Comparison of Antioxidant Capability after Isopropanol Salting-Out Pretreatment and n-Butanol Partition Extraction, and Identification and Evaluation of Antioxidants of Sedum formosanum N.E.Br.
Abstract
:1. Introduction
2. Results and Discussion
2.1. Comparison of the Antioxidant Capability of n-Butanol Partition and Isopropanol Salting-Out Pretreatment Extraction Technology
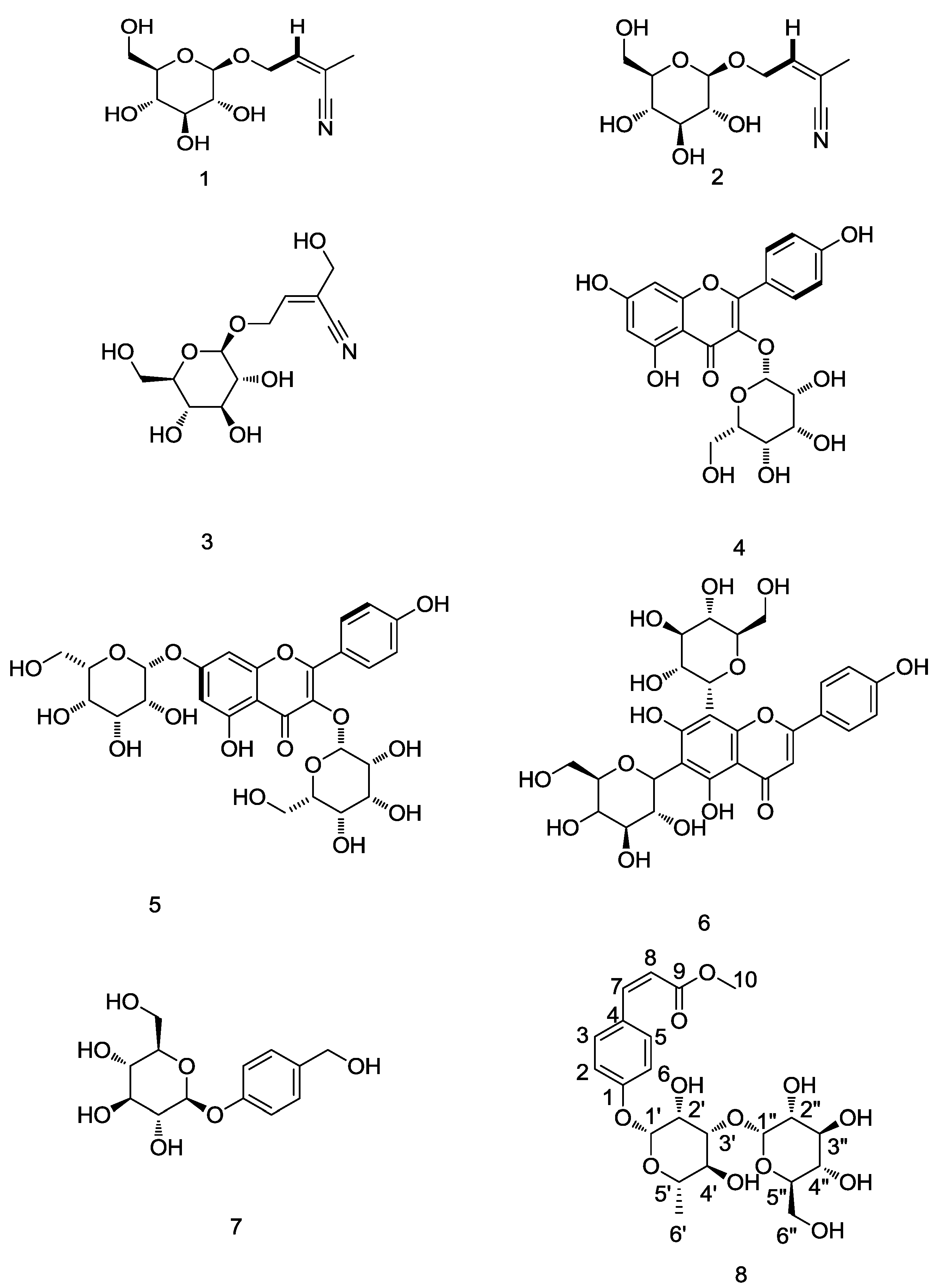
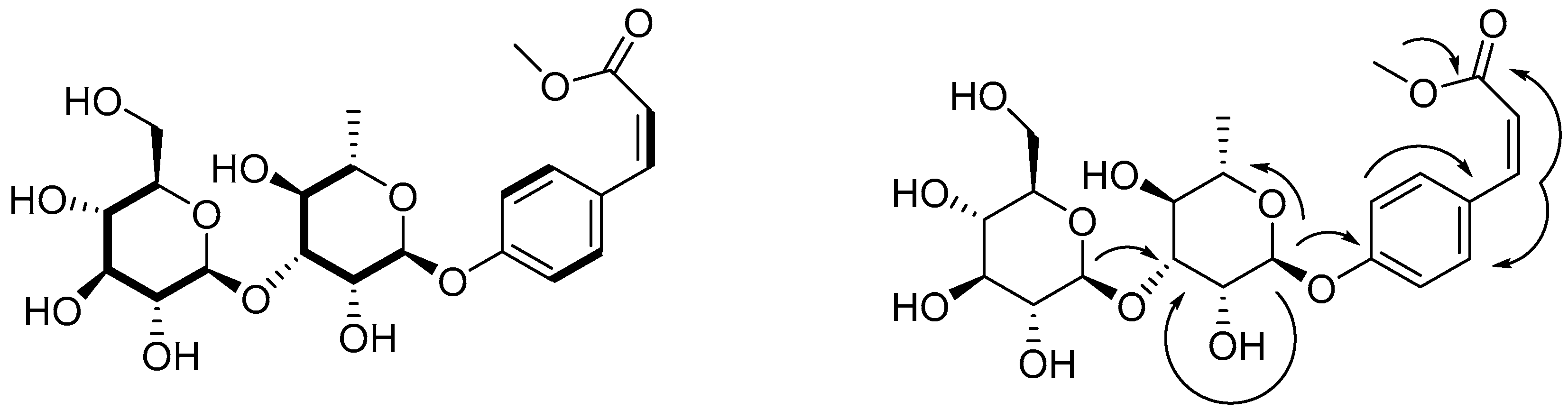
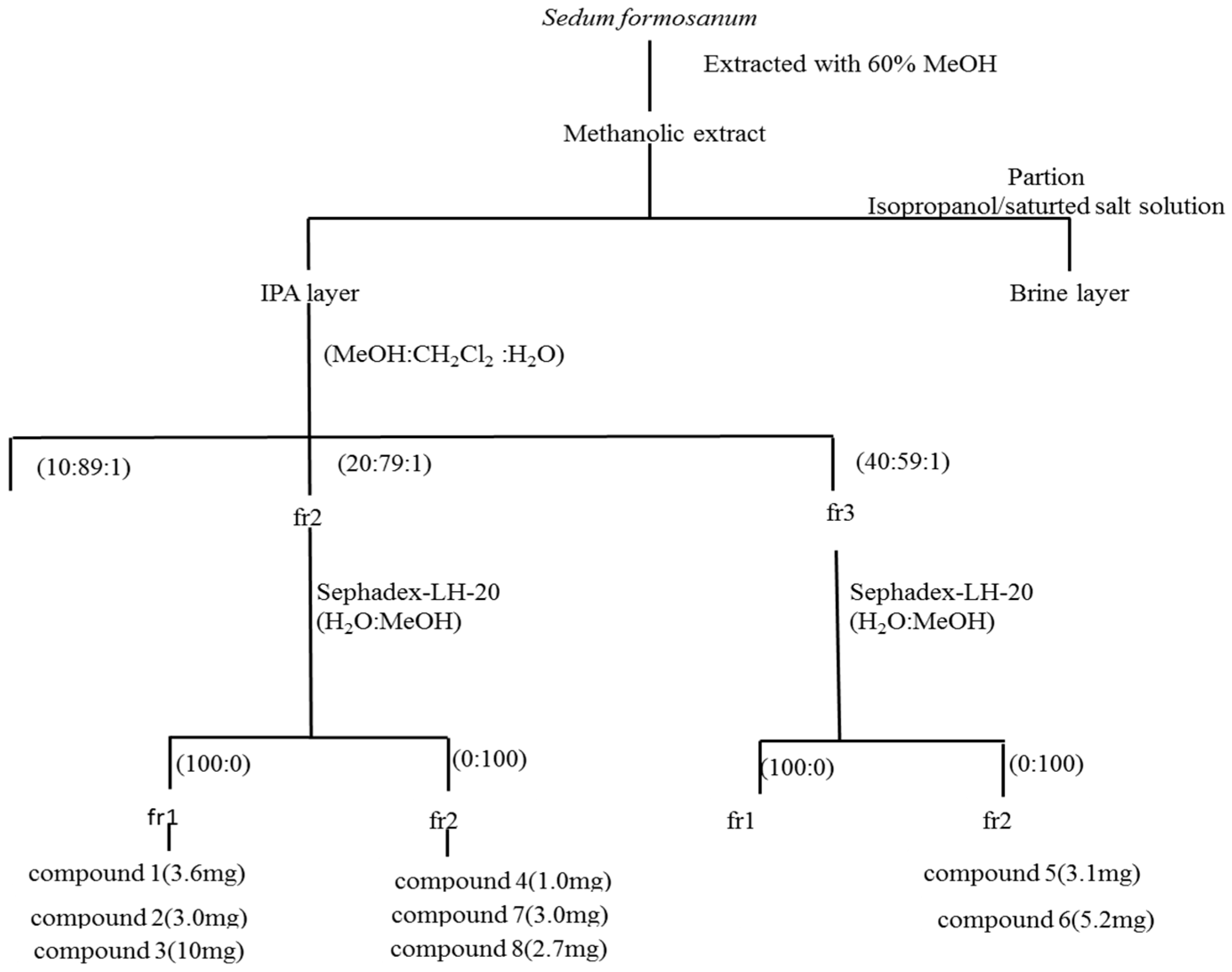
2.2. Identification of Compounds 1–8 Using Isopropanol Salting-Out Pretreatment Extraction Technology
2.3. Antioxidant Activity Potentials of Identified Compounds
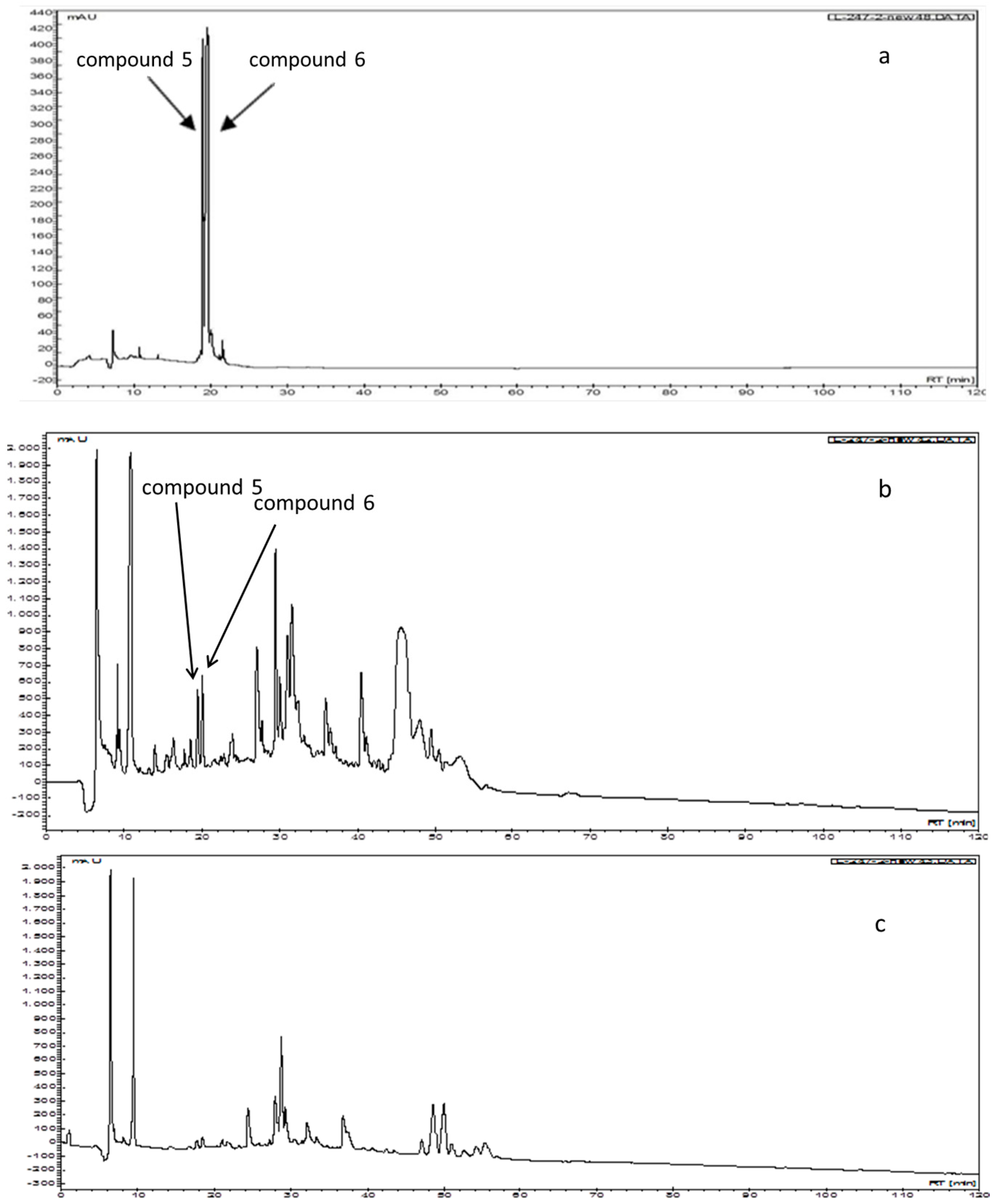
2.4. Comparison of the Chromatograms after Isopropanol Salting-Out Pretreatment and n-Butanol Partition Extraction Technology Using HPLC Separation
3. Materials and Methods
3.1. General Procedures
3.2. Sources of Sedum formosanum
3.3. Determination of the Two Types of Extraction Technology
3.3.1. Isopropanol Salting-Out Pretreatment Extraction Technology
3.3.2. n-Butanol Partition Extraction Technology
3.4. Extraction and Purification
3.5. Compound Characterization
3.6. Measurement of Antioxidant Activity
3.6.1. Superoxide Radical Scavenging Activity Assay
3.6.2. Oxygen Radical Absorbance Capacity Assay
3.6.3. Assay on Chelation of Ferrous Ions
3.6.4. Assay on Inhibition of Nitric Oxide Radical
3.7. Comparison of Chromatograms after Isopropanol Salting-Out Pretreatment and n-Butanol Partition Extractions Using HPLC Separation
