Purification and Characterization of a White Laccase with Pronounced Dye Decolorizing Ability and HIV-1 Reverse Transcriptase Inhibitory Activity from Lepista nuda
Abstract
:1. Introduction
2. Results and Discussion
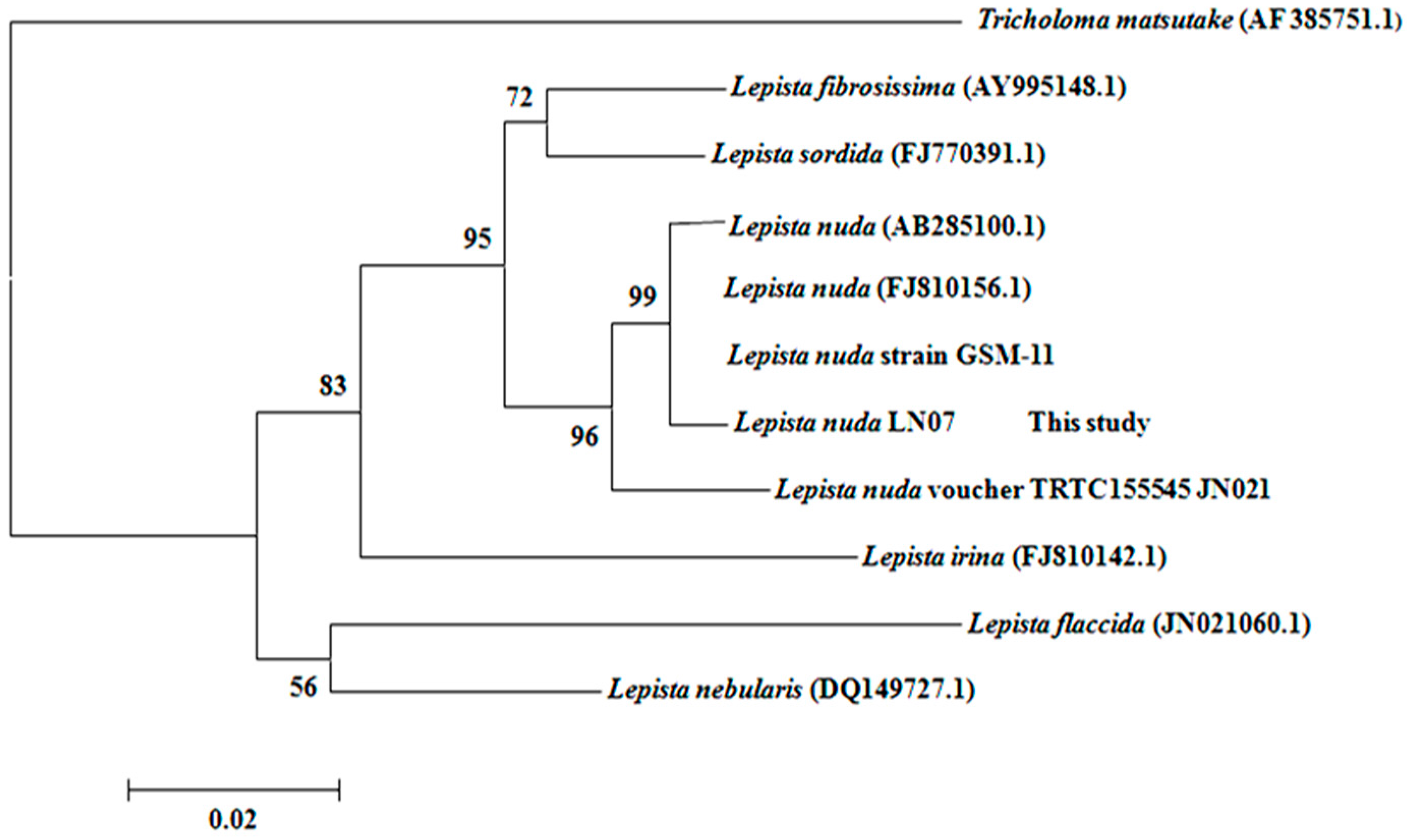
2.1. The Classification and Determination of the Isolated Strain LN07
