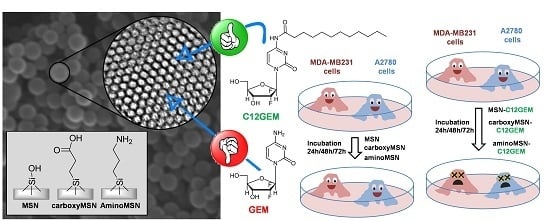
Delivery of Gemcitabine Prodrugs Employing Mesoporous Silica Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
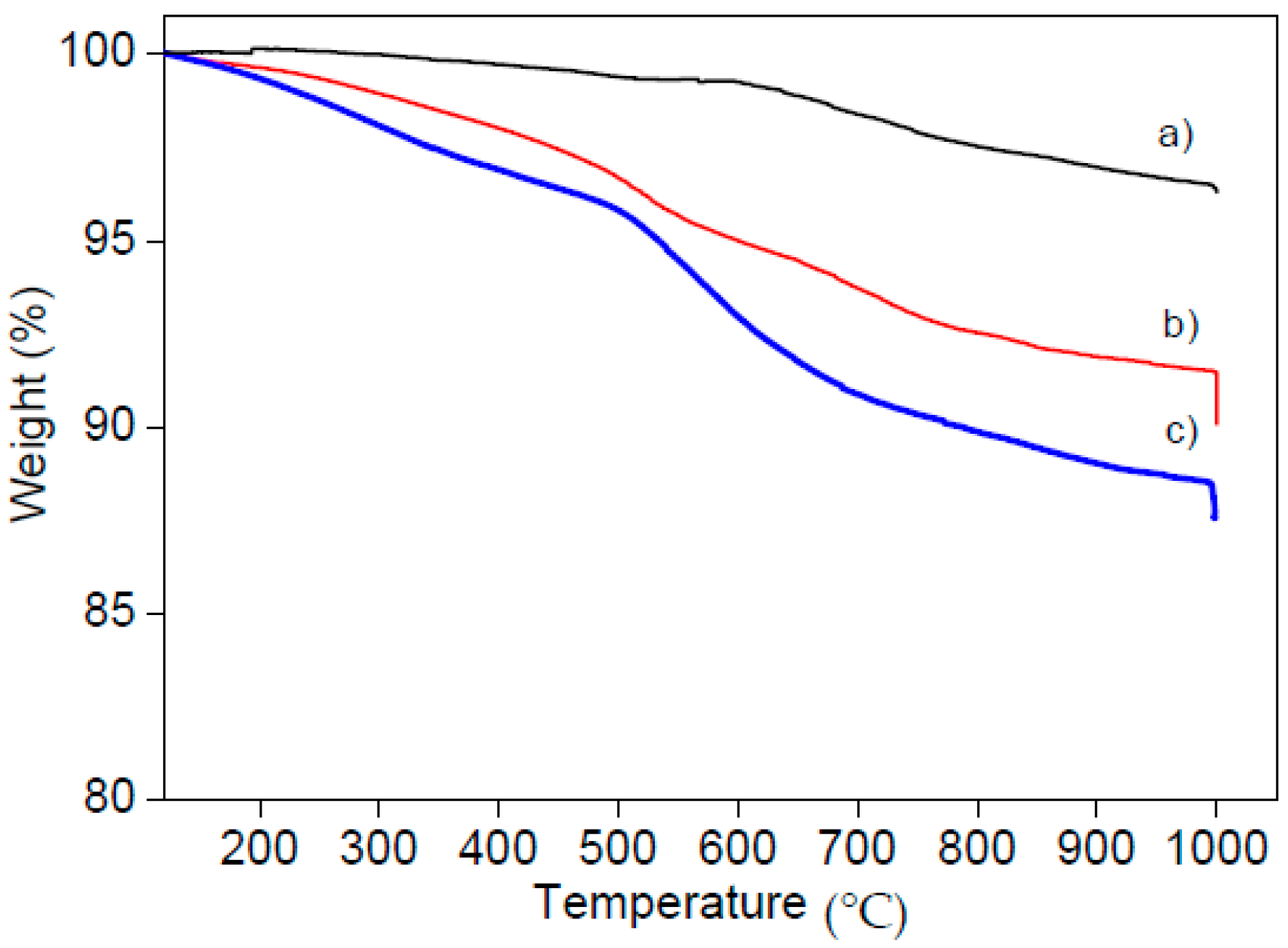
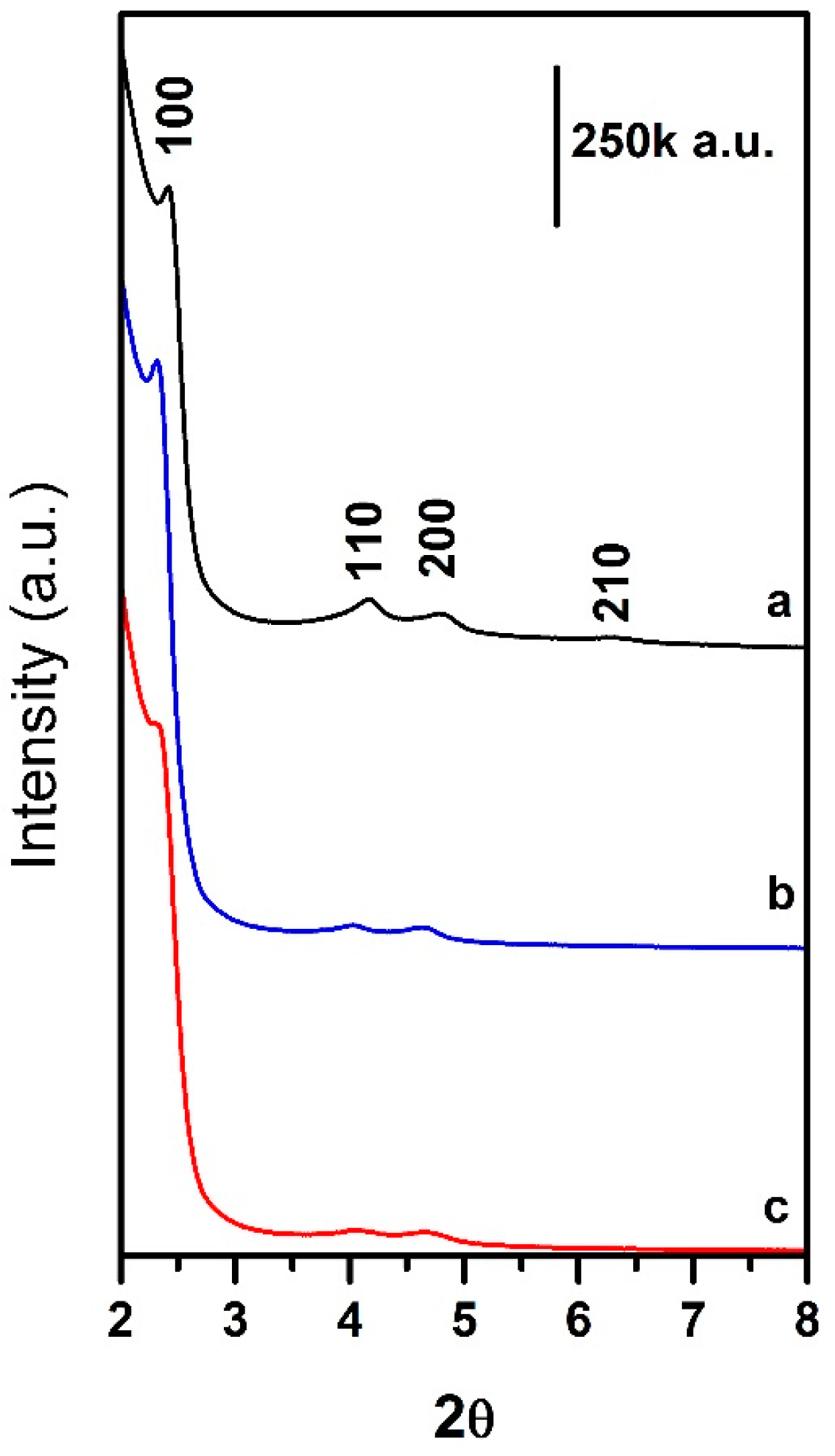
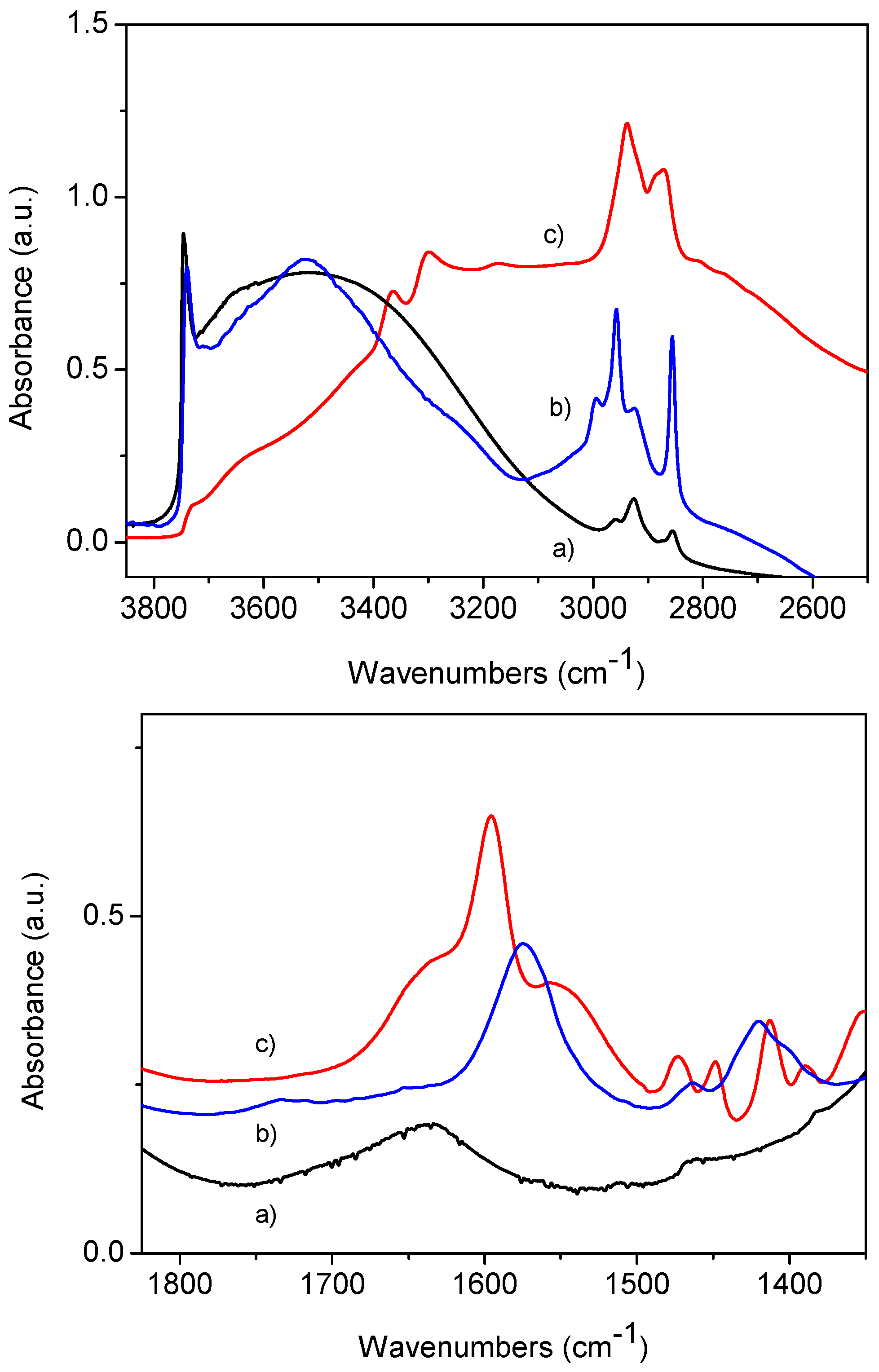
2.1. Synthesis and Characterization of Mesoporous Silica Nanoparticles (MSNs)
2.2. Drug Loading
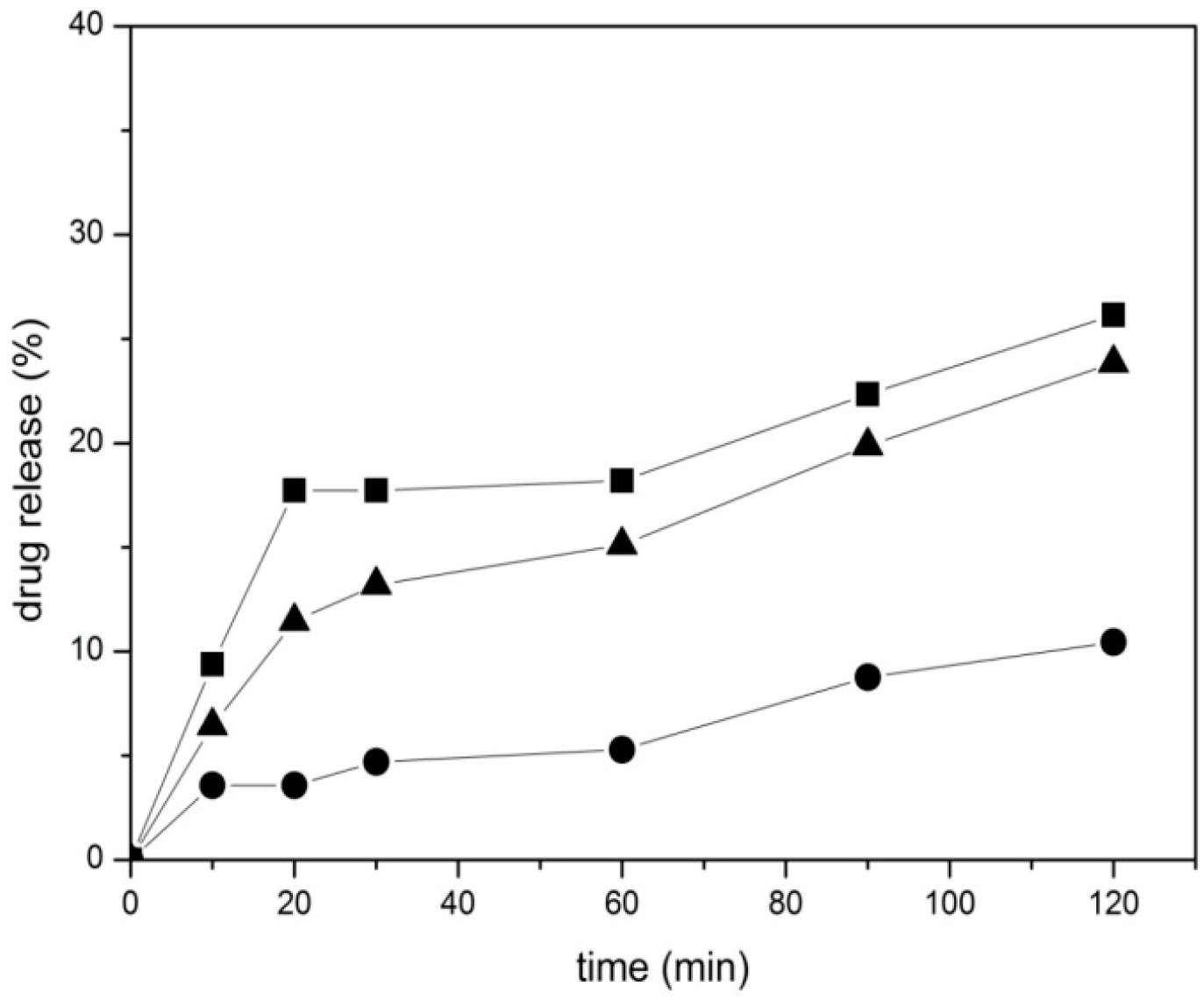
2.3. In Vitro Drug Release Study
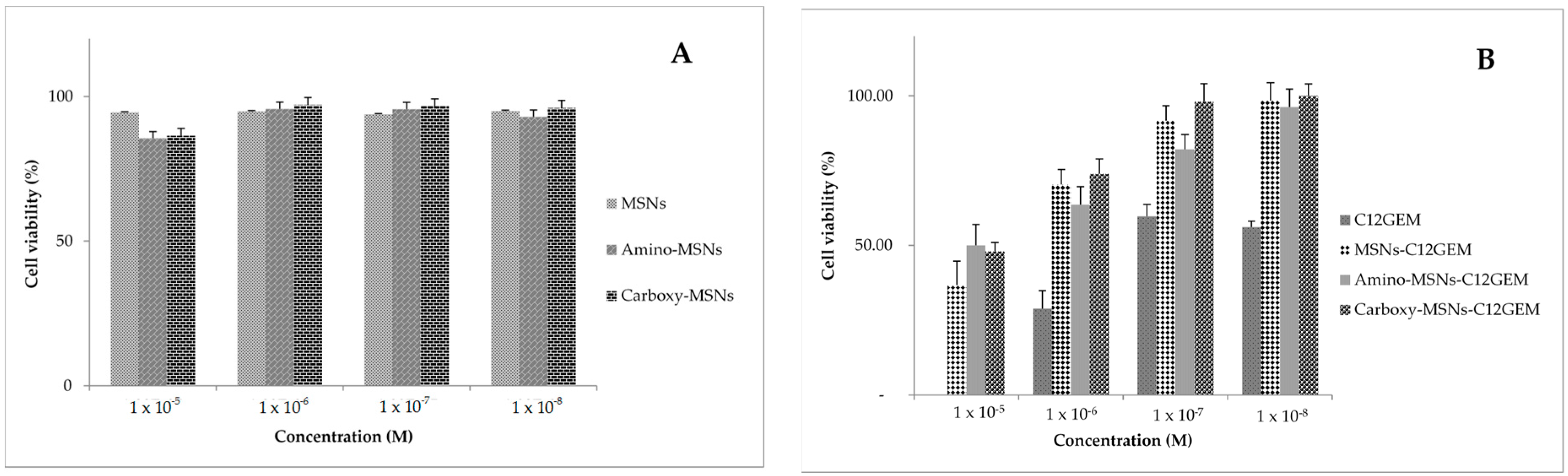
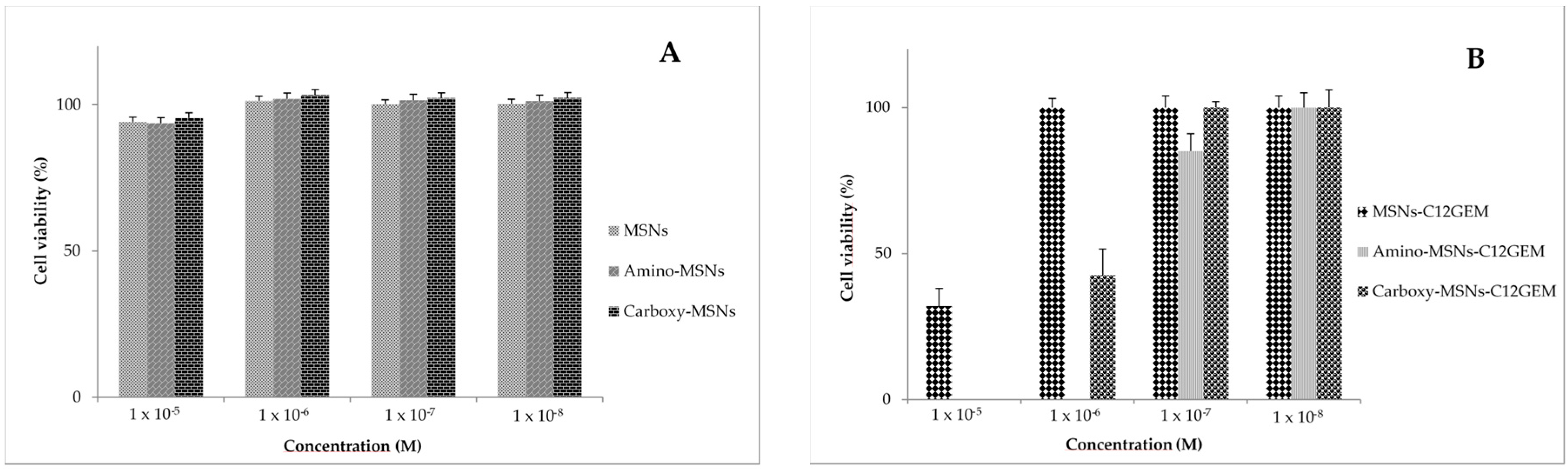
2.4. Cytotoxicity
3. Materials and Methods
3.1. Materials
3.2. Synthesis and Functionalization of MSN
3.3. Characterization of MSNs
3.4. Gemcitabine (GEM) and GEM Prodrugs Loading
3.5. In Vitro Drug Release
3.6. Tumor Cell Lines and Cell Culture
3.7. Cell Proliferation Assays
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| APTS | 3-aminopropyl triethoxysilane |
| BET | Brunauer–Emmett–Teller |
| BJH | Barrett–Joyner–Halenda |
| CTAB | cetyltrimethylammonium bromide |
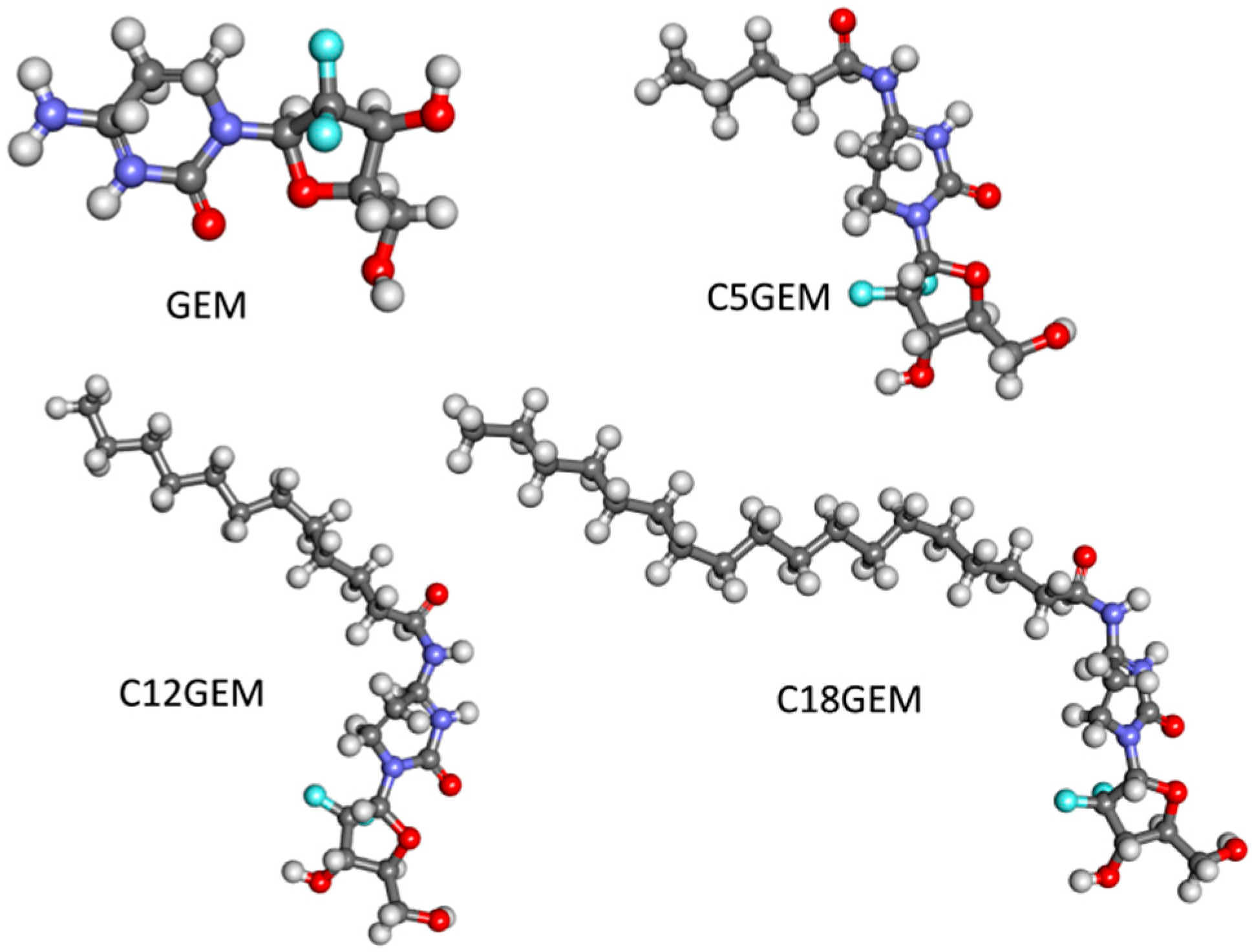
| C5GEM | 4-(N)-valeroyl-gemcitabine |
| C12GEM | 4-(N)-lauroyl-gemcitabine |
| C18GEM | 4-(N)-stearoyl-gemcitabine |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GEM | gemcitabine |
| HPLC | High Performance Liquid Chromatography |
| HRTEM | High Resolution Transmission Electron Microscopy |
| MSN | mesoporous silica nanoparticle |
| PBS | phosphate buffer saline |
| RPMI | Roswell Park Memorial Institute Medium |
| RT | room temperature |
| SDA | structure direct agent |
| SSA | specific surface area |
| STD | standard deviation |
| TEOS | tetraethyl orthosilicate |
| TGA | thermogravimetric analysis |
| XRD | X-ray diffraction |
References
- Are, C.; Rajaram, S.; Are, M.; Raj, H.; Anderson, B.O.; Chaluvarya Swamy, R.; Vijayakumar, M.; Song, T.; Pandey, M.; Edney, J.A.; et al. A review of global cancer burden: Trends, challenges, strategies, and a role for surgeons. J. Surg. Oncol. 2013, 107, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Hassett, M.J.; O’Malley, A.J.; Pakes, J.R.; Newhouse, J.R.; Earle, C.C. