Anti-Cancer Activity of Solanum nigrum (AESN) through Suppression of Mitochondrial Function and Epithelial-Mesenchymal Transition (EMT) in Breast Cancer Cells
Abstract
:1. Introduction
2. Results
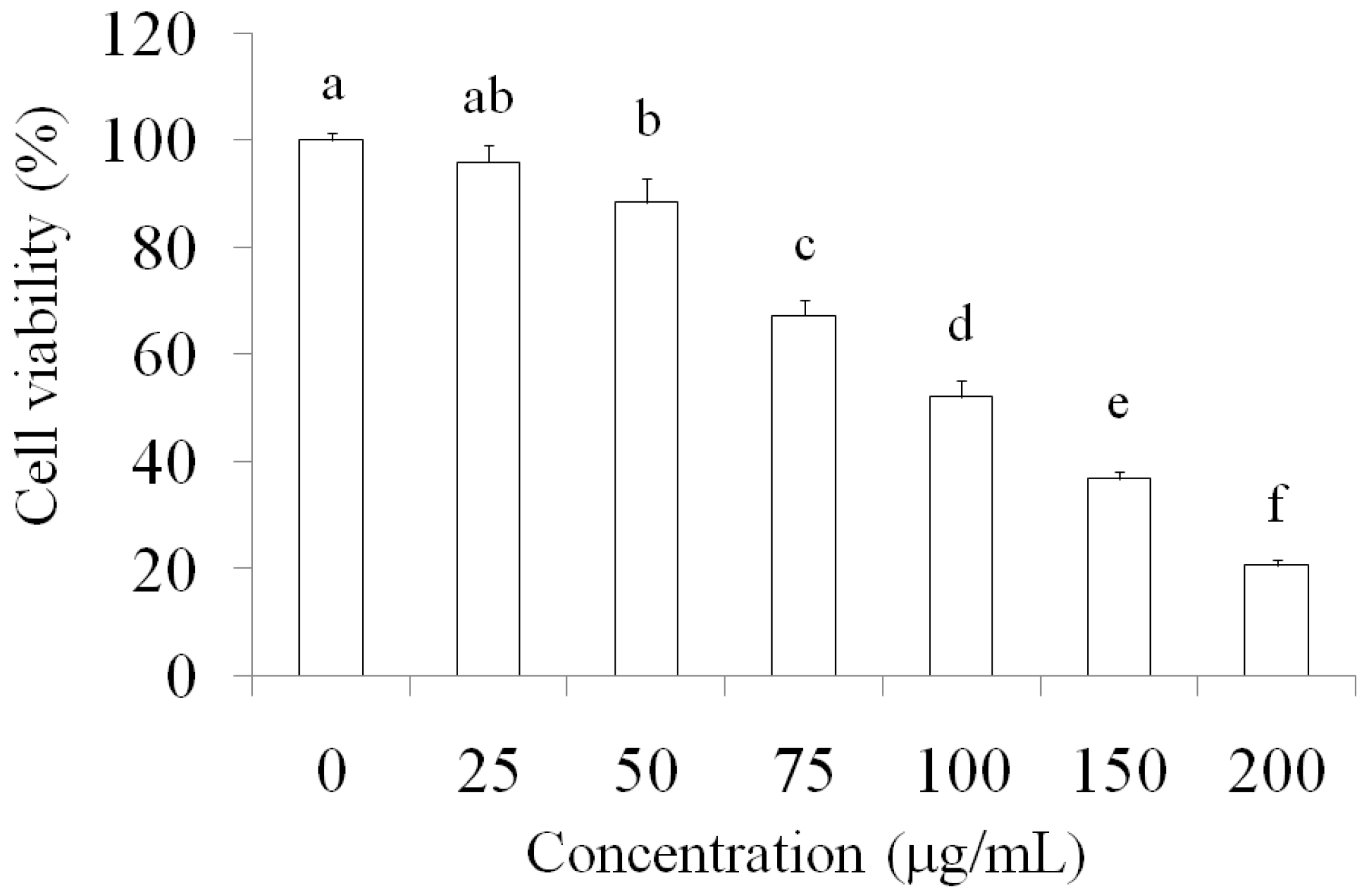
2.1. Suppression of MCF-7 Breast Cancer Cells through AESN Treatment
2.2. Apoptosis Induction by AESN Treatment in MCF-7 Breast Cancer Cells
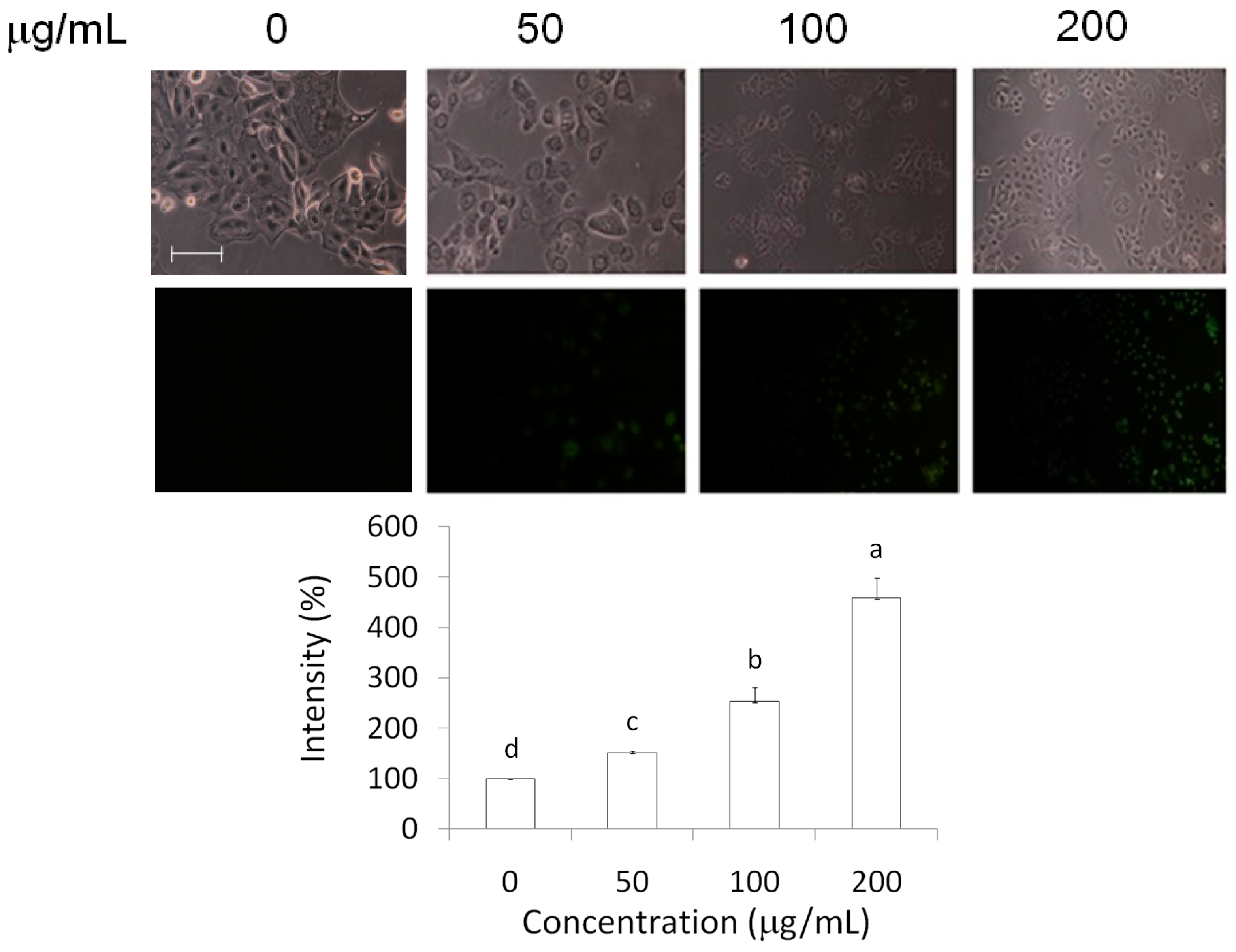
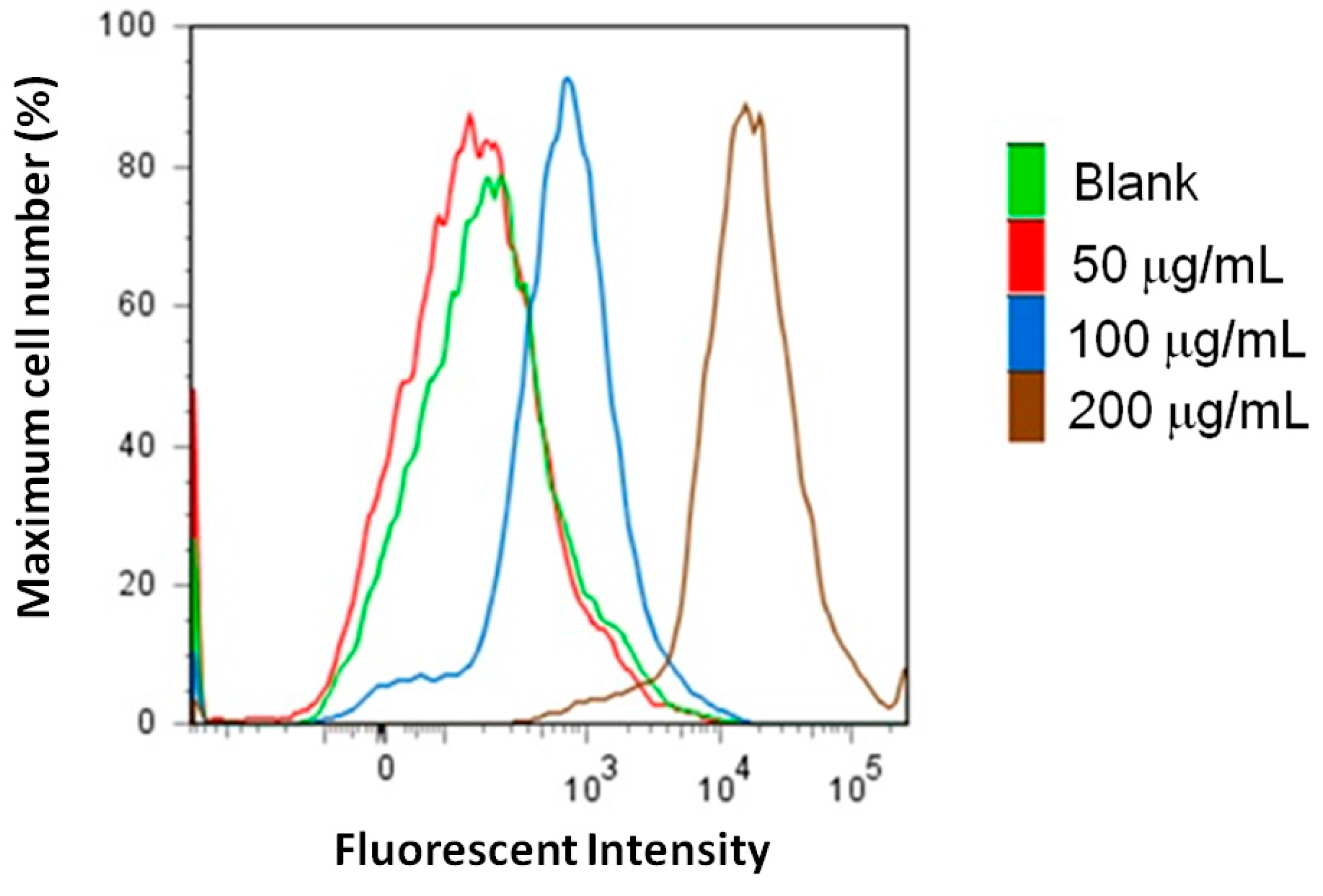
2.3. Measurements of Caspase-3 and Reactive Oxygen Species (ROS) Level
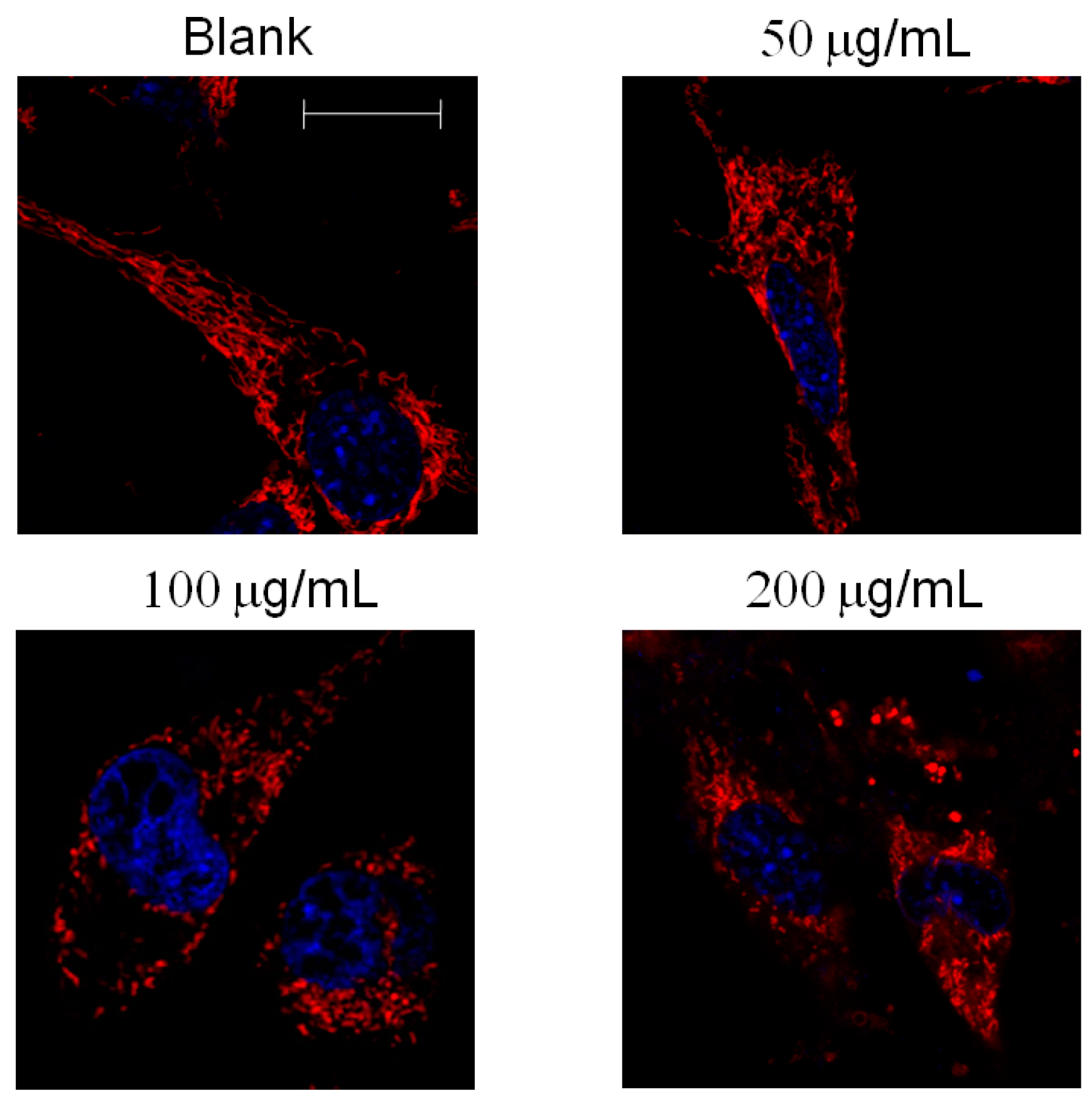
2.4. Mitochondrial Morphology
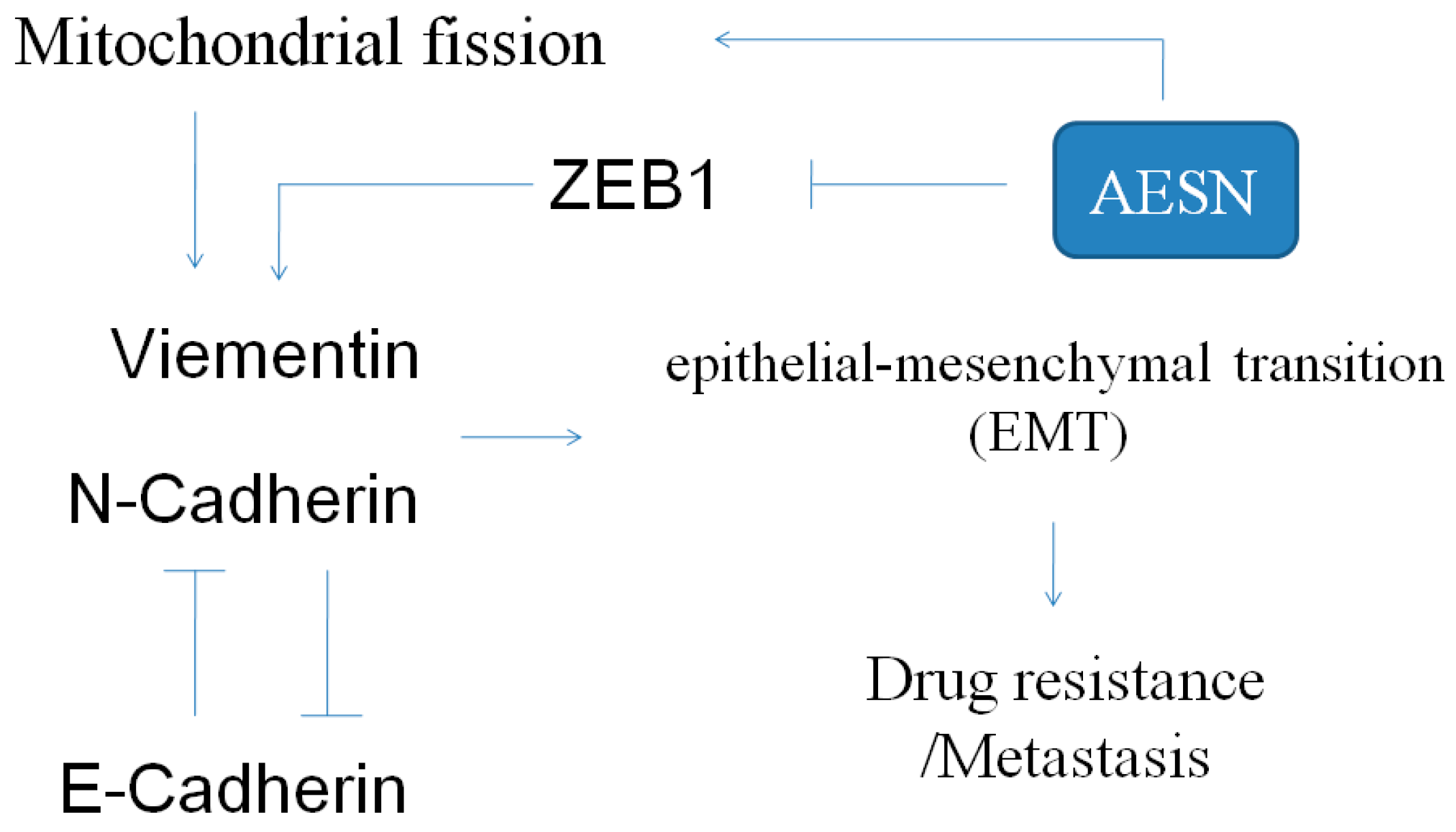
2.5. EMT Marker
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Cell Viablitiy
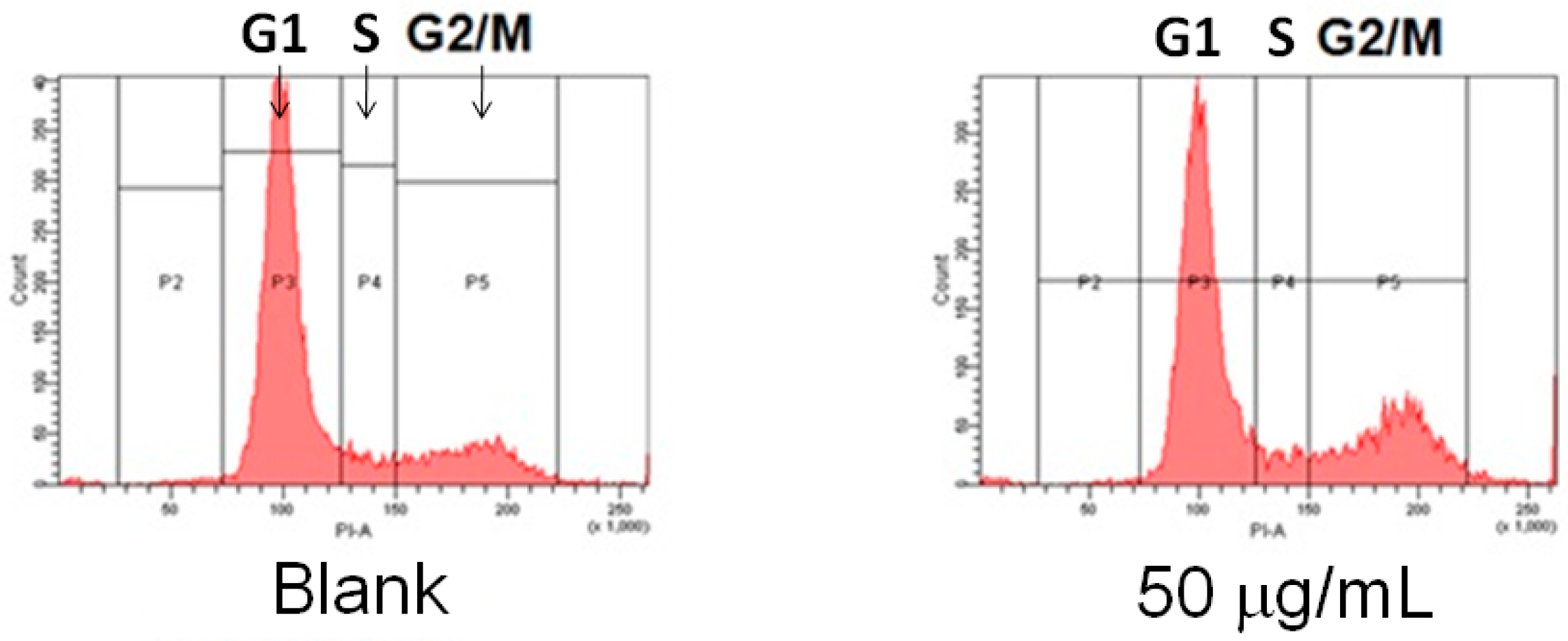
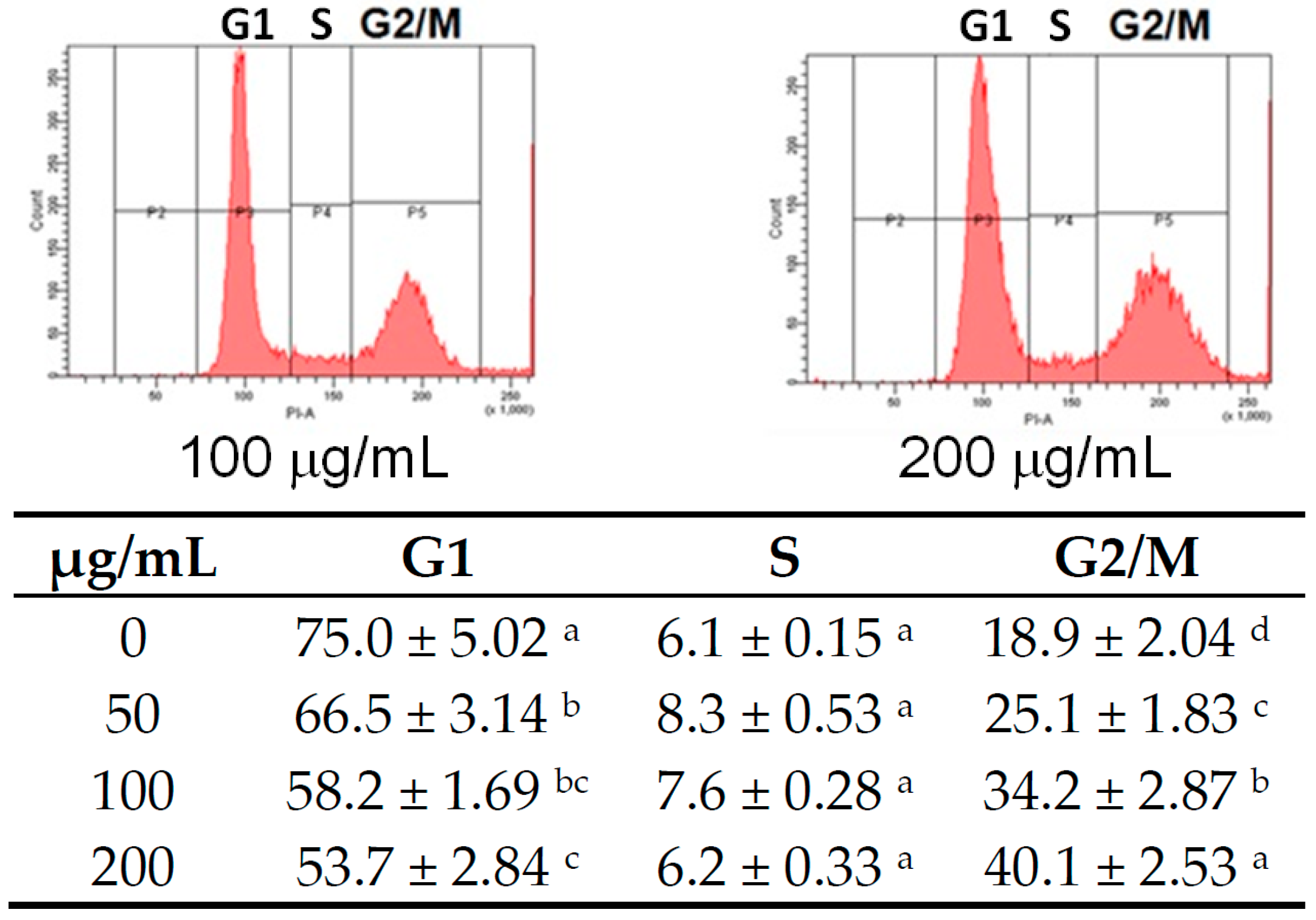
4.4. Cell Cycle
4.5. Apoptosis
4.6. Western Blot
4.7. Immune Fluorescent Stain
4.8. ROS Production
4.9. Mitochondrial Fission
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AESN | aqueous extract of Solanum nigrum |
| DCFH-DA | dichlorodihydrofluorescin diacetate |
| EMT | epithelial-mesenchymal transition |
| FBS | fetal bovine serum |
| PBS | phosphate-buffered saline |
| PI | propidium iodide |
| ROS | reactive oxygen species |
| SDS | sodium dodecyl sulfate |
References
