Inhibition of Listeria monocytogenes in Fresh Cheese Using Chitosan-Grafted Lactic Acid Packaging
Abstract
:1. Introduction
2. Results and Discussion
2.1. Grafting of LA onto Chitosan
2.2. Mechanical Properties of Films
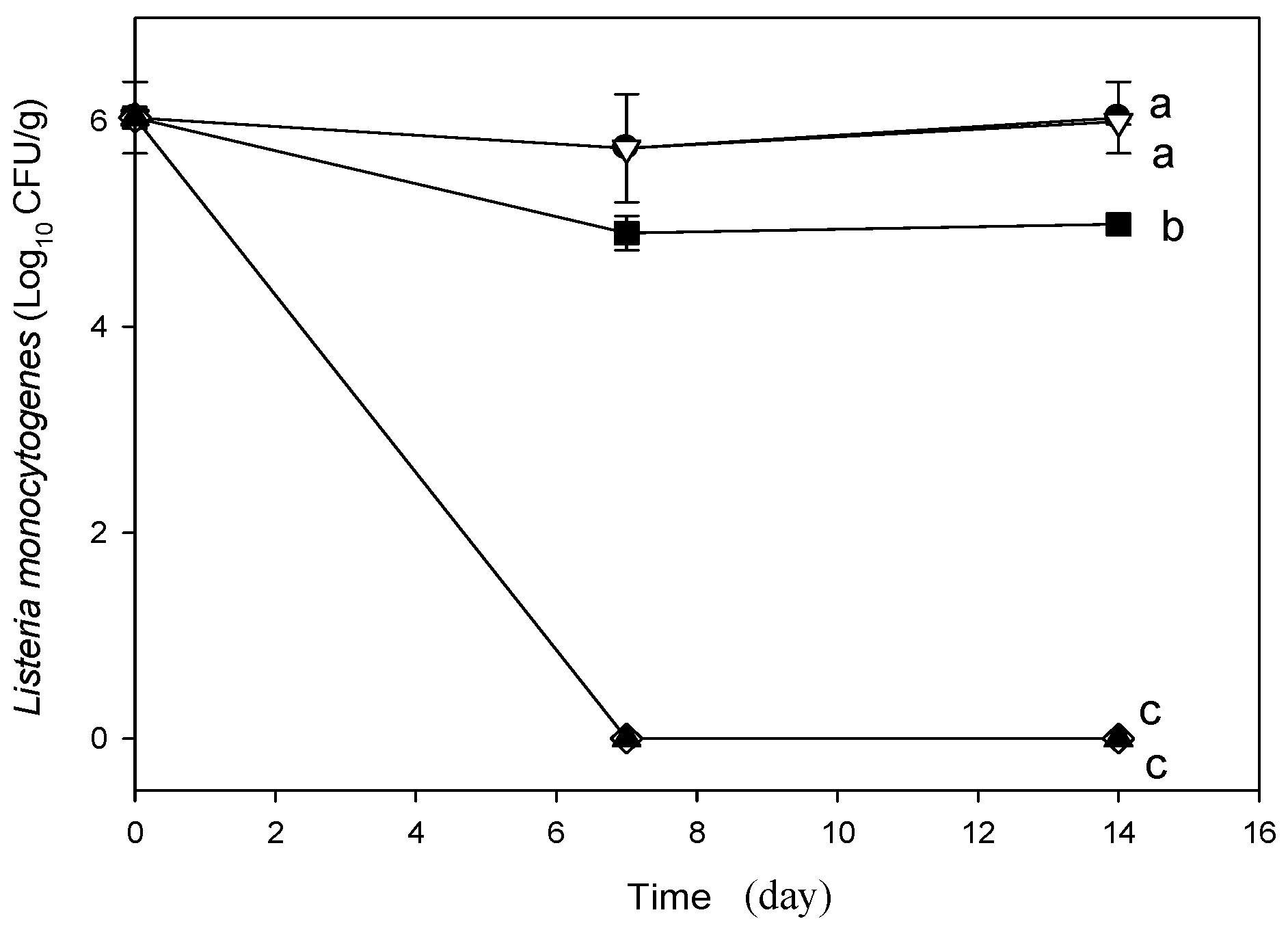
2.3. Analyzes of Fresh Cheese Inoculated with L. monocytogenes
2.4. SEM Analyses of Samples
3. Materials and Methods
3.1. Materials
3.2. Preparation of Chitosan Films
3.3. Grafting of Chitosan with LA and Production Films
3.4. Chitosan Characterization and Characterization of Films
3.5. Analysis of Coated Cheese Samples
3.6. Scanning Electron Microscopy Analyses
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferreira, V.; Wiedmann, M.; Teixeira, P.; Stasiewicz, M.J. Listeria monocytogenes persistence in food-associated environments: Epidemiology, strain characteristics, and implications for public health. J. Food Protect. 2014, 1, 150–170. [Google Scholar] [CrossRef] [PubMed]
- Farber, J.M.; Peterkin, P.I. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 1991, 55, 476–511. [Google Scholar] [PubMed]
- Genigeorgis, C.; Carniciu, M.; Dutulescu, D.; Farver, T.B. Growth and survival of Listeria monocytogenes in market cheeses stored at 4 to 30 °C. J. Food Protect. 1991, 54, 662–668. [Google Scholar]
- Stessl, B.; Fricker, M.; Fox, E.; Karpiskova, R.; Demnerova, K.; Jordan, K.; Ehling-Schulz, M.; Wagner, M. Collaborative survey on the colonization of different types of cheese-processing facilities with Listeria monocytogenes. Foodborne Pathog. Dis. 2013, 11, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Melo, J.; Andrew, P.W.; Faleiro, M.L. Listeria monocytogenes in cheese and the dairy environment remains a food safety challenge: The role of stress responses. Food Res. Int. 2015, 67, 75–90. [Google Scholar] [CrossRef]
- Tenenhaus-Aziza, F.; Daudin, J.J.; Maffre, A.; Sanaa, M. Risk-based approach for microbiological food safety management in the dairy industry: The case of Listeria monocytogenes in soft cheese made from pasteurized milk. Risk Anal. 2014, 34, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vitela, M.R.; Mendoza-Bernardo, M.; Castro-Rosas, J.; Gomez-Aldapa, C.A.; Garay-Martínez, L.E.; Navarro-Hidalgo, V.; Villarruel-López, A. Incidence of Salmonella, Listeria monocytogenes, Escherichia coli O157:H7, and staphylococcal enterotoxin in two types of Mexican fresh cheeses. J. Food Protect. 