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J. Natl. Cancer Inst. 2006, 98, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Thorley, A.J.; Tetley, T.D. New perspectives in nanomedicine. Pharmacol. Ther. 2013, 140, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Heidel, J.; Davis, M. Clinical developments in nanotechnology for cancer therapy. Pharm. Res. 2011, 28, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.P.E.; Langer, R.; Farokhzad, O.C. Nanoparticle technologies for cancer therapy. Handb. Exp. Pharmacol. 2010, 197, 55–86. [Google Scholar]
- Gary-Bobo, M.; Vaillant, O.; Maynadier, M.; Basile, I.; Gallud, A.; El Cheikh, K.; Bouffard, E.; Morere, A.; Rebillard, X.; Puche, P.; et al. Targeting multiplicity: THE key factor for anti-cancer nanoparticles. Curr. Med. Chem. 2013, 20, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Nazir, S.; Hussain, T.; Ayub, A.; Rashid, U.; MacRobert, A.J. Nanomaterials in combating cancer: Therapeutic applications and developments. Nanomedicine: NBM 2014, 10, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.P.; Zeng, Q.H.; Lu, G.Q.; Yu, A.B. Inorganic nanoparticles as carriers for efficient cellular delivery. Chem. Eng. Sci. 2006, 61, 1027–1040. [Google Scholar] [CrossRef]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, B.S.; Kamboj, S.R. Inorganic nanomedicine—Part 1. Nanomedicine: NBM 2010, 6, 516–522. [Google Scholar]
- Sekhon, B.S.; Kamboj, S.R. Inorganic nanomedicine—Part 2. Nanomedicine: NBM 2010, 6, 612–618. [Google Scholar]
- Chen, Y.; Chen, H.; Shi, J. Drug delivery/imaging multifunctionality of mesoporous silica-based composite nanostructures. Expert Opin. Drug Deliv. 2014, 11, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, M.; Zhao, J.X. Recent development of silica nanoparticles as delivery vectors for cancer imaging and therapy. Nanomedicine: NBM 2014, 10, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Vivero-Escoto, J.L.; Slowing, I.I.; Trewyn, B.G.; Lin, V.S.Y. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small 2010, 6, 1952–1967. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Hung, Y.; Mou, C.-Y. Mesoporous silica nanoparticles as nanocarriers. Chem. Commun. 2011, 47, 9972–9985. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Mamaeva, V.; Sahlgren, C.; Lindèn, M. Nanoparticles in targeted cancer therapy: Mesoporous silica nanoparticles entering preclinical development stage. Nanomedicine 2012, 7, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Douroumis, D.; Onyesom, I.; Maniruzzaman, M.; Mitchell, J. Mesoporous silica nanoparticles in nanotechnology. Crit. Rev. Biotechnol. 2013, 33, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Mamaeva, V.; Sahlgren, C.; Lindèn, M. Mesoporous silica nanoparticles in medicine—Recent advances. Adv. Drug Deliv. Rev. 2013, 65, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Szegedi, A.; Popova, M.; Goshev, I.; Mihàly, J. Effect of amine functionalization of spherical MCM-41 and SBA-15 on controlled drug release. J. Solid State Chem. 2011, 184, 1201–1207. [Google Scholar] [CrossRef]
- Trewyn, B.G.; Giri, S.; Slowing, I.I.; Lin, V.S.Y. Mesoporous silica nanoparticle based controlled release, drug delivery, and biosensor systems. Chem. Commun. 2007, 31, 3236–3245. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Colilla, M.; Vallet-Regì, M. Drug delivery from ordered mesoporous matrices. Expert Opin. Drug Deliv. 2009, 6, 1383–1400. [Google Scholar] [CrossRef] [PubMed]
- Gary-Bobo, M.; Mir, Y.; Rouxel, C.; Brevet, D.; Basile, I.; Maynadier, M.; Vaillant, O.; Mongin, O.; Blanchard-Desce, M.; Morère, A.; et al. Mannose-functionalized mesoporous silica nanoparticles for efficient two-photon photodynamic therapy of solid tumors. Angew. Chem. Int. Ed. 2011, 50, 11425–11429. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Wu, C.-W.; Vivero-Escoto, J.L.; Lin, V.S.Y. Mesoporous silica nanoparticles for reducing hemolytic activity towards mammalian red blood cells. Small 2009, 5, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Haynes, C.L. Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity. J. Am. Chem. Soc. 2010, 132, 4834–4842. [Google Scholar] [CrossRef] [PubMed]
