Identification and Synthesis of (Z,Z)-8,11-Heptadecadienyl Formate and (Z)-8-Heptadecenyl Formate: Unsaturated Aliphatic Formates Found in the Unidentified Astigmatid Mite, Sancassania sp. Sasagawa (Acari: Acaridae)
Abstract
:1. Introduction
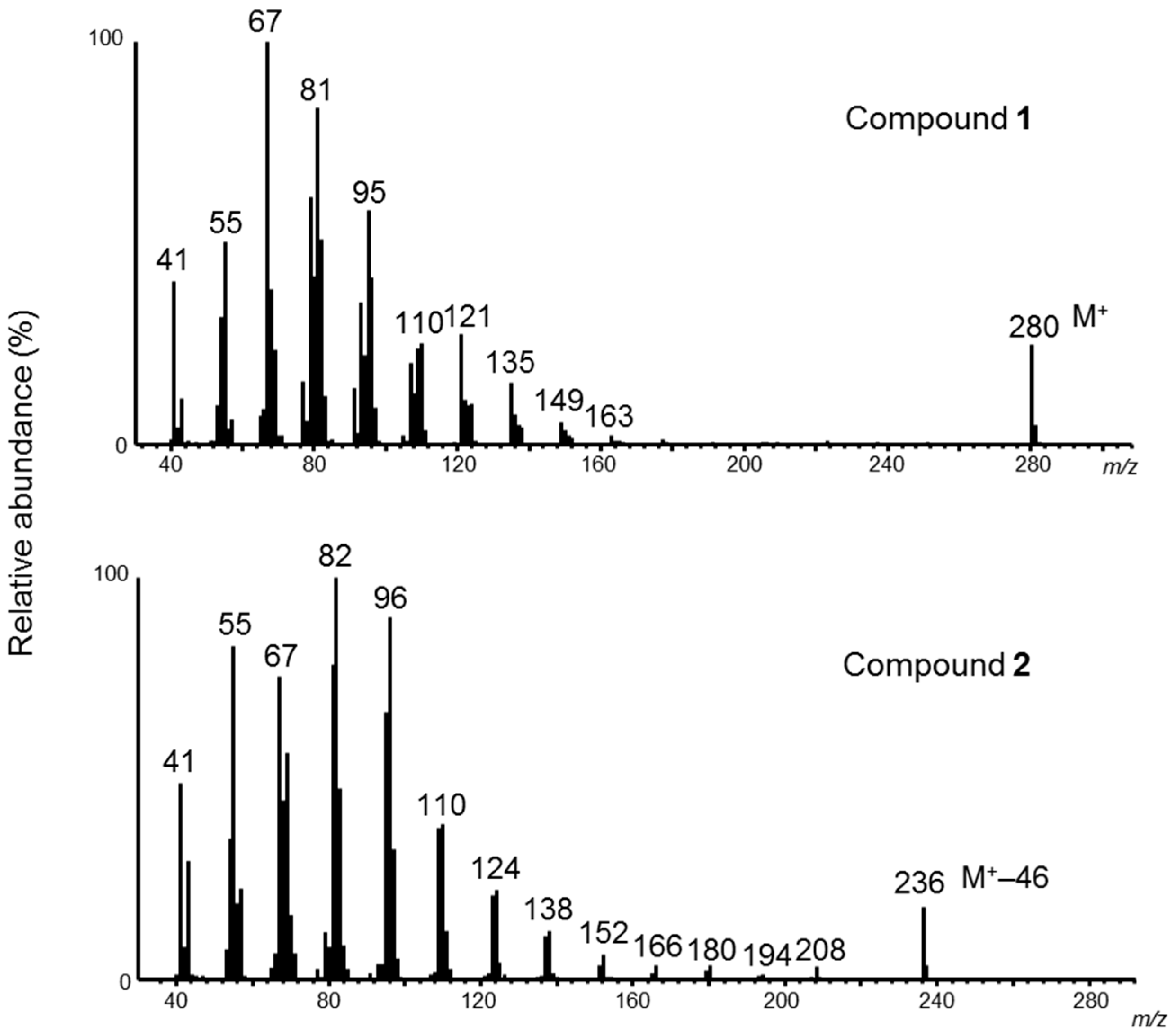
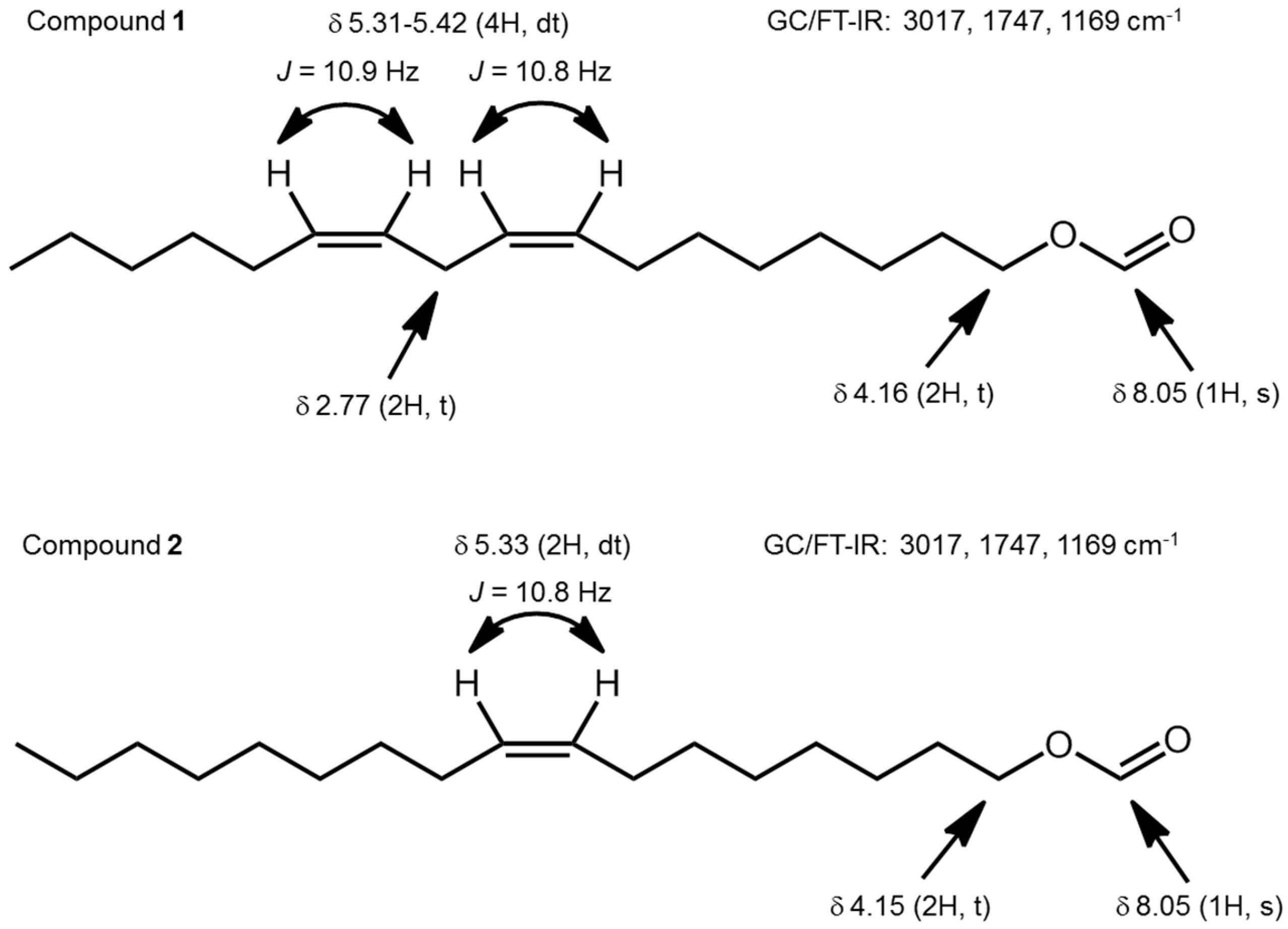
2. Results and Discussion
3. Experimental
3.1. General Procedures
3.2. Mites
3.3. Extraction and Isolation
3.4. Determination of Double-Bond Positions in Formates 1 and 2
3.5. Synthesis
3.5.1. 4-Methyl-2-thioxothiazol-3(2H)-yl(Z,Z)-9,12-octadecadienoate (3)
3.5.2. 4-Methyl-2-thioxothiazol-3(2H)-yl(Z)-9-octadecenoate (5)
3.5.3. (Z,Z)-8,11-Octadecadienol (4)
3.5.4. (Z)-8-Octadecenol (6)
3.5.5. (Z,Z)-8,11-Heptadecadienyl Formate (1)
3.5.6. (Z)-8-Heptadecenyl formate (2)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuwahara, Y. Chemical ecology of astigmatid mites. In Advances in Insect Chemical Ecology; Cardè, R.T., Millar, J.G., Eds.; Cambridge University Press: Cambridge, UK, 2004; pp. 76–109. [Google Scholar]
- Kuwahara, Y.; Leal, W.S.; Kurosa, K.; Sato, M.; Matsuyama, S.; Suzuki, T. Chemical ecology on astigmatid mites XXXIII. Identification of (Z,Z)-6,9-heptadecadiene in the secretion of Carpoglyphus lactis (Acarina, Carpoglyphidae) and its distribution among astigmatid mites. J. Acarol. Soc. Jpn. 1992, 1, 95–104. [Google Scholar]
- Shimizu, N.; Naito, M.; Mori, N.; Kuwahara, Y. De novo biosynthesis of linoleic acid and its conversion to the hydrocarbon (Z,Z)-6,9-heptadecadiene in the astigmatid mite, Carpoglyphus lactis: Incorporation experiments with 13C-labeled glucose. Insect Biochem. Mol. Biol. 2014, 45, 51–57. [Google Scholar] [PubMed]
- Kuwahara, Y.; Ishii, S.; Fukami, H. Neryl formate: Alarm pheromone of the cheese mite, Tyrophagus putrescentiae (Schrank) (Acarina, Acaridae). Experientia 1975, 31, 1115–1116. [Google Scholar] [CrossRef]
