Lignan Glucosides from the Stem Barks of Illicium difengpi
Abstract
:1. Introduction
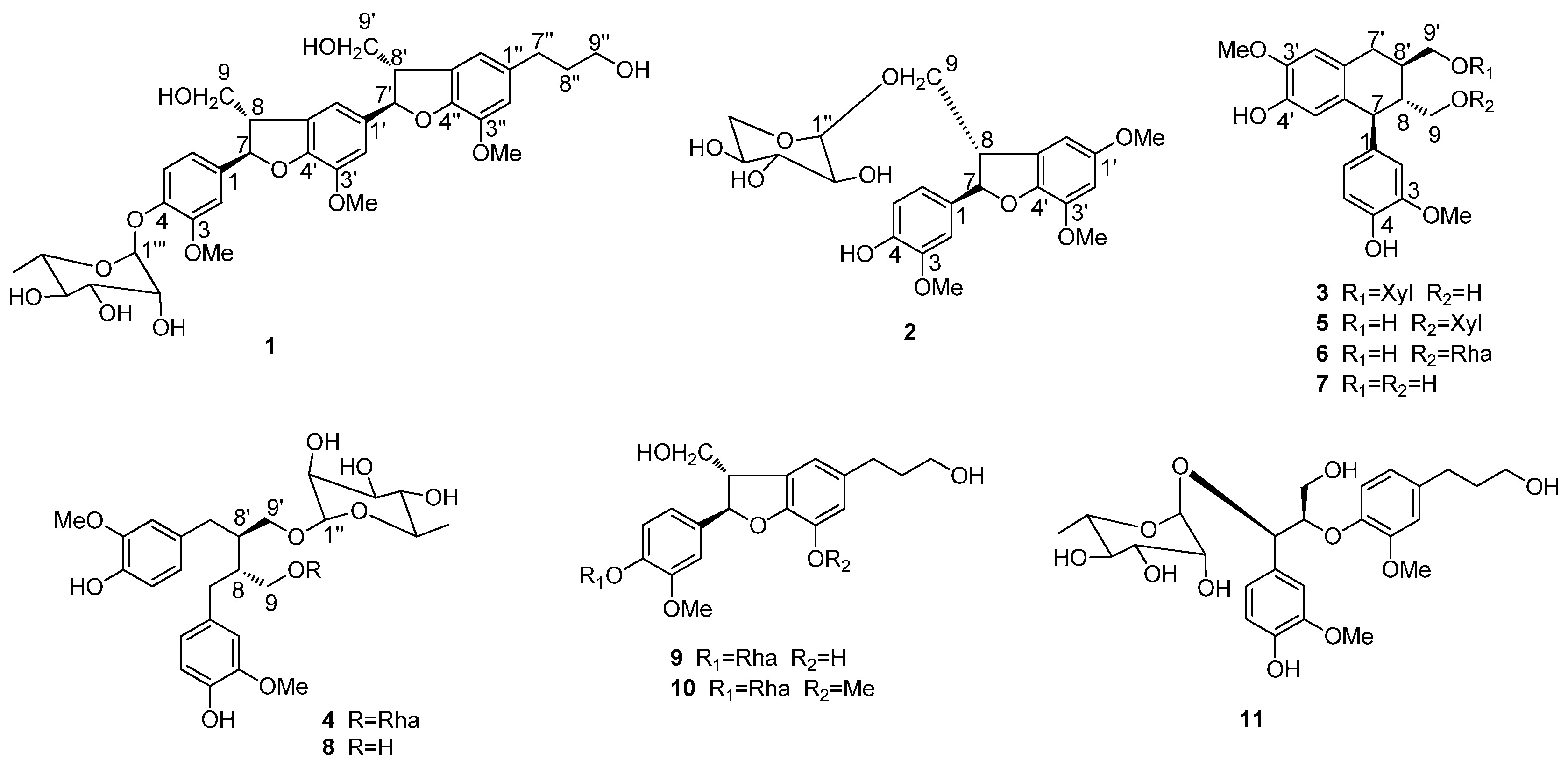
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Compound Characterization
3.5. Determination of the Absolute Configuration of the Sugars in Compounds 1−4
3.6. NO Production Inhibition Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Editorial Committee of Chinese Pharmacopoeia. Chinese Pharmacopoeia, 2015 ed.; China Medical Science Press: Beijing, China, 2015; p. 123. [Google Scholar]
- Huang, P.; Xi, Z.M.; Zheng, X.Z.; Lai, M.X.; Zhong, X.Q. Studies on the chemical constituents of the Traditional Chinese Medicine “DIFENGPI”. Acta Pharm. Sin. 1996, 31, 278–281. [Google Scholar]
- Huang, P.; Xi, Z.M.; Zheng, X.Z.; Lai, M.X.; Zhong, X.Q. Triterpene acids from the barks of Illicium difengpi. Acta Pharm. Sin. 1997, 32, 704–707. [Google Scholar]
- Kouno, I.; Yanagida, Y.; Shimono, S.; Shintomi, M.; Yang, C.S. Phenylpropanoids from the barks of Illicium difengpi. Chem. Pharm. Bull. 1992, 40, 2461–2464. [Google Scholar] [CrossRef]
- Kouno, I.; Yanagida, Y.; Shimono, S.; Shintomi, M.; Ito, Y.; Yang, C.S. Neolignans and a phenylpropanoid glucoside from Illicium difengpi. Phytochemistry 1993, 32, 1573–1577. [Google Scholar] [CrossRef]
- Fang, L.; Du, D.; Ding, G.Z.; Si, Y.K.; Yu, S.S.; Liu, Y.; Wang, W.J.; Ma, S.G.; Xu, S.; Qu, J.; et al. Neolignans and glycosides from the stem barks of Illicium difengpi. J. Nat. Prod. 2010, 73, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Wang, X.J.; Ma, S.G.; Yu, S.S. A new sesquiterpene lactone and a new aromatic glycoside from Illicium difengpi. Acta Pharm. Sin. B 2011, 1, 178–183. [Google Scholar] [CrossRef]
- Gu, Q.; Zhang, X.M.; Zhou, J.; Qiu, S.X.; Chen, J.J. One new dihydrobenzofuran lignan from Vitex trifolia. J. Asian Nat. Prod. Res. 2008, 10, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Iliefski, T.; Lundquist, K.; Wallis, A.F.A. Reassignment of relative stereochemistry at C-7 and C-8 in arylcoumaran neolignans. Phytochemistry 1997, 46, 929–934. [Google Scholar] [CrossRef]
