenging activity of an aqueous extract of potato peel. Food Chem. 2004, 85, 611–616. [Google Scholar] [CrossRef]
- Lee, Y.L.; Huang, G.W.; Liang, Z.C.; Mau, J.L. Antioxidant properties of three extracts from Pleurotus citrinopileatus. LWT Food Sci. Technol. 2007, 40, 823–833. [Google Scholar] [CrossRef]
- Lee, Y.L.; Huang, M.T.; Yen, Z.C.; Mau, J.L. Antioxidant properties of various extracts from Hypsizigus marmoreus. Food Chem. 2007, 104, 1–9. [Google Scholar] [CrossRef]
- Kim, M.Y.; Seguin, P.; Ahn, J.K.; Kim, J.J.; Chun, S.C.; Kim, E.H.; Seo, S.H.; Kang, E.Y.; Kim, S.L.; Park, Y.J.; et al. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J. Agric. Food Chem. 2008, 56, 7265–7270. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, D.; Bousova, L.; Wilhelmova, H. Antioxidant and prooxidant properties of flavonoid. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Marchesan, E. Phenolic compounds and antioxidant activity of rice. Braz. Arch. Biol. Technol. 2011, 54, 371–377. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, B.; Wang, J.; Li, B.; Jiang, Y. Identification of the major flavonoids from pericarp tissues of lychee fruit in relation to their antioxidant activities. Food Chem. 2006, 98, 539–544. [Google Scholar] [CrossRef]
- Santhoshkumar, S.; Nagarajan, N. In vitro antioxidant and antidiabetic activity of methanol extract of wild’ mushroom Ganoderma lucidum (Curtis) P. Karst. Int. J. Biosci. Nanosci. 2014, 1, 77–85. [Google Scholar]
- Pandimeena, M.; Prabu, M.; Sumath, R.; Kumuthakalavalli, R. Evaluation of phytochemicals and in vitro anti-inflammatory, anti-diabetic activity of the white oyster mushroom, Pleurotus florida. Int. Res. J. Pharmaceut. Appl. Sci. 2015, 5, 16–21. [Google Scholar]
- Agarwal, A.A.; Jadhav, P.R.; Deshmukh, Y.A. Prescribing pattern and efficacy of anti-diabetic drugs in maintaining optimal glycemic levels in diabetic patients. J. Basic Clin. Pharm. 2014, 5, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhang, W.; Feng, F.; Zhang, Y.; Kang, W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Human Wellness 2014, 3, 136–174. [Google Scholar] [CrossRef]
- Su, C.H.; Lu, T.M.; Lai, M.N.; Ng, L.T. Inhibitory effects of medicinal mushrooms on α-amylase and α-glucosidase -enzymes related to hyperglycemia. Food Func. 2013, 4, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D. Anticholinesterase activities of selected polyphenols—A short report. Pol. J. Food Nutr. Sci. 2013, 64, 59–64. [Google Scholar] [CrossRef]
- Ahmad, W.; Ahmad, B.; Ahmad, M.; Iqbal, Z.; Nisar, M.; Ahmad, M. In vitro inhibition of acetylcholinesterase, butyrylcholinesterase and lipoxygenase by crude extract of Myricaria elegans Royle. J. Biol. Sci. 2003, 11, 1046–1049. [Google Scholar]
- Orhan, I.; Kartal, M.; Tosun, F.; Sener, B. Screening of various phenolic acids and flavonoid derivatives for their anticholinesterase potential. Z. Naturforschung C 2007, 62, 829–832. [Google Scholar]
- Cong, L.; Cao, C.; Cheng, Y.; Qin, X.Y. Green tea polyphenols attenuated glutamate excitotoxicity via antioxidative and antiapoptotic pathway in the primary cultured cortical neurons. Oxid. Med. Cell. Longev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Song, D.K.; Jung, C.H.; Shin, D.H.; Park, J.; Kwon, T.K.; Jang, B.C.; Mun, K.C.; Kim, S.P.; Suh, S.I.; et al. (−)-Epigallocatechin gallate attenuates glutamate-induced cytotoxicity via intracellular Ca2+ modulation in PC12 cells. Clin. Exp. Pharmacol. Physiol. 2004, 31, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.W.; Tham, C.L.; Isaf, D.A.; Lee, S.H.; Kim, M.K. Neuroprotective effects of biochanin A against glutamate-induced cytotoxicity in PC12 cells via apoptosis inhibition. Neurochem. Res. 2013, 38, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Bechman, J.S.; Laudit, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Kim, S.H.; Sa, J.H.; Jin, C.B.; Lim, C.J.; Park, E.H. Anti-angiogenic and inhibitory activity on inducible nitric oxide production of the mushroom Ganoderma lucidum. J. Ethnopharmacol. 2004, 90, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Won, J.; Kim, J.H.; Choi, Y.H.; Park, W.; Park, H.J.; Lee, K.T. In vivo and in vitro anti-inflammatory and anti-nociceptive effects of the methanol extract of Inonotus obliquus. J. Ethnopharmacol. 2005, 101, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.N.; Mejia, E.G.; Wu, J.W.B. Inhibitory effect of a glycoprotein isolated from golden oyster mushroom (Pleurotus citrinopileatus) on the lipopolysaccharide-induced inflammatory reaction in Raw 264.7 macrophage. J. Agric. Food Chem. 2011, 59, 7092–7097. [Google Scholar] [CrossRef] [PubMed]