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stocker, P.; Lesgards, J.F.; Vidal, N.; Chalier, F.; Prost, M. ESR study of a biological assay on whole blood: Antioxidant efficiency of various vitamins. Biochem. Biophys. Acta 2003, 1621, 1–8. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; van Beck, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Tachakittirungrod, S.; Okonogi, S.; Colwwanapoonpohn, S. Study on antioxidant activity of certain plants in Thailand: Mechanism of antioxidant action of guava leaf extract. Food Chem. 2007, 103, 381–388. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Rao, J.L.; Sakariah, K.K. Antioxidant activities of flavidin in different in vitro model systems. Bioorg. Med. Chem. 2004, 12, 5141–5146. [Google Scholar] [CrossRef] [PubMed]
- Moure, A.; Cruz, J.M.; Franco, D.; Dominguez, J.M.; Sineiro, J.; Dominguez, H. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Braca, A.; Sortino, C.; Politi, M.; Morelli, I.; Mendez, J. Antioxidant activity of flavonoids from Licania licaniaeflora. J. Ethnopharmacol. 2002, 79, 379–381. [Google Scholar] [CrossRef]
- Montoro, P.; Braca, A.; Pizza, C.; de Tommasi, N. Structure-antioxidant activity relationships of flavonoids isolated from different plant species. Food Chem. 2005, 92, 349–355. [Google Scholar] [CrossRef]
- Kayano, S.; Kikuzaki, H.; Fukutsuka, N.; Mitani, T.; Nakatain, N. Antioxidant activity of prune (Prunus domestica L.) Constituenys and a new synergist. J. Agric. Food. Chem. 2002, 50, 3708–3712. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Son, K.H.; Chang, K.B.; Kang, S.S.; Kim, H.P. Anti-inflammatory activity of Sedum Kamtschaticum. J. Ethnopharmacol. 2004, 90, 409–414. [Google Scholar] [CrossRef] [PubMed]