- Kim, T.H.; Ito, H.; Hayashi, K.; Hasegawa, T.; Machiguchi, T.; Yoshida, T. Aromatic constituents from the heartwood of Santalum album L. Chem. Pharm. Bull. 2005, 53, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Ma, Y.P.; Yuan, J.Z.; Sun, Q.S. Isolation and identification of lignans from pine needles of Pinus koraiensis Sieb. et Zucc. J. Shenyang Pharm. Univ. 2010, 27, 797–802. [Google Scholar]
- Baderschneider, M.; Winterhalter, P. Isolation and characterization of novel benzoates, cinnamates, flavonoids, and lignans from Riesling wine and screening for antioxidant activity. J. Agric. Food Chem. 2001, 49, 2788–2798. [Google Scholar] [CrossRef] [PubMed]
- Bu, P.B.; Li, Y.R.; Jiang, M.; Wang, X.L.; Wang, F.; Lin, S.; Zhu, C.G.; Shi, J.G. Lignans from Machilus robusta. Chin. J. Chin. Mater. Med. 2013, 38, 1740–1746. [Google Scholar]
- Qiu, S.X.; Lu, Z.Z.; Luyengi, L.; Lee, S.K.; Pezzuto, J.M.; Farnsworth, N.R.; Thompson, L.U.; Fong, H.H.S. Isolation and characterization of Flaxseed (Linum usitatissimum) constituents. Pharm. Biol. 1999, 37, 1–7. [Google Scholar] [CrossRef]
- Xie, L.H.; Akao, T.; Hamasaki, K.; Deyama, T.; Hattori, M. Biotransformation of pinoresinol diglucoside to mammalian lignans by human intestinal microflora, and isolation of enterococcus faecalis strain PDG-1 responsible for the transformation of (1)-pinoresinol to (1)-lariciresinol. Chem. Pharm. Bull. 2003, 51, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Zhang, X.M.; Shi, Y.; Jiang, Z.Y.; Ma, Y.B.; Chen, J.J. Chemical constituents from rhizomes of Illicium henryi. Chin. J. Chin. Mater. Med. 2010, 35, 2281–2284. [Google Scholar]
- Chen, X.C.; Jia, Z.J. Two new glycosides from Rubus amabilis. Chinese Chem. Lett. 2000, 11, 897–900. [Google Scholar]
