Bioconversion of Biomass-Derived Phenols Catalyzed by Myceliophthora thermophila Laccase
Abstract
:1. Introduction
2. Results and Discussion
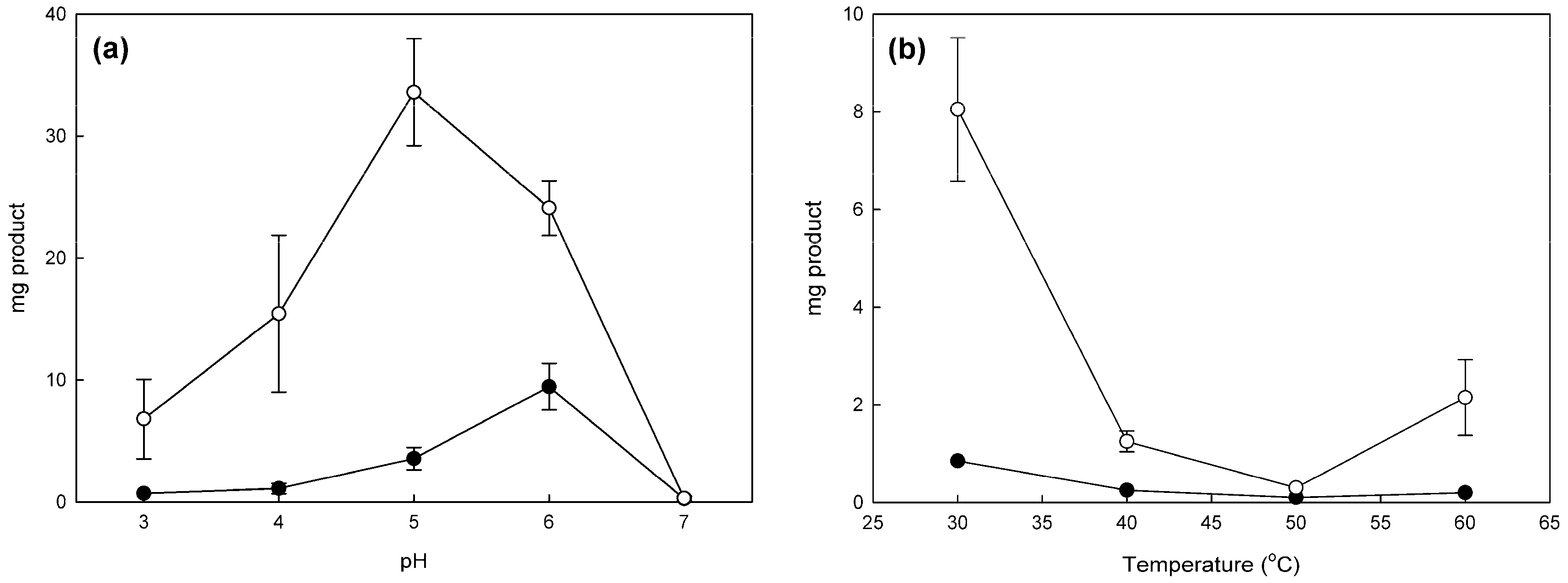
2.1. Effect of pH and Temperature in Phenol Bioconversion
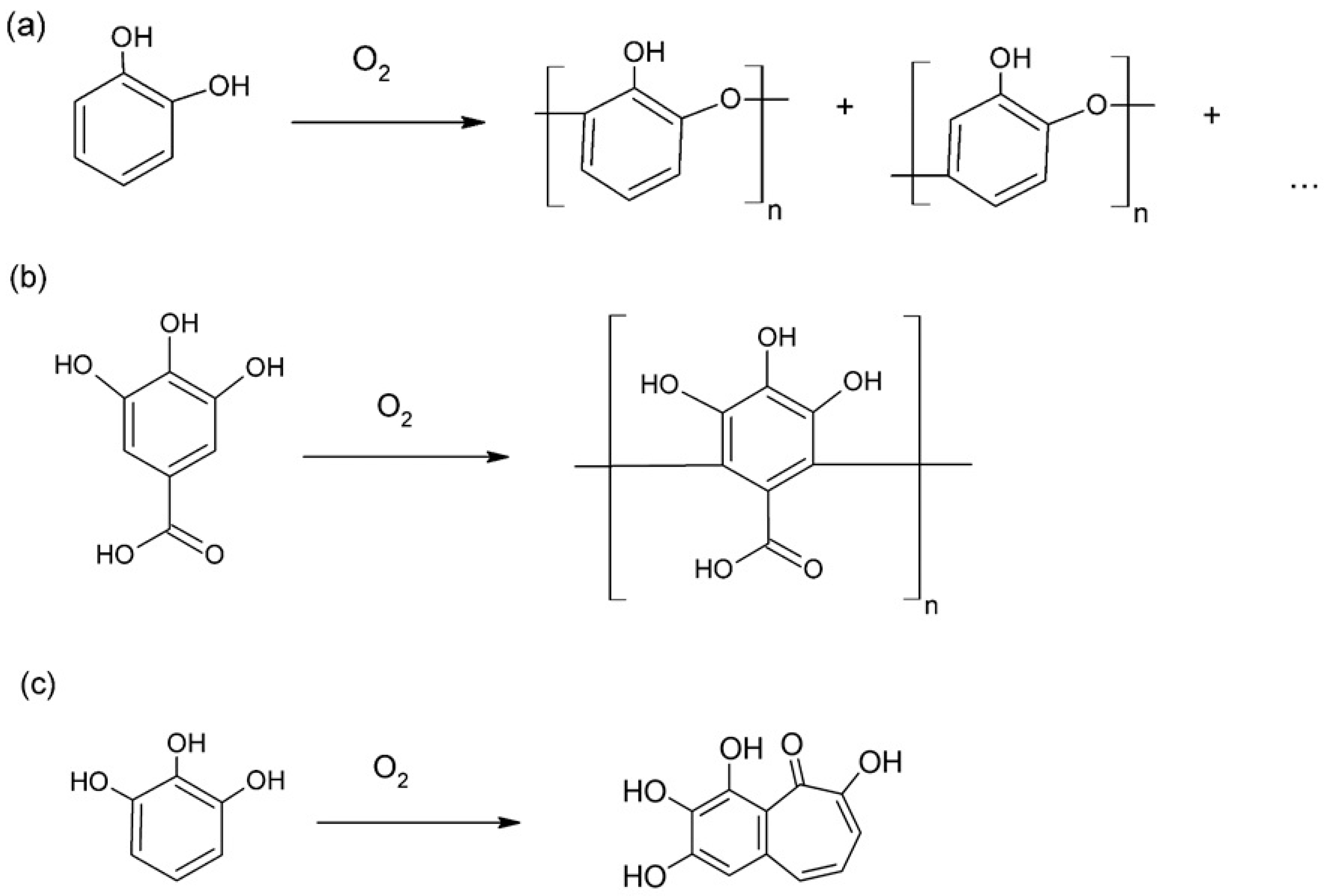
2.2. Preparative Synthesis and Product Characterization
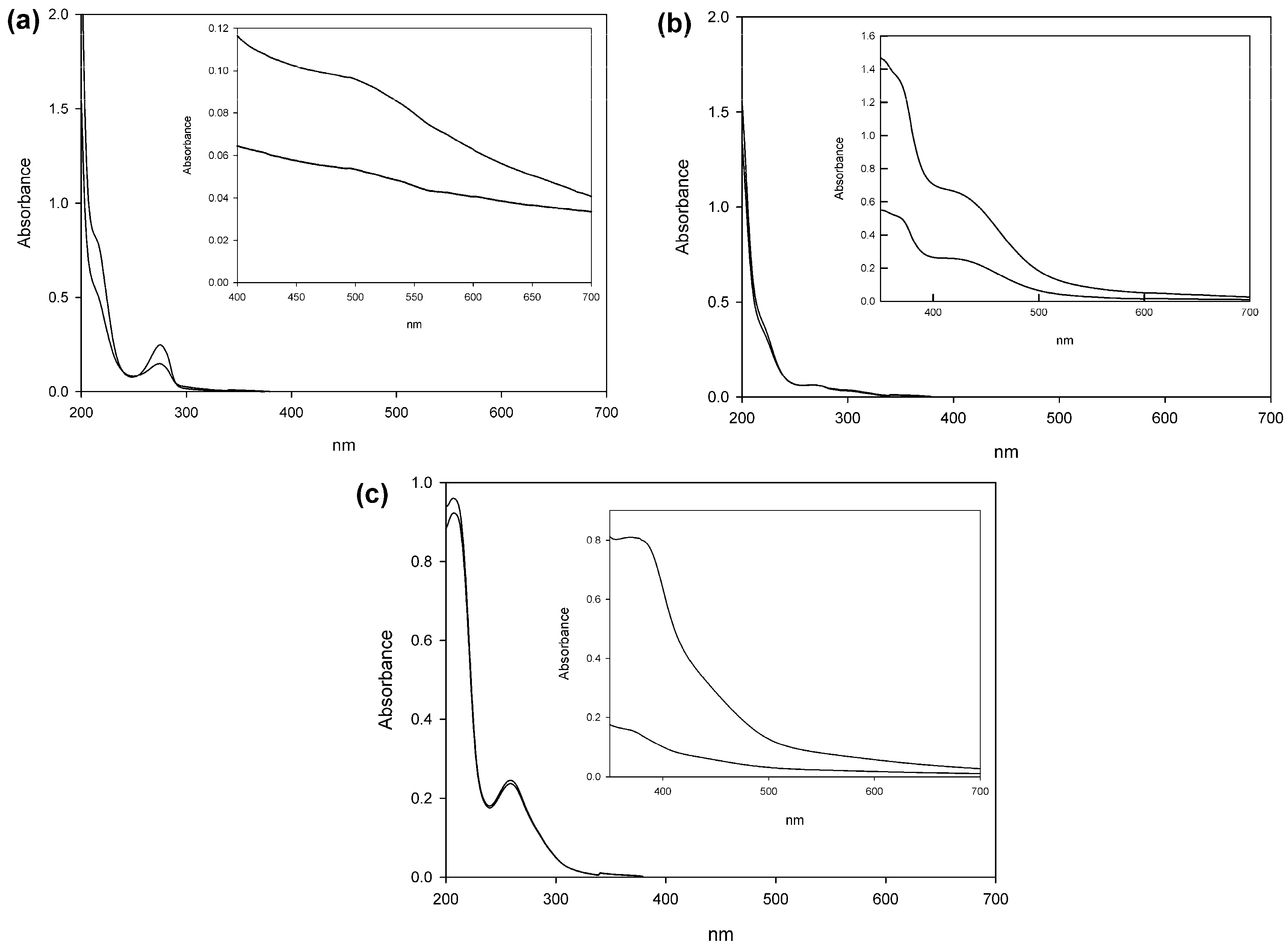
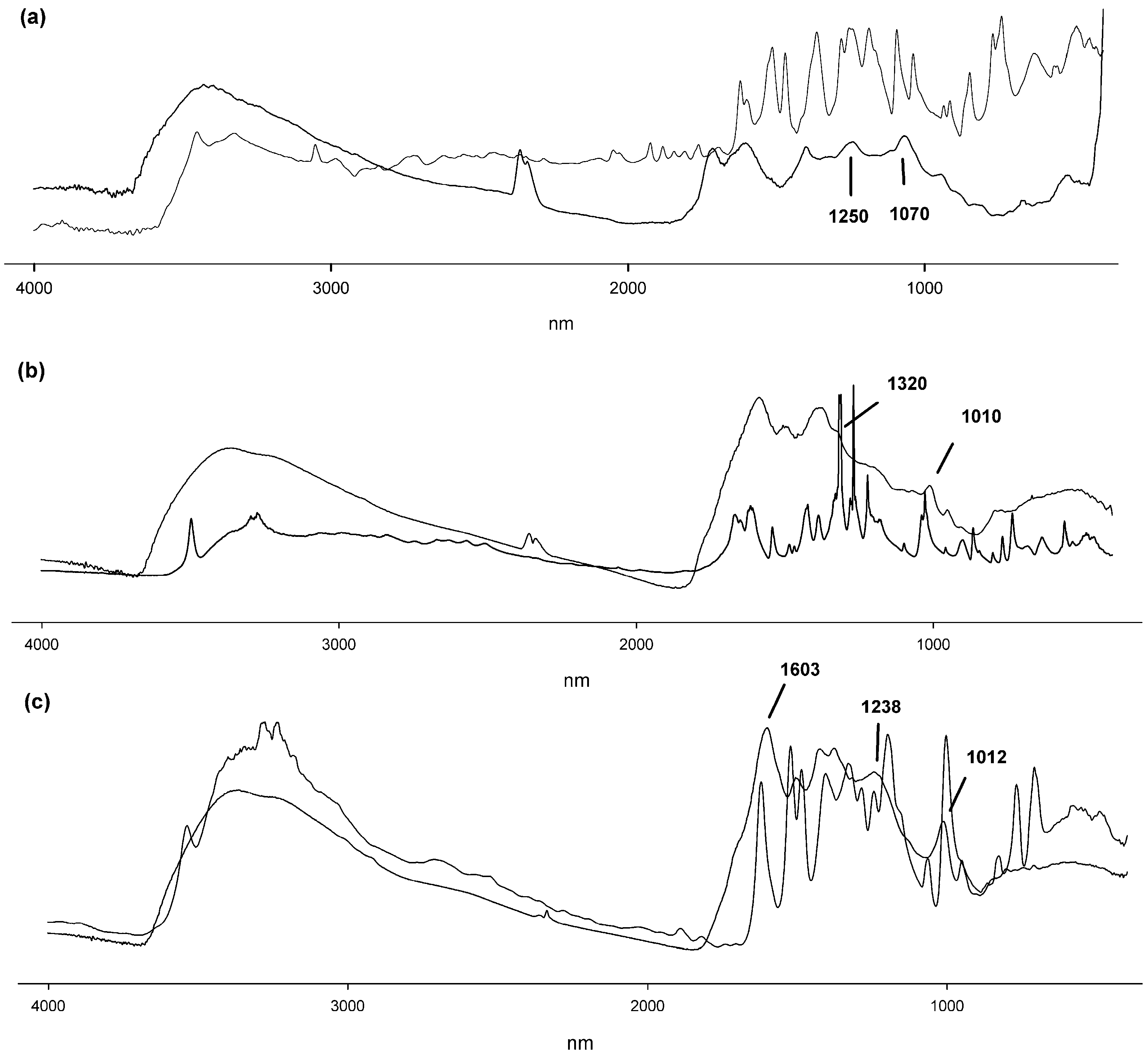
2.3. Spectroscopic Analysis of Products
2.4. Molecular Weight Distributions from GPC and Viscosity Measurements
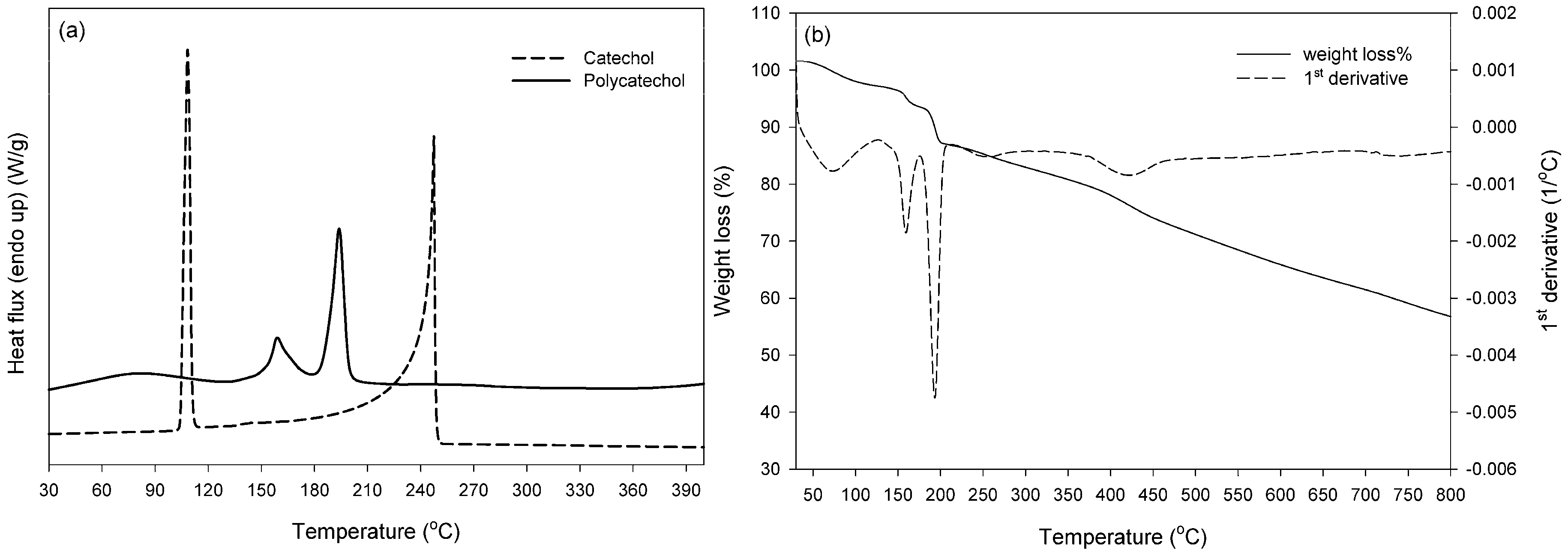
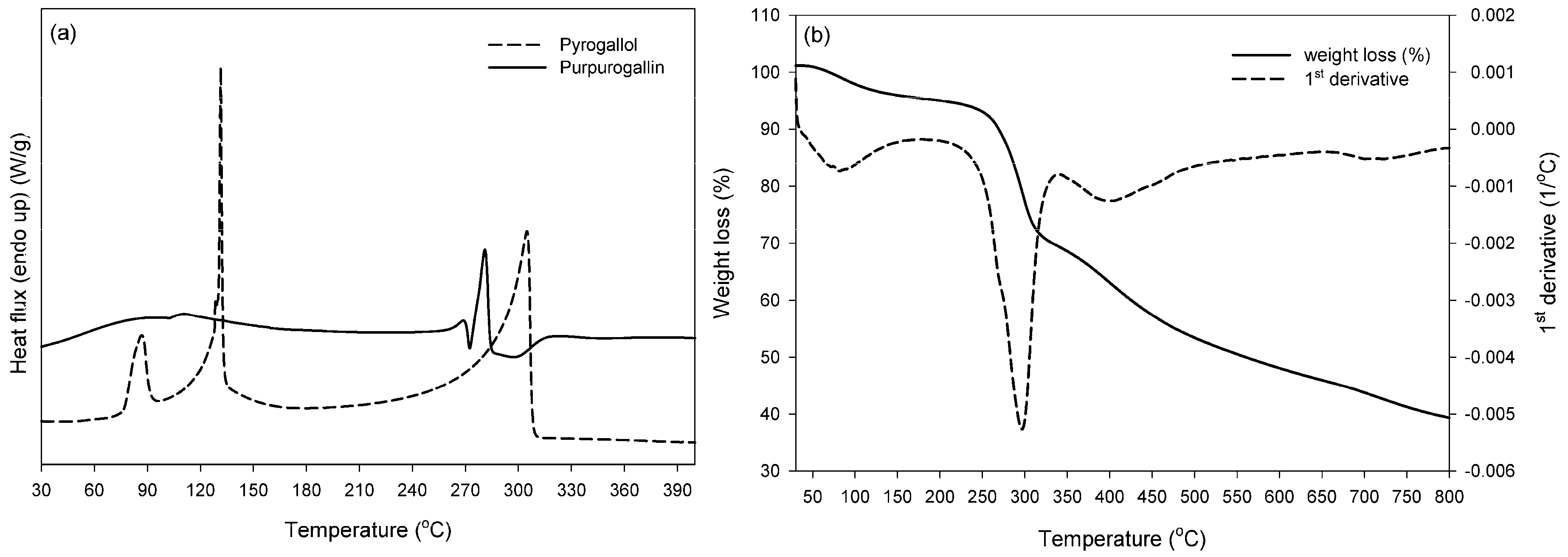
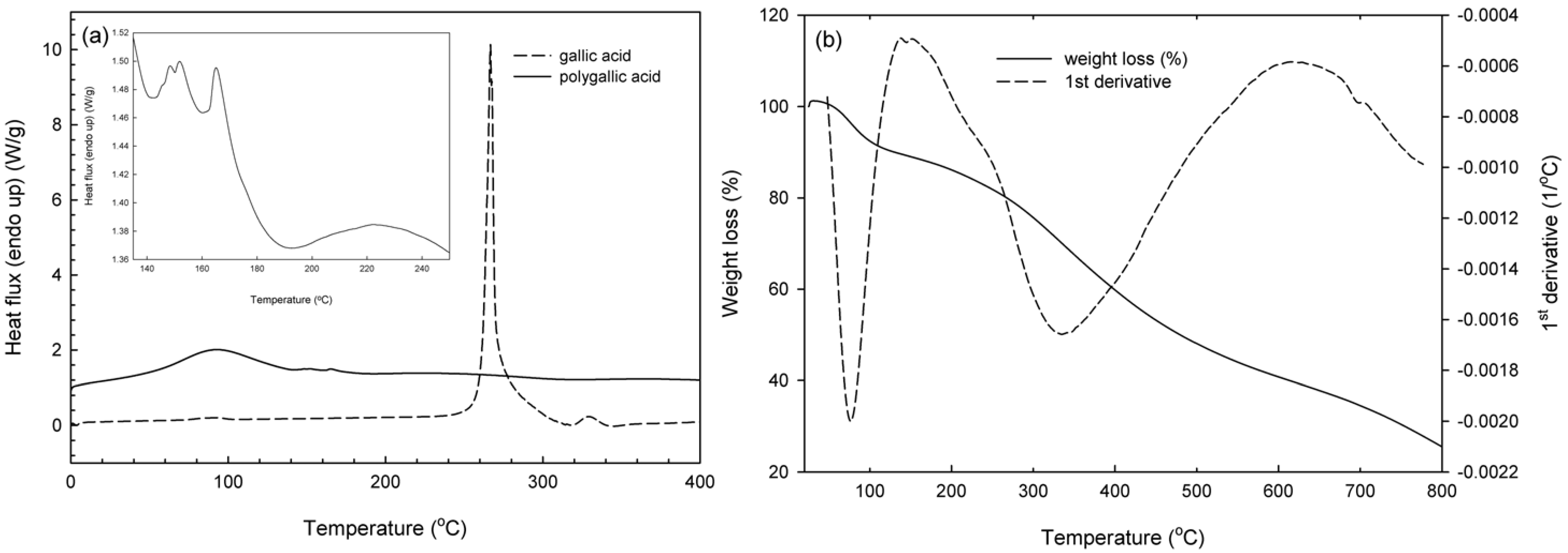
2.5. Thermal Properties
3. Materials and Methods
3.1 Enzymes and Chemicals
3.2 Determination of Laccase Activity and Protein Concentration
3.3 Bioconversion Reactions
3.4 Product Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ward, G.; Parales, R.E.; Dosoretz, C.G. Biocatalytic synthesis of polycatechols from toxic aromatic compounds. Environ. Sci. Technol. 2004, 38, 4753–4757. [Google Scholar] [CrossRef] [PubMed]