- Asefa, T.; Tao, Z. Biocompatibility of mesoporous silica nanoparticles. Chem. Res. Toxicol. 2012, 25, 2265–2284. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, H.; Shi, J. In vivo bio-safety evaluations and diagnostic/therapeutic applications of chemically designed mesoporous silica nanoparticles. Adv. Mater. 2013, 25, 3144–3176. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Barnes, J.C.; Bosoy, A.; Stoddart, J.F.; Zink, J.I. Mesoporous silica nanoparticles in biomedical applications. Chem. Soc. Rev. 2012, 41, 2590–2605. [Google Scholar] [CrossRef] [PubMed]
- Plunkett, W.; Huang, P.; Gandhi, V. Preclinical characteristics of gemcitabine. Anti-Cancer Drugs 1995, 6, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Hertel, L.W.; Kroin, J.S.; Misner, J.W.; Tustin, J.M. Synthesis of 2-deoxy-2, 2-difluoro-D-ribose and 2-deoxy-2, 2′-difluoro-D-ribofuranosyl nucleosides. J. Org. Chem. 1988, 53, 2406–2409. [Google Scholar] [CrossRef]
- Hertel, L.W.; Boder, G.B.; Kroin, J.S.; Rinzel, S.M.; Poore, G.A.; Todd, G.C.; Grindey, G.B. Evaluation of the antitumor activity of gemcitabine (2′,2′-difluoro-2′-deoxycytidine). Cancer Res. 1990, 50, 4417–4422. [Google Scholar] [PubMed]
- Heinemann, V. Gemcitabine: Progress in the treatment of pancreatic cancer. Oncology 2001, 60, 8–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plunkett, W.; Huang, P.; Xu, Y.Z.; Heinemann, V.; Grunewald, R.; Gandhi, V. Gemcitabine metabolism, mechanism of action, and self-potentiation. Semin. Oncol. 1995, 22, 3–10. [Google Scholar] [PubMed]
- Plunkett, W.; Huang, P.; Searcy, C.E.; Gandhi, V. Gemcitabine: Preclinical pharmacology and mechanisms of action. Semin. Oncol. 1996, 23, 3–15. [Google Scholar] [PubMed]
- Bouffard, D.Y.; Lalibertà, J.; Momparler, R.L. Kinetic studies on 2′, 2′-difluorodeoxycytidine (gemcitabine) with purified human deoxycytidine kinase and cytidine deaminase. Biochem. Pharmacol. 1993, 45, 1857–1861. [Google Scholar] [CrossRef]