- Liu, J.F.; Jiang, Z.Y.; Geng, C.A.; Zhang, Q.; Shi, Y.; Ma, Y.B.; Zhang, X.M.; Chen, J.J. Two new lignans and anti-HBV constituents from Illicium henryi. Chem. Biodivers. 2011, 8, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, J.F., Jr.; Lancaster, J.R., Jr.; Feldman, P.L. Nitric oxide: A new paradigm for second messengers. J. Med. Chem. 1995, 38, 4343–4362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, L.; Huang, J.; Liu, M.S.; Li, G.Y.; Zhang, C.; Zhang, K.; Wang, J.H. 3, 4-seco-Labdane diterpenoids from the leaves of Callicarpa nudiflora and their inhibitory effects on nitric oxide production. Fitoterapia 2013, 89, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1−11 are not available from the authors.

| No. | δC | δH | No. | δC | δH |
|---|---|---|---|---|---|
| 1 | 138.6 | 1′′ | 137.1 | ||
| 2 | 111.4 | 7.01 d (2.0) | 2′′ | 112.0 | 6.72 s |
| 3 | 152.2 | 3′′ | 145.3 | ||
| 4 | 146.7 | 4′′ | 147.5 | ||
| 5 | 119.7 | 7.07 d (8.5) | 5′′ | 129.9 | |
| 6 | 119.2 | 6.89 dd (8.5, 2.0) | 6′′ | 117.9 | 6.72 s |
| 7 | 88.8 | 5.58 d (6.0) | 7′′ | 32.9 | 2.61 t (7.5) |
| 8 | 55.5 | 3.49 m | 8′′ | 35.8 | 1.80 m |
| 9 | 65.1 | 3.83 m | 9′′ | 62.3 | 3.55 t (6.5) |
| 1′ | 137.0 | 1′′′ | 101.5 | 5.33 d (1.5) | |
| 2′ | 112.0 | 6.92 s | 2′′′ | 72.1 | 4.04 dd (3.5, 2.0) |
| 3′ | 145.6 | 3′′′ | 72.3 | 3.86 m | |
| 4′ | 149.3 | 4′′′ | 73.9 | 3.43 t (9.5) | |
| 5′ | 130.1 | 5′′′ | 70.9 | 3.78 m | |
| 6′ | 115.8 | 6.92 s | 6′′′ | 18.0 | 1.20 d (6.0) |
| 7′ | 89.2 | 5.52 d (6.5) | 3-MeO | 56.5 | 3.80 s |
| 8′ | 55.5 | 3.49 m | 3′-MeO | 56.8 | 3.85 s |
| 9′ | 64.9 | 3.75 m | 3′′-MeO | 56.9 | 3.85 s |
| No. | 2 | 3 | 4 | |||
|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | |
| 1 | 134.7 | 138.6 | 133.4 | |||
| 2 | 110.6 | 6.97 d (1.5) | 113.9 | 6.67 d (2.0) | 113.3 | 6.52 s |
| 3 | 149.0 | 148.9 | 148.9 | |||
| 4 | 147.4 | 145.9 | 145.6 | |||
| 5 | 116.1 | 6.76 d (8.0) | 116.0 | 6.72 d (8.0) | 115.9 | 6.65 d (8.5) |
| 6 | 119.7 | 6.83 dd (8.0, 1.5) | 123.2 | 6.59 dd (8.0, 2.0) | 122.7 | 6.50 d (8.5) |
| 7 | 89.0 | 5.44 d (6.5) | 47.8 | 3.84 br d (11.5) | 36.2 | 2.62 d (7.5) |
| 8 | 52.7 | 3.57 dd (13.5, 6.5) | 48.1 | 1.75 m | 41.6 | 2.05 m |