- De Souza, G.C.; Haas, A.P.; Poser, G.L.; Schapoval, E.E.; Elisabetsky, E. Ethnopharmacological studies of antimicrobial remedies in the south of Brazil. J. Ethnopharmacol. 2004, 90, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Kan, W.S. Manual of Vegetable Drugs in Taiwan; The Chinese Medicine Publishing Inc.: Taipei, Taiwan, 1968; Volume 3, p. 30. [Google Scholar]
- Yoshikawa, M.; Shimada, H.; Shimoda, H.; Murakami, N.; Yamahara, J.; Matsuda, H. Bioactive constituents of Chinese natural medicines. II. Rhodiocyanosides A and B from the underground part of Rhodiola (R.) quadrifida (PALL) FISCH. Et MEY. (Crassulaceae). Chem. Pharm. Bull. 1996, 44, 2086–2091. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Wang, M.; Hao, H.; Zhang, D.; Lee, K.H. Hepatoprotective triterpenes from Sedum sarmentosum. Phtochemistry 1998, 49, 2607–2610. [Google Scholar] [CrossRef]
- Morikawa, T.; Ninomiya, K.; Zhang, Y.; Yamada, T.; Nakamura, S.; Matsuda, H.; Muraoka, O.; Hayakawa, T.; Yoshikawa, M. Flavonol glycosides with lipid accumulation inhibitory activity from Sedum sarmentosum. Phytochem. Lett 2012, 5, 53–58. [Google Scholar] [CrossRef]
- Kang, T.H.; Pae, H.O.; Yoo, J.C.; Kim, N.Y.; Kim, Y.C.; Ko, G.I.; Chung, H.T. Antiproliferative effects of alkaloids from Sedum sarmentosum on murine and human hepatoma cell limes. J. Ethnopharmacol. 2000, 70, 177–182. [Google Scholar] [CrossRef]
- Zhang, Y.; Morikawa, T.; Nakamura, S.; Ninomiya, K.; Matsuda, H.; Muraoka, O.; Yoshikawa, M. Bioactive constituents from Chinese natural medicines. XXV. New flavonol bisdesmosides, sarmenosides I, II, III and IV, with hepato- protective activity from Sedum sarmentosum. Heterocycles 2007, 71, 1565–1567. [Google Scholar]