- Heinemann, V.; Xu, Y.-Z.; Chubb, S.; Sen, A.; Hertel, L.W.; Grindey, G.B.; Plunkett, W. Cellular elimination of 2′,2′-difluorodeoxycytidine 5-triphosphate: A mechanism of self-potentiation. Cancer Res. 1992, 52, 533–539. [Google Scholar] [PubMed]
- Matsuda, A.; Sasaki, T. Antitumor activity of sugar-modified cytosine nucleosides. Cancer Sci. 2004, 95, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Abbruzzese, J.L.; Grunewald, R.; Weeks, E.A.; Gravel, D.; Adams, T.; Nowak, B.; Mineishi, S.; Tarassoff, P.; Satterlee, W.; Raber, M.N. A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J. Clin. Oncol. 1991, 9, 491–498. [Google Scholar] [PubMed]
- Grunewald, R.; Kantarjian, H.; Du, M.; Faucher, K.; Tarassoff, P.; Plunkett, W. Gemcitabine in leukemia: A phase I clinical, plasma, and cellular pharmacology study. J. Clin. Oncol. 1992, 10, 406–413. [Google Scholar] [PubMed]
- Storniolo, A.M.; Allerheiligen, S.R.; Pearce, H.L. Preclinical, pharmacologic, and phase I studies of gemcitabine. Semin. Oncol. 1997, 24, S7-2–S7-7. [Google Scholar] [PubMed]
- Yu, X.; Zhang, Y.; Chen, C.; Yao, Q.; Li, M. Targeted drug delivery in pancreatic cancer. BBA Rev. Cancer 2010, 1805, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Myhren, F.; Borretzen, B.; Dalen, A.; Sandvold, M.L. Gemcitabine Derivatives. WO 98/32762, 30 July 1998. [Google Scholar]
- Meng, H.; Zhao, Y.; Dong, J.; Xue, M.; Lin, Y.S.; Ji, Z.; Mai, W.X.; Zhang, H.; Chang, C.H.; Brinker, C.J.; et al. Two-wave nanotherapy to target the stroma and optimize gemcitabine delivery to a human pancreatic cancer model in mice. ACS Nano 2013, 11, 10048–10065. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Zhang, D.; Alsaiari, S.; Lu, J.; Deng, L.; Tamanoi, F.; AlMalik, A.M.; Zink, J.I.; Khashab, N.M. Protein-gold clusters-capped mesoporous silica nanoparticles for high drug loading, autonomous gemcitabine/doxorubicin co-delivery, and in vivo tumor imaging. J. Control. Release 2016, 229, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Tehrani, Z.M.; Moghanaki, A.A. Folate-conjugated pH-responsive nanocarrier designed for active tumor targeting and controlled release of gemcitabine. Pharm. Res. 2016, 33, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Wang, M.; Liu, H.; Liu, X.; Situ, A.; Wu, B.; Ji, Z.; Chang, C.H.; Nel, A.E. Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano 2015, 9, 3540–3557. [Google Scholar] [CrossRef] [PubMed]
- Gurka, M.K.; Pender, D.; Chuong, P.; Fouts, B.L.; Sobelov, A.; McNally, M.W.; Mezera, M.; Woo, S.Y.; McNally, L.R. Identification of pancreatic tumors in vivo with ligand-targeted, pH responsive mesoporous silica nanoparticles by multispectral optoacoustic tomography. J. Control. Release 2016. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Tehrani, Z.M. Mesoporous silica nanoparticles with bilayer coating of poly(acrylic acid-co-itaconic acid) and human serum albumin (HSA): A pH-sensitive carrier for gemcitabine delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Miletto, I.; Massa, A.; Ugazio, E.; Musso, G.; Caputo, G.; Berlier, G. The protective effect of the mesoporous host on the photo oxidation of fluorescent guests: A UV-Vis spectroscopy study. Phys. Chem. Chem. Phys. 2014, 16, 12172–12177. [Google Scholar] [CrossRef] [PubMed]
- Sapino, S.; Ugazio, E.; Gastaldi, L.; Miletto, I.; Berlier, G.; Zonari, D.; Oliaro-Bosso, S. Mesoporous silica as topical nanocarriers for quercetin: Characterization and in vitro studies. Eur. J. Pharm. Biopharm. 2015, 89, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Rouquerol, J.; Rouquerol, F.; Sing, K.S.W. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, 1st ed.; Academic Press: London, UK, 1999. [Google Scholar]
- Kozlova, S.A.; Kirik, S.D. Post-synthetic activation of silanol covering in the mesostructured silicate materials MCM-41 and SBA-15. Microporous Microporous Mater. 2010, 133, 124–133. [Google Scholar] [CrossRef]