2012, 75, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Romero, M.C.; Murphy, T.; Morris, M.; Cummins, E.; Kerry, J.P. Antimicrobial activity of chitosan, organic acids and nano-sized solubilisates for potential use in smart antimicrobially-active packaging for potential food applications. Food Control 2013, 34, 393–397. [Google Scholar] [CrossRef]
- Suyatma, N.; Copinet, A.; Copinet, E.; Fricoteaux, F.; Coma, V. Different Pla grafting techniques on chitosan. J. Polym. Environ. 2011, 19, 166–171. [Google Scholar] [CrossRef]
- Di Pierro, P.; Sorrentino, A.; Mariniello, A.; Concetta, V. Chitosan/whey protein as active coating to extend Ricotta cheese shelf-life. Food Sci. Technol. 2011, 44, 2224–2327. [Google Scholar] [CrossRef]
- Miranda, P.; Cardenas, G.; Lopez, D.; Sagahon, A. Comportamiento de peliculas de quitosano compuesto en un modelo de almacenamiento de aguacate. J. Mex. Chem. Soc. 2003, 47, 1456–1467. [Google Scholar]
- Leceta, I.; Guerrero, P.; de la Caba, K. Functional properties of chitosan-based films. Carbohydr. Polym. 2013, 93, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Wirsén, A.; Albertsson, A. Synthesis and characterization of pH-sensitive hydrogels based on chitosan and d,l-lactic acid. J. Appl. Polym. Sci. 1999, 74, 3193–3202. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Khoshgozaran, S.; Hossain-Azizi, M.H.; Hamidy, Z.; Bagheripoor-Fallah, F. Mechanical, physicochemical and color properties of chitosan based-films as a function of Aloe vera gel incorporation. Carbohydr. Polym. 2012, 87, 2058–2062. [Google Scholar] [CrossRef]
- Bonilla, J.; Fortunati, E.; Vargas, M.; Chiralt, A.; Kenny, J.M. Effects of chitosan on the physicochemical and antimicrobial properties of PLA films. J. Food Eng. 2013, 119, 236–243. [Google Scholar] [CrossRef]
- Han, H. Innovations in Food Packaging. J. Food Sci. Technol. 2010, 47, 1765–1770. [Google Scholar]