- Taofiq, O.; Calhelha, R.C.; Heleno, S.; Barros, L.; Martins, A.; Santos-Buelga, C.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. The contribution of phenolic acids to the anti-inflammatory activity of mushrooms: Screening in phenolic extracts, individual parent molecules and synthesized glucuronated and methylated derivatives. Food Res. Int. 2015, 76, 821–827. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.J.; Tsai, J.Y.; Lai, M.N.; Ng, L.T. Armillariella mellea shows anti-inflammatory activity by inhibiting the expression of NO, iNOS, COX-2 and cytokines in THP-1 cells. Am. J. Chin. Med. 2007, 35, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Posadas, I.; Bucci, M.; Roviezzo, F.; Rossi, A.; Parente, L.; Sautebin, L.; Cirino, G. Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br. J. Pharmacol. 2004, 142, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Galvez, M.; Martin-Cordero, C.; Houghton, P.J.; Ayuso, M.J. Antioxidant activity of Plantago bellardii All. Phytother. Res. 2005, 19, 1074–1076. [Google Scholar] [CrossRef] [PubMed]
- Sowndhararajan, K.; Kang, S.C. Free radical scavenging activity from different extracts of leaves of Bauhinia vahlii Wight & Arn. Saudi J. Biol. Sci. 2013, 20, 319–325. [Google Scholar] [PubMed]
- Gulcin, I.; Buyukokuroglu, M.E.; Oktay, M.; Kufrevioglu, I.O. Antioxidant and analgesic activities of turpentine of Pinus nigraarn. Subsp. Pallsiana (Lamb.) Holmboe. J. Ethnopharmacol. 2003, 86, 51–58. [Google Scholar] [CrossRef]
- Im, K.H.; Nguyen, T.K.; Shin, D.B.; Lee, K.R.; Lee, T.S. Appraisal of antioxidant and anti-inflammatory activities of various extracts from the fruiting bodies of Pleurotus florida. Molecules 2014, 19, 3310–3326. [Google Scholar] [CrossRef] [PubMed]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Sener, B.; Choudhary, M.I.; Khalid, A. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some Turkish medicinal plants. J. Ethnopharmacol. 2004, 91, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Liu, H.; Jiao, H.; Wang, L.; Chen, L.; Liang, J.; Zhao, M.; Zhang, X. Neuroprotective effect of ginkgolide K on glutamate-induced cytotoxicity in PC 12 cells via inhibition of ROS generation and Ca2+ influx. NeuroToxicology 2012, 33, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Cha, D.S.; Lee, Y.J.; Ko, S.H.; Park, H.J.; Lee, S.Y.; Choi, J.H.; Jeon, H. Effects of Vitex rotundifolia on radical scavenging and nitric oxide production. Orient. Pharm. Exp. Med. 2010, 10, 51–58. [Google Scholar] [CrossRef]
- Coruzzi, G.; Adami, M.; Guaita, E.; de Esch, I.J.; Leurs, R. Antiinflammatory and antinociceptive effects of the selective histamine H4-receptor antagonists JNJ7777120 and VUF6002 in a rat model of carrageenan-induced acute inflammation. Eur. J. Pharmacol. 2007, 563, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Fereidoni, M.; Ahmadiani, A.; Semnanian, S.; Javan, M. An accurate and simple method for measurement of paw oedema. J. Pharmacol. Toxicol. Methods 2000, 43, 11–14. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the dried fruitting bodies powder are available from the authors.




© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, K.H.; Nguyen, T.K.; Choi, J.; Lee, T.S. In Vitro Antioxidant, Anti-Diabetes, Anti-Dementia, and Inflammation Inhibitory Effect of Trametes pubescens Fruiting Body Extracts. Molecules 2016, 21, 639. https://doi.org/10.3390/molecules21050639
Im KH, Nguyen TK, Choi J, Lee TS. In Vitro Antioxidant, Anti-Diabetes, Anti-Dementia, and Inflammation Inhibitory Effect of Trametes pubescens Fruiting Body Extracts. Molecules. 2016; 21(5):639. https://doi.org/10.3390/molecules21050639
Chicago/Turabian StyleIm, Kyung Hoan, Trung Kien Nguyen, Jaehyuk Choi, and Tae Soo Lee. 2016. "In Vitro Antioxidant, Anti-Diabetes, Anti-Dementia, and Inflammation Inhibitory Effect of Trametes pubescens Fruiting Body Extracts" Molecules 21, no. 5: 639. https://doi.org/10.3390/molecules21050639