- Musso, G.E.; Bottinelli, E.; Celi, L.; Magnacca, G.; Berlier, G. Influence of surface functionalization on the hydrophilic character of mesoporous silica nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 13882–13894. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.J.; Shen, J.Y.; Li, Z.Y.; Zhang, D.R.; Zhang, Q.; Liu, G.P.; Zheng, D.D.; Tian, X.N. In vitro and in vivo evaluation of paclitaxel-loaded mesoporous silica nanoparticles with three pore sizes. Int. J. Pharm. 2013, 445, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.H.; Jambhrunkar, S.; Thorn, P.; Chen, J.Z.; Gu, W.Y.; Yu, C.Z. Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanoscale 2013, 5, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Brusa, P.; Rocco, F.; Arpicco, S.; Ceruti, M.; Cattel, L. Preparation, characterization, cytotoxicity and pharmacokinetics of liposomes containing lipophilic gemcitabine prodrugs. J. Control. Release 2004, 100, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Dalla Pozza, E.; Lerda, C.; Costanzo, C.; Donadelli, M.; Dando, I.; Zoratti, E.; Scupoli, M.T.; Beghelli, S.; Scarpa, A.; Fattal, E.; et al. Targeting gemcitabine containing liposomes to CD44 expressing pancreatic adenocarcinoma cells causes an increase in the antitumoral activity. BBA Biomembr. 2013, 1828, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Arpicco, S.; Lerda, C.; Dalla Pozza, E.; Costanzo, C.; Tsapis, N.; Stella, B.; Donadelli, M.; Dando, I.; Fattal, E.; Cattel, L.; et al. Hyaluronic acid-coated liposomes for active targeting of gemcitabine. Eur. J. Pharm. Biopharm. 2013, 85, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Stella, B.; Arpicco, S.; Rocco, F.; Marsaud, V.; Renoir, J.M.; Cattel, L.; Couvreur, P. Encapsulation of gemcitabine lipophilic derivatives into polycyanoacrylate nanospheres and nanocapsules. Int. J. Pharm. 2007, 344, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regì, M.; Ramila, A.; del Real, R.P.; Parez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Radu, D.R.; Lai, C.-Y.; Jeftinija, K.; Rowe, E.W.; Jeftinija, S.; Lin, V.S.Y. A polyamidoamine dendrimer-capped mesoporous silica nanosphere-based gene transfection reagent. J. Am. Chem. Soc. 2004, 126, 13216–13217. [Google Scholar] [CrossRef] [PubMed]