- Yen, L.T.M.; Wada, Y.; Matsumoto, K.; Kuwahara, Y. Pheromone study on acarid mites VI. Demonstration and isolation of an aggregation pheromone in Lardoglyphus konoi Sasa et Asanuma. Jpn. J. Sanit. Zool. 1980, 31, 249–254. [Google Scholar]
- Kuwahara, Y.; Yen, L.T.M.; Tominaga, Y.; Matsumoto, K.; Wada, Y. 1,3,5,7-Tetramethyldecyl Formate, Lardolure: Aggregation Pheromone of the Acarid Mite, Lardoglyphus konoi (Sasa et Asanuma) (Acarina: Acaridae). Agric. Biol. Chem. 1982, 46, 2283–2291. [Google Scholar]
- Kuwahara, Y.; Matsumoto, K.; Wada, Y.; Suzuki, T. Chemical ecology on astigmatid mites. XXIX. Aggregation pheromone and kairomone activity of synthetic lardolure (1R,3R,5R,7R)-1,3,5,7-tetramethyldecyl formate and its optical isomers to Lardoglyphus konoi and Carpoglyphus lactis (Acari: Astigmata). Appl. Entomol. Zool. 1991, 26, 85–89. [Google Scholar]
- Vincenti, M.; Gugliemetti, G.; Cassani, G.; Tonini, C. Determination of double bond position in diunsaturated compounds by mass spectrometry of dimethyl disulfide derivatives. Anal. Chem. 1987, 59, 694–699. [Google Scholar] [CrossRef]
- Howard, R.; Kuwahara, Y.; Suzuki, H.; Suzuki, T. Pheromone study on acarid mites. XII. Characterization of the hydrocarbons and external gland morphology of the opisthonotal glands of six species of mites (Acari: Astigmata). Appl. Entomol. Zool. 1988, 23, 58–66. [Google Scholar]
- Kuwahara, Y.; Leal, W.S.; Akimoto, A.; Nakano, Y.; Suzuki, T. Pheromone study on acarid mites. XVI. Identification of hexyl linoleate in acarid mites and its distribution among the genus Tyrophagus. Appl. Entomol. Zool. 1988, 23, 338–344. [Google Scholar]
- Carballeira, N.M.; Shalabi, F.; Cruz, C. Thietane, tetrahydrothiophene and tetrahydrothiopyran formation in reaction of methylene-interrupted dienoates with dimehyl disulfide. Tetrahedron Lett. 1994, 35, 5575–5578. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Crich, D.; Kretzschmar, G. The invention of new radical chain reactions. Part 9. Further radical chemistry of thiohydroxamic esters; Formation of carbon-carbon bonds. J. Chem. Soc. Perkin Trans. 1986, 1, 39–53. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Géro, S.D.; Holliday, P.; Quiclet-Sire, B.; Zard, S.Z. A practical decarboxylative hydroxylation of carboxylic acids. Tetrahedron 1998, 54, 6751–6756. [Google Scholar] [CrossRef]
- Akakabe, Y.; Washizu, K.; Matsui, K.; Kajiwara, T. Concise synthesis of (8Z,11Z,14Z)-8,11,14-heptadecatrienel, (7Z,10Z,13Z)-7,10,13-hextadecatrienel, and (8Z,11Z)-8,11-heptadecadienel, components of the essential oil of marine green alga Ulva pertusa. Biosci. Biotechnol. Biochem. 2005, 69, 1348–1352. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.-P.; Burger, B.V.; Le Roux, M.; Spies, H.S.C. Mammalian exocrine secretion: IX. Constituents of preorbital secretion of oribi, Ourebia, ourebi. J. Chem. Ecol. 1995, 21, 1191–1215. [Google Scholar] [CrossRef] [PubMed]
- Burger, B.V.; Tien, F.-C.; Le Roux, M.; Mo, W.–P. Mammalian exocrine secretions: X. Constituents of preorbital secretion of grysbok, Raphicerus melanotis. J. Chem. Ecol. 1996, 22, 739–764. [Google Scholar] [CrossRef] [PubMed]
- Stander, M.A.; Burger, B.V.; Le Roux, M. Mammalian exocrine secretions. XVII: Chemical characterization of preorbital secretion of male suni, Neotragus, moschatus. J. Chem. Ecol. 2002, 28, 89–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, J.R.; Vanderwel, D.; Choi, S.; Pomonis, J.G.; Reitz, R.C.; Blomquist, G.J. Unusual mechanism of hydrocarbon formation in the housefly: Cytochrome P450 converts aldehyde to the sex pheromone component (Z)-9-tricosene and CO2. PNAS 1994, 91, 10000–10004. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.R.; Quilici, D.R.; Blomquist, G.J.; Reitz, R.C. Proposed mechanism for the cytochrome P450-catalyzed conversion of aldehydes to hydrocarbons in the house fly, Musca domestica. Biochemistry 1995, 34, 16221–16227. [Google Scholar] [CrossRef] [PubMed]