- Aktas, N.; Tanyolac, A. Kinetics of laccase-catalyzed oxidative polymerization of catechol. J. Mol. Catal. B Enzym. 2003, 22, 61–69. [Google Scholar] [CrossRef]
- Sun, X.; Bai, R.; Zhang, Y.; Wang, Q.; Fan, X.; Yuan, J.; Cui, L.; Wang, P. Laccase-catalyzed oxidative polymerization of phenolic compounds. Appl. Biochem. Biotechnol. 2013, 171, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Reihmann, M.; Ritter, H. Synthesis of phenol polymers using peroxidases. In Enzyme-Catalyzed Synthesis of Polymers; Kobayashi, S., Ritter, H., Kaplan, D., Eds.; Springer: Berlin, Heidelberg, Germany, 2006; Volume 194, pp. 1–49. [Google Scholar]
- Zerva, A.; Christakopoulos, P.; Topakas, E. Characterization and application of a novel class II thermophilic peroxidase from Myceliophthora thermophila in biosynthesis of polycatechol. Enzym. Microb. Technol. 2015, 75–76, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Desentis-Mendoza, R.M.; Hernández-Sánchez, H.; Moreno, A.; Rojas del C., E.; Chel-Guerrero, L.; Tamariz, J.; Jaramillo-Flores, M.E. Enzymatic