- Martins, J.; Cerqueira, M.; Souza, B.; Carmo, A.; Vicente, A. Shelf Life extension of Ricotta cheese using coatings of galactomannans from nonconventional sources incorporating nisin against Listeria monocytogenes. J. Agric. Food Chem. 2010, 58, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariani, C.; Oulahal, N.; Chamba, J.; Dubois-Brissonnet, F.; Notz, E.; Briandet, R. Inhibition of Listeria monocytogenes by resident biofilms present on wooden shelves used for cheese ripening. Food Control 2011, 22, 45–51. [Google Scholar] [CrossRef]
- Saxer, S.; Schwenninger, S.M.; Lacroix, C. Characterization of the microflora of industrial Mexican cheeses produced without added chemical preservatives. LWT Food Sci. Technol. 2013, 53, 314–320. [Google Scholar] [CrossRef]
- Martin, A.H.; Goff, H.D.; Smith, A.; Dalgleish, D.G. Immobilization of casein micelles for probing their structure and interactions with polysaccharides using scanning electron microscopy (SEM). Food Hydrocolloid 2006, 20, 817–824. [Google Scholar] [CrossRef]
- Kougoulos, E.; Marziano, I.; Miller, P.R. Lactose particle engineering: Influence of ultrasound and anti-solvent on crystal habit and particle size. J. Cryst. Growth 2010, 312, 3509–3520. [Google Scholar] [CrossRef]
- Razmi, M.; Divsalara, A.; Saboury, A.A.; Izadi, Z.; Haertlé, T.; Mansuri-Torshizidi, H. β-casein and its complexes with chitosan as nanovehicles for delivery of a platinum anticancer drug. Colloids Surfaces B 2013, 112, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Espadín, A.; Vázquez, N.; Tecante, A.; Tamay de Dios, L.; Gimeno, M.; Velasquillo, C.; Shirai, K. Fibroblast viability and inhibitory activity against Pseudomonas aeruginosa in lactic acid-grafted chitosan hydrogels. J. Appl. Polym. Sci. 2014, 131, 14. [Google Scholar] [CrossRef]
- Pacheco, N.; Gárnica-González, M.; Gimeno, M.; Bárzana, E.; Trombotto, S.; David, L.; Shirai, K. Structural characterization of chitin and chitosan obtained by biological and chemical methods. Biomacromolecules 2011, 12, 3285–3290. [Google Scholar] [CrossRef] [PubMed]
- American Society for Testing and Materials. Standard test method for tensile properties of thin plastic sheeting. In Standards Designations: D882-02. Annual Book of ASTM Standards; ASTM D882-02; American Society for Testing and Materials: Philadelphia, PA, USA, 1991; pp. 194–202. [Google Scholar]