- Wang, H.C.; Wu, D.H.; Chang, Y.C.; Li, Y.J.; Wang, C.J. Solanum nigrum Linn. water extract inhibits metastasis in mouse melanoma cells in vitro and in vivo. J. Agric. Food Chem. 2010, 58, 11913–11923. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Syu, K.Y.; Lin, J.K. Chemical composition of Solanum nigrum linn extract and induction of autophagy by leaf water extract and its major flavonoids in AU565 breast cancer cells. J. Agric. Food Chem. 2010, 58, 8699–8708. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Q.; Feng, T.; Li, K. Aqueous extract of Solanum nigrum inhibit growth of cervical carcinoma (U14) via modulating immune response of tumor bearing mice and inducing apoptosis of tumor cells. Fitoterapia 2008, 79, 548556. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.M.; Tseng, H.C.; Wang, C.J.; Chyau, C.C.; Liao, K.K.; Peng, P.L.; Chou, F.P. Induction of autophagy and apoptosis by the extract of Solanum nigrum Linn in HepG2 cells. J. Agric. Food Chem. 2007, 55, 3620–3628. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.; Lin, Y.F.; Tai, C.J.; Wang, C.W.; Chang, Y.J.; Choong, C.Y.; Lin, C.S.; Tai, C.J.; Chang, C.C. Integrated treatment of aqueous extract of Solanum nigrum-potentiated cisplatin- and doxorubicin-induced cytotoxicity in human hepatocellular carcinoma cells. Evid. Based Complement. Altern. Med. 2015, 2015, 675270. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Chen, C.L.; Wang, C.K.; Chang, Y.J.; Jian, J.Y.; Lin, C.S.; Tai, C.J.; Tai, C.J. Cisplatin-, doxorubicin- and docetaxel-induced cell death promoted by the aqueous extract of Solanum nigrum in human ovarian carcinoma cells. Integr. Cancer Ther. 2015, 14, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.J.; Wang, C.K.; Chang, Y.J.; Lin, C.S.; Tai, C.J. Aqueous extract of Solanum nigrum leaf activates autophagic cell death and enhances docetaxel-induced cytotoxicity in human endometrial carcinoma cells. Evid. Based Complement. Altern. Med. 2012, 2012, 859185. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.J.; Wang, C.K.; Tai, C.J.; Lin, Y.F.; Lin, C.S.; Jian, J.Y.; Chang, Y.J.; Chang, C.C. Aqueous extract of Solanum nigrum leaves induces autophage and enhances cytotoxicity of cisplatin, doxorubicin, docetaxel, and 5-fluorouracil in human colorectal carcinoma cells. Evid. Based Complement. Altern. Med. 2013, 2013, 514719. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Aktas, B.; Tewes, M.; Fehm, T.; Hauch, S.; Kimmig, R.; Kasimir-Bauer, S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009, 11. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Blick, T.; Widodo, E.; Hugo, H.; Waltham, M.; Lenburg, M.E.; Neve, R.M.; Thompson, E.W. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin. Exp. Metastasis 2008, 25, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Lee, B.H.; Pan, T.M. Red mold dioscorea-induced G2/M arrest and apoptosis in human oral cancer cells. J. Sci. Food Agric. 2010, 90, 2709–2715. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Hsu, W.H.; Hsu, Y.W.; Pan, T.M. Dimerumic acid attenuates receptor for advanced glycation endproducts signal to inhibit inflammation and diabetes mediated by Nrf2 activation and promotes methylglyoxal metabolism into d-lactic acid. Free Radic. Biol. Med. 2013, 60, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Lee, B.H.; Pan, T.M. Leptin-induced mitochondrial fusion mediates hepatic lipid accumulation. Int. J. Obes. 2015, 39, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, H.; Wang, X.; Zhao, Y.; Zhao, D.; Wang, C.; Li, Y.; Yang, Z.; Lu, S.; Zeng, Q.; et al. Molecular mechanisms of polyphllin I-induced apoptosis and reversal of the epithelial-mesenchymal transition in human osteosarcoma cells. J. Ethnopharmacol. 2015, 170, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Cooke, V.G.; LeBleu, V.S.; Keskin, D.; Khan, Z.; O’Connell, J.T.; Teng, Y.; Duncan, M.B.; Xie, L.; Maeda, G.; Vong, S.; et al. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell 2012, 21, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, J.T.; Henry, M.D. Epithelial-to-mesenchymal transition in prostate cancer: Paradigm or puzzle? Nat. Rev. Urol. 2011, 8, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.D.; Camp, E.R.; Fan, F.; Shen, L.; Gray, M.J.; Liu, W.; Somcio, R.; Bauer, T.W.; Wu, Y.; Hicklin, D.J.; et al. Vascular endothelial growth factor receptor-1 activation mediates epithelial to mesenchymal transition in human pancreatic carcinoma cells. Cancer Res. 2006, 66, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.R.; Saha, S.; Wang, E.; Robertson, J.D.; Bhattacharya, R.; Mukherjee, P. Inhibition of tumor growth and metastasis by a self-therapeutic nano particle. Proc. Natl. Acad. Sci. USA 2013, 110, 6700–6705. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Dedhar, S.; Kalluri, R.; Thompson, E.W. The epithelial-mesenchymal transition: New insights in signaling, development, and disease. J. Cell Biol. 2006, 172, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Nakagawa, T.; Fukushima, C.; Torii, M.; Sueoka, K.; Nawata, S.; Takeda, O.; Ishikawa, H.; Sugino, N. Relationship between decreased expression of squamous cell carcinoma antigen 2 and E-cadherin in primary cervical cancer lesion and lymph node metastasis. Oncol. Rep. 2008, 19, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, D.J.; Kobayashi, N.; Ruscetti, M.; Zhi, A.; Tran, L.M.; Huang, J.; Gleave, M.; Wu, H. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res. 2012, 72, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Byles, V.; Zhu, L.; Lovaas, J.D.; Chmilewski, L.K.; Wang, J.; Faller, D.V.; Dai, Y. SIRT1 induces EMT by cooperating with EMT transcription factors and enhances prostate cancer cell migration and metastasis. Oncogene 2012, 31, 4619–4629. [Google Scholar] [CrossRef] [PubMed]
- Darai, E.; Scoazec, J.Y.; Walker-Combrouze, F.; Mlika-Cabanne, N.; Feldmann, G.; Madelenat, P.; Potet, F. Expression of cadherins in benign, borderline, and malignant ovarian epithelial tumors: A clinicopathologic study of 60 cases. Hum. Pathol. 1997, 28, 922–928. [Google Scholar] [CrossRef]
- Veatch, A.L.; Carson, L.F.; Ramakrishnan, S. Differential expression of the cell-cell adhesion molecule E-cadherin in ascites and solid human ovarian tumor cells. Int. J. Cancer 1994, 58, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Haslehurst, A.M.; Koti, M.; Dharsee, M.; Nuin, P.; Evans, K.; Geraci, J.; Childs, T.; Chen, J.; Li, J.; Weberpals, J.; et al. EMT transcription factors snail and slug directly contribute to cisplatin resistance in ovarian cancer. BMC Cancer 2012, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.J.; Cheng, C.W.; Su, H.Y.; Chen, W.Y.; Wu, C.T.; Lin, F.Y.; Wang, C.K.; Tai, C.J.; Wei, P.L. Thrombomodulin