- De Melo, G.O.; Malvar, D.C.; Vanderlinde, F.A.; Pires, P.A.; Côrtes, W.S.; Filho, G.P.; Muzitano, M.F.; Kaiser, C.R.; Costa, S.S. Phytochemical and pharmacological study of Sedum dendroideun leaf juice. J. Ethnopharmacol. 2005, 102, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Morikawa, T.; Zhang, Y.; Nakamura, S.; Muraoka, O.; Matsuda, H. Megastigmanes and their glucosides from the whole plant of Sedum sarmentosum. J. Nat. Prod. 2007, 70, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Shimada, H.; Horikawa, S.; Murakami, T.; Shimada, H.; Yamahara, J.; Matsuda, H. Bioactive Constituents of Chinese Natural Medicones. IV. Rhodiolae radix. (2): On the Histamine Release Inhibitor from the Underground Part of Rhodiola sacra (PRAIN ex. HAMET) S. H. Fu (Crassulaceae): Chemical Structure of Rhodiocyanoside D and Sacranosides A and B. Chem. Pharm. Bull. 1997, 45, 1498–1503. [Google Scholar] [PubMed]
- Nishida, R.; Rothschild, M.; Mummery, R. Acyanoglucoside, sarmentosin, from the magpie moth, Abraxas grossulariata, geometridae: Lepidoptera. Phytochemistry 1994, 36, 37–38. [Google Scholar] [CrossRef]
- Yang, Z.; Tu, Y.; Baldermann, S.; Dong, F.; Xu, Y.; Watanabe, N. Isolation and identification of compounds from the ethanolic extract of flowers of the tea (Camellia sinensis) plant and their contribution to the antioxidant capacity. LWT-Food Sci. Technol. 2009, 42, 1439–1443. [Google Scholar] [CrossRef]