| 9 | 72.8 | 3.99 dd (9.5, 7.5) 3.81 overlap | 61.6 | 3.35 dd (11.5, 3.0) 3.68 dd (11.5, 3.0) | 69.4 | 3.34 dd (9.5, 5.5) 3.80 dd (9.5, 6.5) |
| 1′ | 151.6 | 128.9 | ||||
| 2′ | 96.1 | 6.56 s | 112.4 | 6.63 s | ||
| 3′ | 144.9 | 147.2 | ||||
| 4′ | 155.6 | 145.2 | ||||
| 5′ | 119.0 | 117.4 | 6.17 s | |||
| 6′ | 111.6 | 7.02 s | 134.1 | |||
| 7′ | 33.7 | 2.81 br d (7.5) | ||||
| 8′ | 37.4 | 2.13 m | ||||
| 9′ | 73.9 | 3.63 dd (10.0, 6.0) 3.90 dd (10.0, 6.0) | ||||
| 1′′ | 105.0 | 4.31 d (7.5) | 105.3 | 4.22 d (7.5) | 102.3 | 4.63 d (1.5) |
| 2′′ | 74.9 | 4.04 dd (8.5, 7.5) | 74.9 | 3.19 m | 72.4 | 3.81 dd (3.5, 1.5) |
| 3′′ | 77.9 | 3.86 dd (9.0, 4.0) | 77.8 | 3.30 m | 72.6 | 3.67 dd (9.5, 3.5) |
| 4′′ | 71.2 | 3.50 m | 71.2 | 3.48 m | 73.9 | 3.36 t (9.5) |
| 5′′ | 66.9 | 3.87 dd (11.0, 5.0) 3.20 d (11.0) | 66.9 | 3.20 m 3.85 br d (11.5) | 70.1 | 3.63 dd (9.5, 6.0) |
| 6′′ | 18.0 | 1.25 d (6.0) | ||||
| 3-MeO | 56.4 | 3.83 s | 56.3 | 3.77 s | 56.3 | 3.73 s |
| 1′-MeO | 56.6 | 3.82 s | ||||
| 3′-MeO | 57.8 | 3.79 s | 56.4 | 3.79 s | 56.3 | 3.73 s |
| Compound | Conc. (μM) | Inhibitory Rate (%) |
|---|---|---|
| 1 | 25 | 7.24 |
| 2 | 25 | 5.69 |
| 3 | 25 | 4.61 |
| 4 | 25 | −0.65 |
| 5 | 25 | 10.53 |
| 6 | 25 | 3.95 |
| 7 | 25 | 3.29 |
| 8 | 25 | 1.32 |
| 9 | 25 | 3.30 |
| 10 | 25 | 4.05 |
| 11 | 25 | 0.66 |
| MG132 a | 0.125 | 91.19 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Z.-H.; Ning, D.-S.; Huang, S.-S.; Cheng, L.; Xia, M.-W.; Peng, L.-Y.; Li, D.-P. Lignan Glucosides from the Stem Barks of Illicium difengpi. Molecules 2016, 21, 607. https://doi.org/10.3390/molecules21050607
Pan Z-H, Ning D-S, Huang S-S, Cheng L, Xia M-W, Peng L-Y, Li D-P. Lignan Glucosides from the Stem Barks of Illicium difengpi. Molecules. 2016; 21(5):607. https://doi.org/10.3390/molecules21050607
Chicago/Turabian StylePan, Zheng-Hong, De-Sheng Ning, Si-Si Huang, Ling Cheng, Meng-Wen Xia, Li-Yan Peng, and Dian-Peng Li. 2016. "Lignan Glucosides from the Stem Barks of Illicium difengpi" Molecules 21, no. 5: 607. https://doi.org/10.3390/molecules21050607