mediates the migration of cervical cancer cells through the regulation of epithelial-mesenchymal transition biomarkers. Tumour Biol. 2013, 35, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Malfettone, A.; Soukupova, J. New insights into the crossroads between EMT and stemness in the context of cancer. J. Clin. Med. 2016, 5, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.; Srivastava, S.K. HER2 mediated de novo production of TGFbeta leads to SNAIL driven epithelial-to-mesenchymal transition and metastasis of breast cancer. Mol. Oncol. 2014, 8, 1532–1547. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhao, N.; Zhao, X.L.; Gu, Q.; Zhang, S.W.; Che, N.; Wang, X.H.; Du, J.; Liu, Y.X.; Sun, B.C. Expression and functional significance of Twist1 in hepatocellular carcinoma: Its role in vasculogenic mimicry. Hepatology 2010, 51, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Westermann, B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Jeong, S.Y.; Karbowski, M.; Smith, C.L.; Youle, R.J. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol. Biol. Cell 2004, 15, 5001–5011. [Google Scholar] [CrossRef] [PubMed]
- Twig, G.; Elorza, A.; Molina, A.J.; Mohamed, H.; Wikstrom, J.D.; Walzer, G.; Stiles, L.; Haigh, S.E.; Katz, S.; Las, G.; et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008, 27, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.C.; Scorrano, L. Mitochondrial elongation during autophagy: A stereotypical response to survive in difficult times. Autophagy 2011, 7, 1251–1253. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.


© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.-J.; Tai, C.-J.; Wang, C.-W.; Choong, C.-Y.; Lee, B.-H.; Shi, Y.-C.; Tai, C.-J. Anti-Cancer Activity of Solanum nigrum (AESN) through Suppression of Mitochondrial Function and Epithelial-Mesenchymal Transition (EMT) in Breast Cancer Cells. Molecules 2016, 21, 553. https://doi.org/10.3390/molecules21050553
Lai Y-J, Tai C-J, Wang C-W, Choong C-Y, Lee B-H, Shi Y-C, Tai C-J. Anti-Cancer Activity of Solanum nigrum (AESN) through Suppression of Mitochondrial Function and Epithelial-Mesenchymal Transition (EMT) in Breast Cancer Cells. Molecules. 2016; 21(5):553. https://doi.org/10.3390/molecules21050553
Chicago/Turabian StyleLai, Ying-Jang, Chen-Jei Tai, Chia-Woei Wang, Chen-Yen Choong, Bao-Hong Lee, Yeu-Ching Shi, and Cheng-Jeng Tai. 2016. "Anti-Cancer Activity of Solanum nigrum (AESN) through Suppression of Mitochondrial Function and Epithelial-Mesenchymal Transition (EMT) in Breast Cancer Cells" Molecules 21, no. 5: 553. https://doi.org/10.3390/molecules21050553