- Le Gall, G.; Dupont, M.S.; Mellon, F.A.; Davis, A.L.; Collins, G.J.; Verhoeyen, M.E. Characterization and content of flavonoid glycosides in genetically modified tomato (Lycopersicon esculentum) fruits. J. Agric. Food. Chem. 2003, 51, 2438–2446. [Google Scholar] [CrossRef] [PubMed]
- Velozo, L.S.M.; Ferreira, M.J.P.; Santos, M.I.S.; Moreira, D.L.; Guimarães, E.F.; Emerenciano, V.P.; Kaplan, M.A.C. C-glycosyl flavones from Peperomia blanda. Fitoterapia 2009, 80, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Yoshioka, I.; Yamasaki, K.; Kim, I.H. Studies on the Constituents of Gastrodia elata. Chem. Pharm. Bull. 1981, 29, 55–62. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure–activity relationships. Free Radic. Biol. Med. 1996, 22, 749–760. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Structure–activity relationships of flavonoids in the cellular antioxidant activity assay. J. Agric. Food. Chem. 2008, 56, 8404–8411. [Google Scholar] [CrossRef] [PubMed]
- Markham, K.R.; Ternai, B.; Stanley, R.; Geiger, H.; Mabry, T.J. Carbon-13 studies of flavonoids-III. Naturally occurring flavonoid glycosides and their acylated derivatives. Tetrahedron 1978, 34, 1389–1397. [Google Scholar] [CrossRef]
- Wiedenfeld, H.; Cetto, A.A.; Amador, C.P. Flavonol glycosides from Equisetum myriochetum. Biochem. Syst. Ecol. 2000, 28, 395–397. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, P.; Xu, Y. Preventive effect of gastrodin on cognitive decline after cardiac surgery with cardiopulmonary bypass: A bouble-blind, randomized controlled study. J. Huazhong Univ. Sci. Technol. 