polymerization of phenolic compounds using laccase and tyrosinase from Ustilago maydis. Biomacromolecules 2006, 7, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Khlupova, M.E.; Vasileva, I.S.; Shumakovich, G.P.; Morozova, O.V.; Chertkov, V.A.; Shestakov, A.K.; Kisin, A.V.; Yaropolov, A.I. Enzymatic polymerization of dihydroquercetin using bilirubin oxidase. Biochemistry 2015, 80, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Aktas, N.; Tanyolac, A. Reaction conditions for laccase catalyzed polymerization of catechol. Bioresour. Technol. 2003, 87, 209–214. [Google Scholar] [CrossRef]
- Mustafa, R.; Muniglia, L.; Rovel, B.; Girardin, M. Phenolic colorants obtained by enzymatic synthesis using a fungal laccase in a hydro-organic biphasic system. Food Res. Int. 2005, 38, 995–1000. [Google Scholar] [CrossRef]
- Kim, S.; Silva, C.; Evtuguin, D.; Gamelas, J.F.; Cavaco-Paulo, A. Polyoxometalate/laccase-mediated oxidative polymerization of catechol for textile dyeing. Appl. Microbiol. Biotechnol. 2011, 89, 981–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, J.R.; Kim, E.J.; Murugesan, K.; Park, H.K.; Kim, Y.M.; Kwon, J.H.; Kim, W.G.; Lee, J.Y.; Chang, Y.S. Laccase-catalysed polymeric dye synthesis from plant-derived phenols for potential application in hair dyeing: Enzymatic colourations driven by homo- or hetero-polymer synthesis. Microb. Biotechnol. 2010, 3, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Nyanhongo, G.S.; Gübitz, G.M.; Cavaco-Paulo, A.; Kim, S. Enzymatic colouration with laccase and peroxidases: Recent progress. Biocatal. Biotransform. 2012, 30, 125–140. [Google Scholar] [CrossRef] [Green Version]
- Polak, J.; Jarosz-Wilkolazka, A. Fungal laccases as green catalysts for dye synthesis. Process Biochem. 2012, 47, 1295–1307. [Google Scholar] [CrossRef]
- Kurisawa, M.; Chung, J.E.; Uyama, H.; Kobayashi, S. Laccase-catalyzed synthesis and antioxidant property of poly(catechin). Macromol. Biosci. 2003, 3, 758–764. [Google Scholar] [CrossRef]
- Wang, P.; Martin, B.D.; Parida, S.; Rethwisch, D.G.; Dordick, J.S. Multienzymic synthesis of poly(hydroquinone) for use as a redox polymer. J. Am. Chem. Soc. 1995, 117, 12885–12886. [Google Scholar] [CrossRef]