- American Society for Testing and Materials. Standard test methods for water vapour transmission of materials. In Standards Designations: E96-95. Annual Book of ASTM Standards; ASTM E96-95; American Society for Testing and Materials: Philadelphia, PA, USA, 1995; pp. 406–413. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.
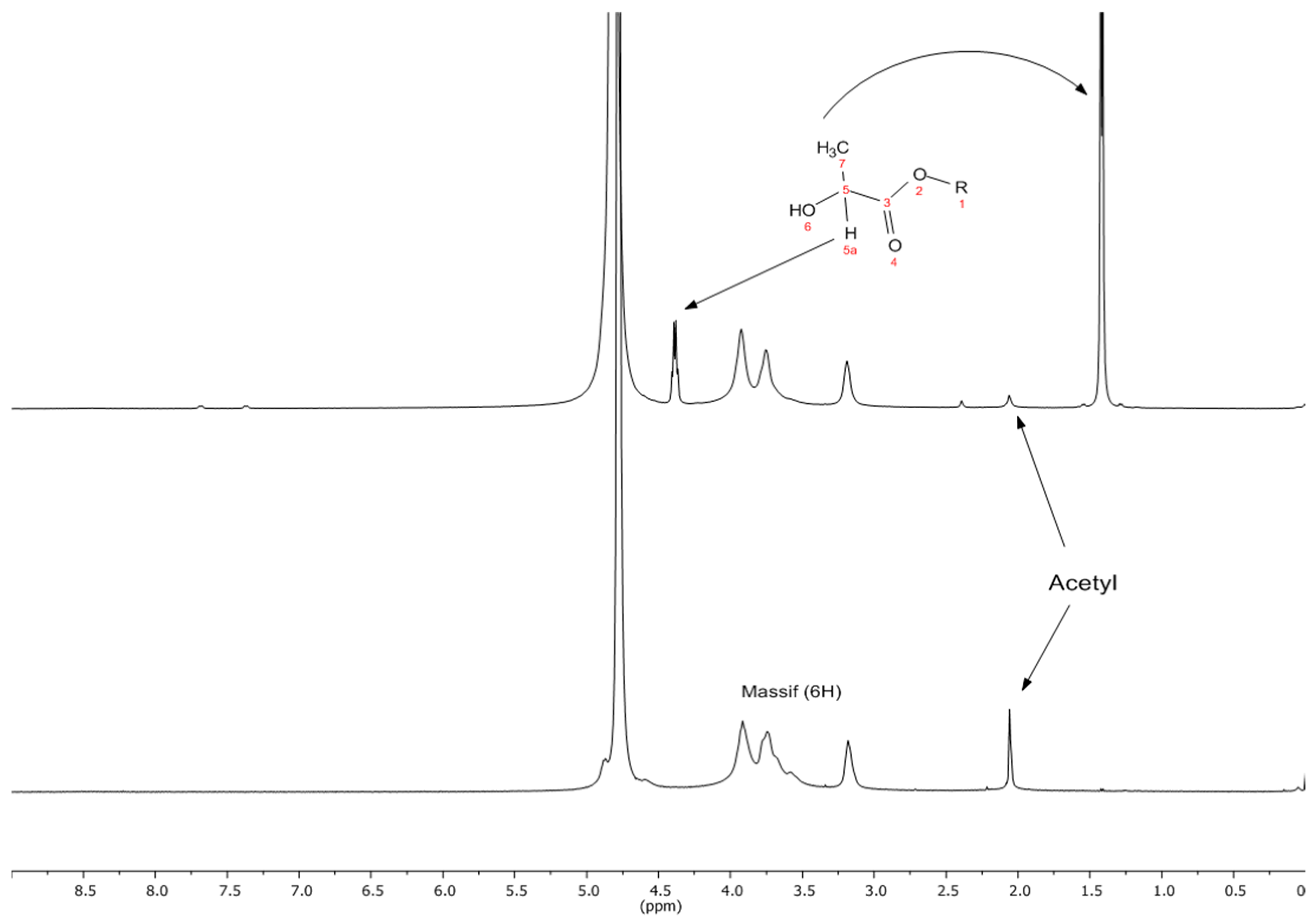


| Material | Yield (%) | Degree of Grafting (%) | Film Thickness (mm) | VWP (g·mm/m2·h·kPa) |
|---|---|---|---|---|
| Ch | - | - | 0.062 ± 0.006 b | 7 × 10−2 ± 2 × 10−3 a |
| ChLA | 51.16 ± 1.96 a | 36.85 ± 2.33 b | 0.074 ± 0.003 b | 1.6 × 10−3 ± 2.6 × 10−4 b |
| ChLA-TSA | 58.77 ± 1.65 a | 43.62 ± 0.90 a | 0.15 ± 0.003 a | 4.2 × 10−5 ± 1.4 × 10−6 c |
| ChLA-TSA-SPI | 48.45 ± 0.58 a | 33.38 ± 0.80 b | 0.15 ± 0.021 a | 4.4 × 10−5 ± 3.1 × 10−6 c |
| LDPE | - | - | 2.07 ± 0.3 c | 7.6 × 10−10 ± 1.4 × 10−9 d |
| Material | Young’s Modulus (MPa) | Elongation at Break (%) | Tensile Strength (MPa) |
|---|---|---|---|
| Ch | 2.2 ± 0.56 d | 57.2 ± 27.0 b | 0.61 ± 0.20 c |
| ChLA | 16.8 ± 0.20 c | 75.4 ± 5.9 b | 31.3 ± 1.12 b |
| ChLA-TSA | 105.6 ± 2.15 b | 67.6 ± 5.6 b | 19.25 ± 1.19 b |
| ChLA-TSA-SPI | 49.26 ± 7.43 c | 78.0 ± 9.5 b | 88.29 ± 2.46 a |
| LDPE | 371.62 ± 12.48 a | 205 ± 24.4 a | 23.58 ± 0.96 b |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandoval, L.N.; López, M.; Montes-Díaz, E.; Espadín, A.; Tecante, A.; Gimeno, M.; Shirai, K. Inhibition of Listeria monocytogenes in Fresh Cheese Using Chitosan-Grafted Lactic Acid Packaging. Molecules 2016, 21, 469. https://doi.org/10.3390/molecules21040469
Sandoval LN, López M, Montes-Díaz E, Espadín A, Tecante A, Gimeno M, Shirai K. Inhibition of Listeria monocytogenes in Fresh Cheese Using Chitosan-Grafted Lactic Acid Packaging. Molecules. 2016; 21(4):469. https://doi.org/10.3390/molecules21040469
Chicago/Turabian StyleSandoval, Laura N., Monserrat López, Elizabeth Montes-Díaz, Andres Espadín, Alberto Tecante, Miquel Gimeno, and Keiko Shirai. 2016. "Inhibition of Listeria monocytogenes in Fresh Cheese Using Chitosan-Grafted Lactic Acid Packaging" Molecules 21, no. 4: 469. https://doi.org/10.3390/molecules21040469