2011, 31, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, Y.M.; Yu, G.Y. Effect of gastrodin injection on blood pressure and vasoactive substances in treatment of old patients with refractory hypertension: A randomized controlled trial. J. Chin. Integr. Med. 2008, 6, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Liau, B.C.; Jong, T.T.; Chang, C.M.J. Extraction and Purification of Flavanone Glycosides and Kaemferol Glycosides from Defatted Camellia Oleifera Seeds by Salting-Out Using Hydrophilic Isopropanol. Sep. Purif. Technol. 2009, 67, 31–37. [Google Scholar] [CrossRef]
- Chen, J.H.; Wu, H.Y.; Liau, B.C.; Chang, C.M.J.; Jong, T.T.; Wu, L.C. Identification and Evaluation of Antioxidants Defatted Camellia Oleifera Seeds by Isopropanol Salting-Out Pretreatment. Food Chem. 2010, 121, 1246–1254. [Google Scholar] [CrossRef]
- Lee, J.C.; Kim, H.R.; Kim, J.; Jang, Y.S. Antioxidant property of an enthanol extract of the stem of Opuntia ficus-indica var. saboten. J. Agric. Food. Chem. 2002, 50, 6490–6496. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.I. High- throughput assay of oxygen radical absorbance capacity (ROAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food. Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]
- Mandal, S.; Hazra, B.; Sarkar, R.; Biswas, S.; Mandal, N. Assessment of the Antioxidant and Reactive Oxygen Species Scavenging of Methanolic Extract of Caesalpinia crista Leaf. Evid. Based Complement. Altern. Med. 2011, 2011, 173768. [Google Scholar]
- Ruch, R.J.; Cheng, S.J.; Klavning, J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogens 1989, 10, 1003–1008. [Google Scholar] [CrossRef]
- Sample Availability: Not Available.