- Tarn, D.; Xue, M.; Zink, J.I. pH-responsive dual cargo delivery from mesoporous silica nanoparticles with a metal-latched nanogate. Inorg. Chem. 2013, 52, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
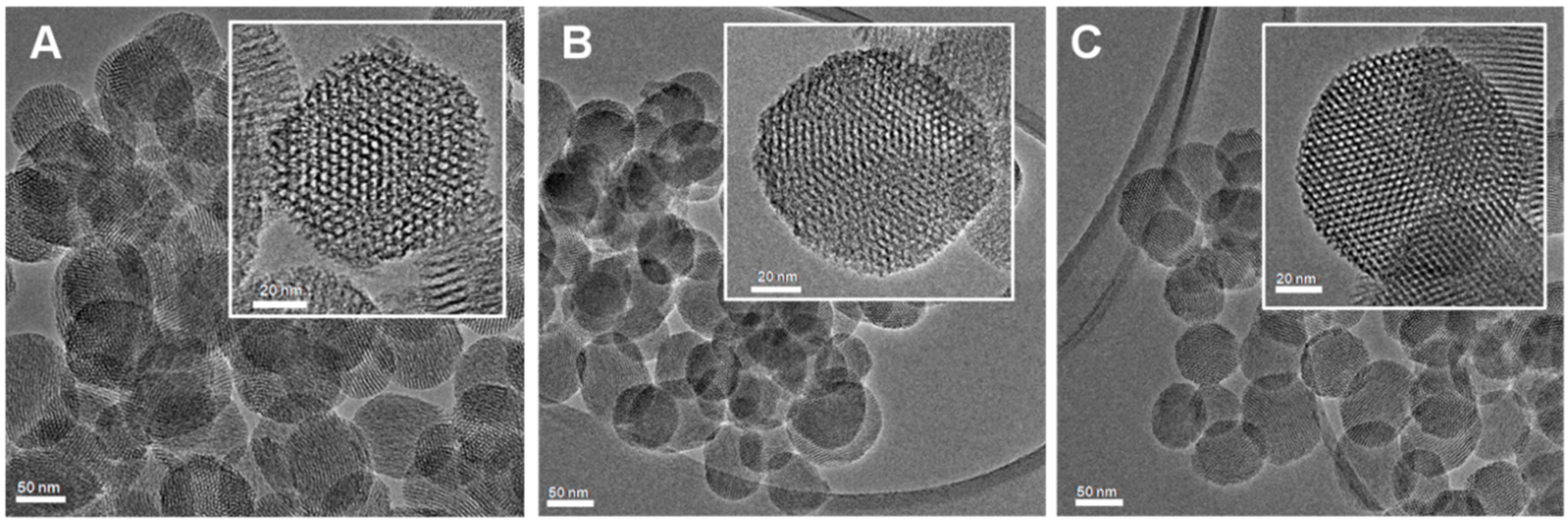

| Sample | Pore Diameter 1 (Å) | SSA 2 (m2·g−1) | Porevolume 1 (cm3·g−1) | Functional Groups 3 wt % |
|---|---|---|---|---|
| MSN | 37 | 1239 | 1.10 | - |
| Amino-MSN | 27 | 704 | 0.54 | 6.0 |
| Carboxy-MSN | 31 | 960 | 0.42 | 4.3 |
| Sample | Incubation Time (h) | ||
|---|---|---|---|
| 2 | 5 | 24 | |
| MSN-C12GEM | 7.4 | 4.8 | 3.5 |
| Amino-MSN-C12GEM | 11 | 5.7 | 6.6 |
| Carboxy-MSN-C12GEM | 23 | 16.4 | 14 |
| Compound | IC50 (M) | |||||
|---|---|---|---|---|---|---|
| MDA MB231 | A2780 | |||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| Gemcitabine | >10−4 | 9.5 × 10−5 | 1.0 × 10−7 | 1.0 × 10−7 | 6.2 × 10−7 | 6.0 × 10−8 |
| C12GEM | 1.0 × 10−4 | 8.0 × 10−5 | 8.5 × 10−6 | 8.0 × 10−6 | <10−9 | <10−10 |
| MSN-C12GEM | >10−5 | >10−5 | 6.0 × 10−5 | >10−5 | 4.0 × 10−6 | 5.5 × 10−6 |
| Amino-MSN-C12GEM | >10−5 | >10−5 | 1.0 × 10−5 | 8.0 × 10−5 | 7.5 × 10−6 | 8.5 × 10−6 |
| Carboxy-MSN-C12GEM | >10−5 | >10−5 | 2 × 10−5 | >10−5 | 1.0 × 10−6 | 2.0 × 10−6 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malfanti, A.; Miletto, I.; Bottinelli, E.; Zonari, D.; Blandino, G.; Berlier, G.; Arpicco, S. Delivery of Gemcitabine Prodrugs Employing Mesoporous Silica Nanoparticles. Molecules 2016, 21, 522. https://doi.org/10.3390/molecules21040522
Malfanti A, Miletto I, Bottinelli E, Zonari D, Blandino G, Berlier G, Arpicco S. Delivery of Gemcitabine Prodrugs Employing Mesoporous Silica Nanoparticles. Molecules. 2016; 21(4):522. https://doi.org/10.3390/molecules21040522
Chicago/Turabian StyleMalfanti, Alessio, Ivana Miletto, Emanuela Bottinelli, Daniele Zonari, Giulia Blandino, Gloria Berlier, and Silvia Arpicco. 2016. "Delivery of Gemcitabine Prodrugs Employing Mesoporous Silica Nanoparticles" Molecules 21, no. 4: 522. https://doi.org/10.3390/molecules21040522