- Nabid, M.; Zamiraei, Z.; Sedghi, R.; Nazari, S. Synthesis and characterization of poly(catechol) catalyzed by porphyrin and enzyme. Polym. Bull. 2010, 64, 855–865. [Google Scholar] [CrossRef]
- He, J.; Guan, Y.; Zhang, Y. Redox-active LBL films via in situ template polymerization of hydroquinone. J. Appl. Polym. Sci. 2013, 129, 3070–3076. [Google Scholar] [CrossRef]
- Marczewska, B.; Przegalinski, M. Poly(catechol) electroactive film and its electrochemical properties. Synth. Met. 2013, 182, 33–39. [Google Scholar] [CrossRef]
- Tanaka, T.; Takahashi, M.; Hagino, H.; Nudejima, S.I.; Usui, H.; Fujii, T.; Taniguchi, M. Enzymatic oxidative polymerization of methoxyphenols. Chem. Eng. Sci. 2010, 65, 569–573. [Google Scholar] [CrossRef]
- Miyazaki, K.; Arai, S.; Iwamoto, T.; Takasaki, M.; Tomoda, A. Metabolism of pyrogallol to purpurogallin by human erythrocytic hemoglobin. Tohoku. J. Exp. Med. 2004, 203, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.K.; Bae, J.S. Antiplatelet