| Extraction Method | Isopropanod Salting-Out Extraction Method | Butanol Partition Extraction Method | |||
|---|---|---|---|---|---|
| Antioxidant Assay | Average Cleaning Factor (%) | Equivalent to Trolox (mmol/g) a | Average Cleaning Factor (%) | Equivalent to Trolox (mmol/g) a | |
| b FRAP | 31.85 | 0.071 ± 0.011 | 21.60 | 0.052 ± 0.005 | |
| c SOD | 77.96 | 0.210 ± 0.005 | 20.41 | 0.057 ± 0.007 | |
| d NO* scavenging | 57.48 | 0.118 ± 0.006 | 25.27 | 0.054 ± 0.007 | |
| e ORAC | 70.56 | 0.198 ± 0.005 | 57.83 | 0.118 ± 0.005 | |
| Position | Compound 8 (in CD3OD) | |
|---|---|---|
| 1H (δ) (ppm) | 13C (δ) (ppm) | |
| 1 | 158.4 | |
| 2 | 7.06 (1H, d, J = 8.8 Hz) | 116.4 |
| 3 | 7.67 (1H, d, J = 8.8 Hz) | 132.7 |
| 4 | 130.9 | |
| 5 | 7.67 (1H, d, J = 8.8 Hz) | 132.7 |
| 6 | 7.06 (1H, d, J = 8.8 Hz) | 116.4 |
| 7 | 6.93 (1H, d, J = 12.8 Hz) | 144.2 |
| 8 | 5.88 (1H, d, J = 12.8 Hz) | 117.8 |
| 9 | 168.3 | |
| 10 | 3.66 (3H, s) | 51.8 |
| Rha | ||
| 1’ | 5.51(1H, d, J = 1.8 Hz) | 99.4 |
| 2’ | 4.30 (1H, m) | 71.3 |
| 3’ | 3.97 (1H, m) | 82.7 |
| 4’ | 3.95 a (1H, m) | 72.6 |
| 5’ | 3.67 a (1H, m) | 70.4 |
| 6’ | 1.23 (3H, d, J = 6.0 Hz) | 18.1 |
| Glc | ||
| 1” | 4.61 (1H, d, J = 7.5 Hz) | 105.9 |
| 2” | 3.35 a (1H, m) | 72.3 |
| 3” | 3.39 a (1H, m) | 77.7 |
| 4” | 3.66 a (1H, m) | 72.6 |
| 5” | 3.37 a (1H, m) | 77.8 |
| 6’’a | 3.74 a (1H, dd, J = 11.9; 4.7 Hz, 1H) | 62.0 |
| 6”b | 3.85 a (1H, dd, J = 11.9; 2.31 Hz, 1H) | |
| Compounds | FRAP (mol of TE/mol) a | SOD (mol of TE/ mol) a | NO* Scavenging (mol of TE/ mol) a | ORAC (mol of TE/mol) a |
|---|---|---|---|---|
| 1 | 0.17 ± 0.01 | 0.25 ± 0.01 | 0.09 ± 0.04 | nt c |
| 2 | 0.33 ± 0.02 | 0.53 ± 0.01 | nd b | nt c |
| 3 | 0.29 ± 0.01 | 0.19 ± 0.01 | nd b | nt c |
| 4 | 0.91 ± 0.04 | 0.81 ± 0.04 | 0.59 ± 0.02 | 0.82 ± 0.04 |
| 5 | 1.14 ± 0.01 | 0.95 ± 0.03 | 1.01 ± 0.05 | 1.03 ± 0.03 |
| 6 | 1.23 ± 0.01 | 1.03 ± 0.04 | 0.83 ± 0.02 | 1.06 ± 0.06 |
| 7 | 0.82 ± 0.02 | 1.21 ± 0.07 | 0.32 ± 0.01 | nt c |
| 8 | 0.60 ± 0.02 | 0.51 ± 0.07 | 0.48 ± 0.05 | 0.64 ± 0.02 |
| Trolox (reference) 1.00 ± 0.01 | ||||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-H.; Lai, W.-H.; Lin, S.-D.; Lan, C.-F.; Hsu, S.-L.; Liao, M.-Y. Comparison of Antioxidant Capability after Isopropanol Salting-Out Pretreatment and n-Butanol Partition Extraction, and Identification and Evaluation of Antioxidants of Sedum formosanum N.E.Br. Molecules 2016, 21, 513. https://doi.org/10.3390/molecules21040513
Chen J-H, Lai W-H, Lin S-D, Lan C-F, Hsu S-L, Liao M-Y. Comparison of Antioxidant Capability after Isopropanol Salting-Out Pretreatment and n-Butanol Partition Extraction, and Identification and Evaluation of Antioxidants of Sedum formosanum N.E.Br. Molecules. 2016; 21(4):513. https://doi.org/10.3390/molecules21040513
Chicago/Turabian StyleChen, Jung-Hui, Wen-Hui Lai, Shang-Dung Lin, Cheng-Fong Lan, Shih-Lan Hsu, and Ming-Yuan Liao. 2016. "Comparison of Antioxidant Capability after Isopropanol Salting-Out Pretreatment and n-Butanol Partition Extraction, and Identification and Evaluation of Antioxidants of Sedum formosanum N.E.Br." Molecules 21, no. 4: 513. https://doi.org/10.3390/molecules21040513