- Mpuru, S.; Reed, J.R.; Reitz, R.C.; Blomquist, G.J. Mechanism of hydrocarbon biosynthesis from aldehyde in selected insect species: Requirement for O2 and NADPH and carbonyl group released as CO2. Insect Biochem. Mol. Biol. 1996, 26, 203–208. [Google Scholar] [CrossRef]
- Qiu, Y.; Tittiger, C.; Wicker-Thomas, C.; Le Goff, G.; Young, S.; Wajnberg, E.; Fricaux, T.; Taquet, N.; Blomquist, G.J.; Feyereisen, R. An insect-specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis. PNAS 2012, 109, 14858–14863. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, Y. Rearing of astigmatid mites by culture medium (in Japanese). J. Acarol. Soc. Jpn. 1999, 8, 58–59. [Google Scholar]
- Sample Availability: Not available.


© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, N.; Sakata, D.; Miyazaki, H.; Shimura, Y.; Kuwahara, Y. Identification and Synthesis of (Z,Z)-8,11-Heptadecadienyl Formate and (Z)-8-Heptadecenyl Formate: Unsaturated Aliphatic Formates Found in the Unidentified Astigmatid Mite, Sancassania sp. Sasagawa (Acari: Acaridae). Molecules 2016, 21, 619. https://doi.org/10.3390/molecules21050619
Shimizu N, Sakata D, Miyazaki H, Shimura Y, Kuwahara Y. Identification and Synthesis of (Z,Z)-8,11-Heptadecadienyl Formate and (Z)-8-Heptadecenyl Formate: Unsaturated Aliphatic Formates Found in the Unidentified Astigmatid Mite, Sancassania sp. Sasagawa (Acari: Acaridae). Molecules. 2016; 21(5):619. https://doi.org/10.3390/molecules21050619
Chicago/Turabian StyleShimizu, Nobuhiro, Daisuke Sakata, Honami Miyazaki, Yasuhiro Shimura, and Yasumasa Kuwahara. 2016. "Identification and Synthesis of (Z,Z)-8,11-Heptadecadienyl Formate and (Z)-8-Heptadecenyl Formate: Unsaturated Aliphatic Formates Found in the Unidentified Astigmatid Mite, Sancassania sp. Sasagawa (Acari: Acaridae)" Molecules 21, no. 5: 619. https://doi.org/10.3390/molecules21050619