and antithrombotic activities of purpurogallin in vitro and in vivo. BMB Rep. 2014, 47, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, H.; Fung, K.P.; Wu, T.W. Purpurogallin as an antioxidant protector of human erythrocytes against lysis by peroxyl radicals. Life Sci. 1993, 53, l39–143. [Google Scholar] [CrossRef]
- López, J.; Hernández-Alcántara, J.M.; Roquero, P.; Montiel, C.; Shirai, K.; Gimeno, M.; Bárzana, E. Trametes versicolor laccase oxidation of gallic acid toward a polyconjugated semiconducting material. J. Mol. Catal. B Enzym. 2013, 97, 100–105. [Google Scholar] [CrossRef]
- Rouet-Mayer, M.A.; Ralambosoa, J.; Philippon, P. Roles of o-quinones and their polymers in the enzymic browning of apples. Phytochemistry 1990, 29, 435–440. [Google Scholar] [CrossRef]
- Aktas, N.; Sahiner, N.; Kantoglu, O.; Salih, B.; Tanyolac, A. Biosynthesis and characterization of laccase catalyzed poly(catechol). J. Polym. Environ. 2003, 11, 123–128. [Google Scholar] [CrossRef]
- Dubey, S.; Singh, D.; Misra, R. A. Enzymatic synthesis and various properties of poly(catechol). Enzym. Microb. Technol. 1998, 23, 432–437. [Google Scholar] [CrossRef]
- Fiege, H.; Voges, H.W.; Hamamoto, T.; Umemura, S.; Iwata, T.; Miki, H.; Fujita, Y.; Buysch, H.J.; Garbe, D.; Paulus, W. Phenol derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar] [CrossRef]
- Jamshidi, K.; Hyon, S.; Ikada, Y. Thermal characterization of polylactides. Polymer 1988, 29, 2229–2234. [Google Scholar] [CrossRef]
- Vouyiouka, S.; Filgueiras, V.; Papaspyrides, C.; Pinto, J.; Lima, E. Morphological changes of poly(ethylene terephthalate-co-isophthalate) during solid state polymerization. J. Appl. Polym. Sci. 2012, 124, 4457–4465. [Google Scholar] [CrossRef]
- Vouyiouka, S.; Theodoulou, P.; Symeonidou, A.; Papaspyrides, C.D.; Pfaendner, R. Solid state polymerization of poly(lactic acid): Some fundamental parameters. Polym. Degrad. Stab. 2013, 98, 2473–2481. [Google Scholar] [CrossRef]
- Gabbott, P. Principles and Applications of Thermal Analysis; Blackwell: Oxford, UK, 2008; p. 101. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Solomon, O.F.; Ciuta, I.Z. Détermination de la viscosité intrinsèque de solutions de polymères par une simple détermination de la viscosité. J. Appl. Polym. Sci. 1962, 6, 683. [Google Scholar] [CrossRef]
- Sample Availability: Not Available.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerva, A.; Manos, N.; Vouyiouka, S.; Christakopoulos, P.; Topakas, E. Bioconversion of Biomass-Derived Phenols Catalyzed by Myceliophthora thermophila Laccase. Molecules 2016, 21, 550. https://doi.org/10.3390/molecules21050550
Zerva A, Manos N, Vouyiouka S, Christakopoulos P, Topakas E. Bioconversion of Biomass-Derived Phenols Catalyzed by Myceliophthora thermophila Laccase. Molecules. 2016; 21(5):550. https://doi.org/10.3390/molecules21050550
Chicago/Turabian StyleZerva, Anastasia, Nikolaos Manos, Stamatina Vouyiouka, Paul Christakopoulos, and Evangelos Topakas. 2016. "Bioconversion of Biomass-Derived Phenols Catalyzed by Myceliophthora thermophila Laccase" Molecules 21, no. 5: 550. https://doi.org/10.3390/molecules21050550