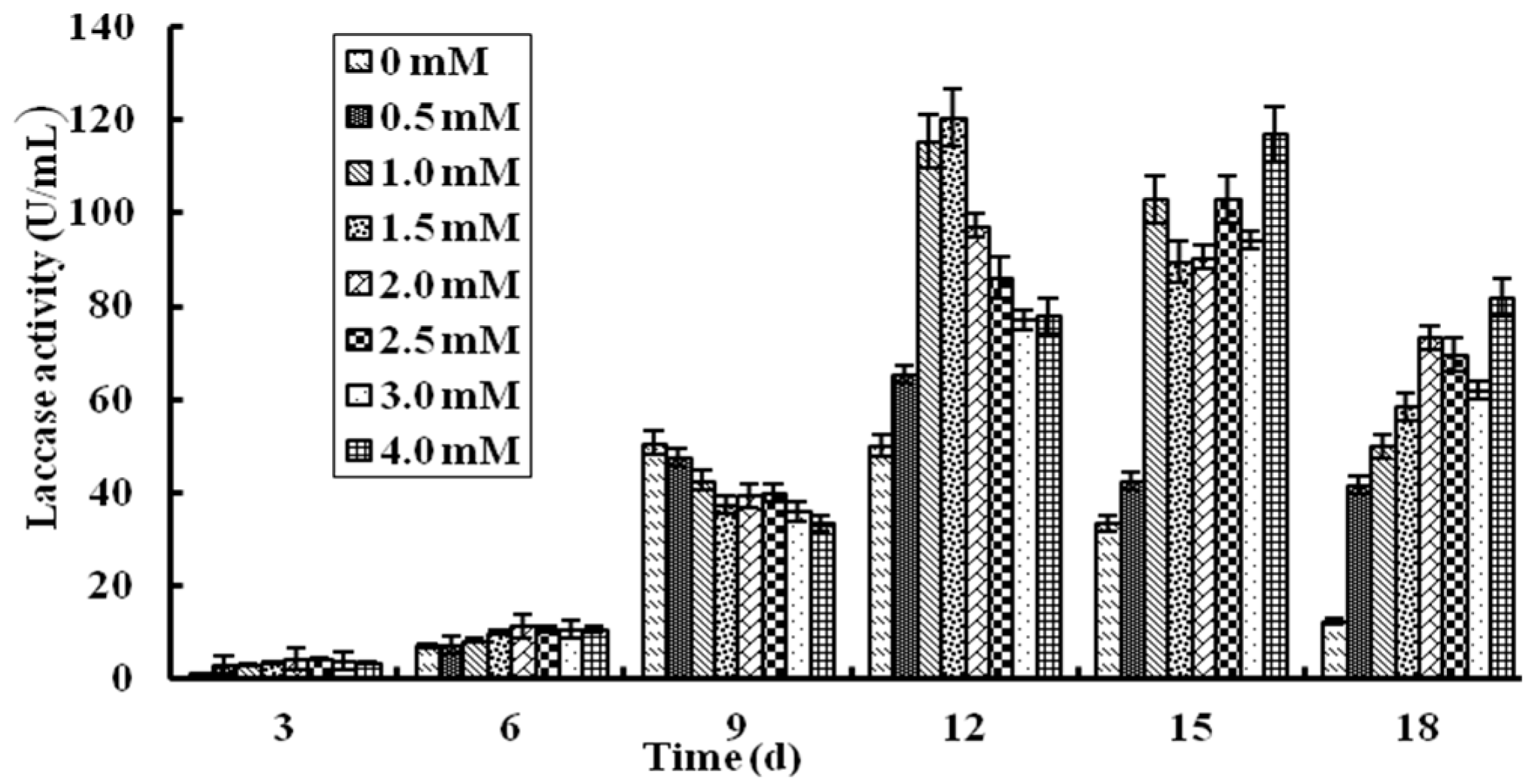
2.2. Effect of Cu2+ Ions on Production of L. nuda Laccase in Liquid Fermentation
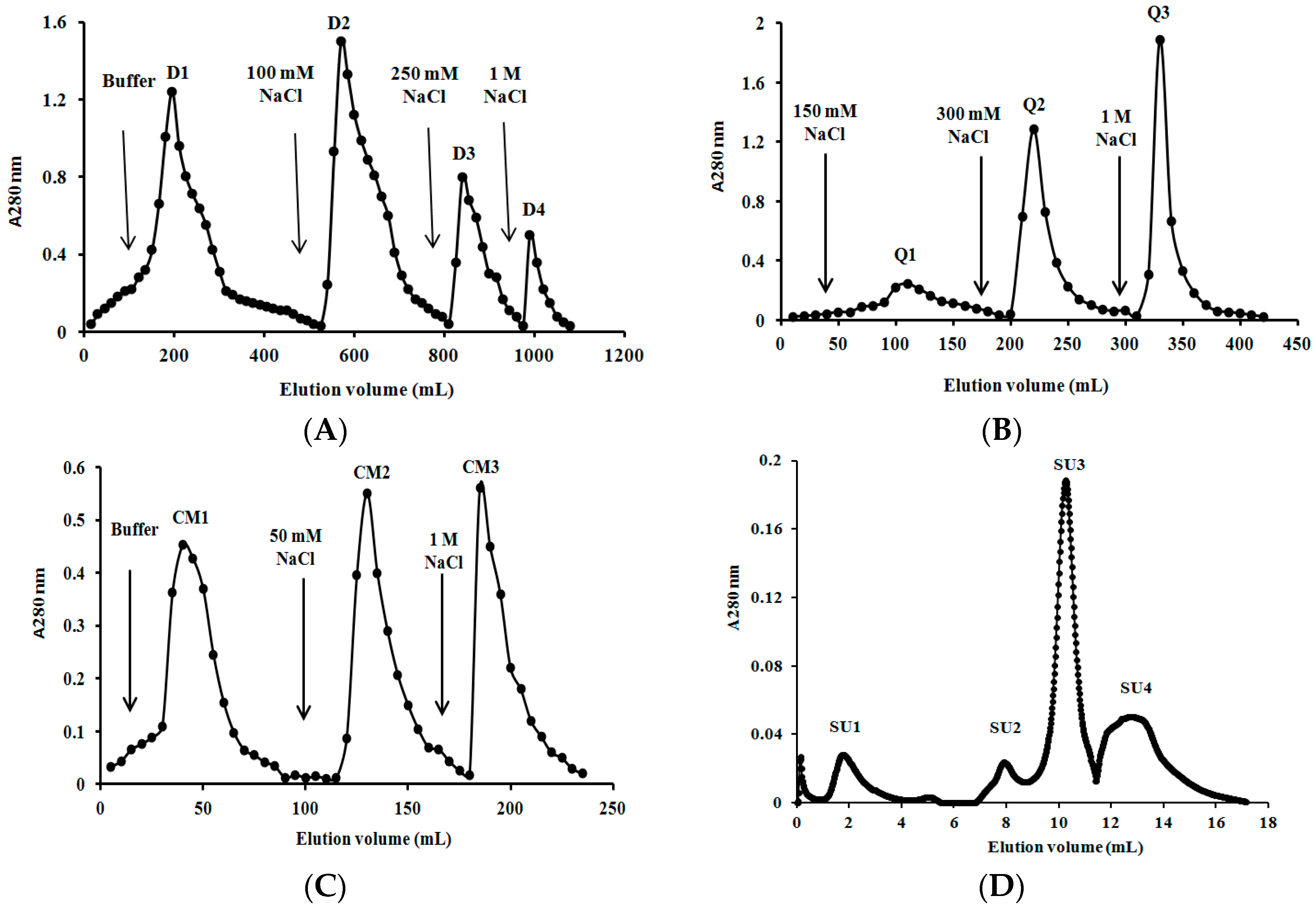
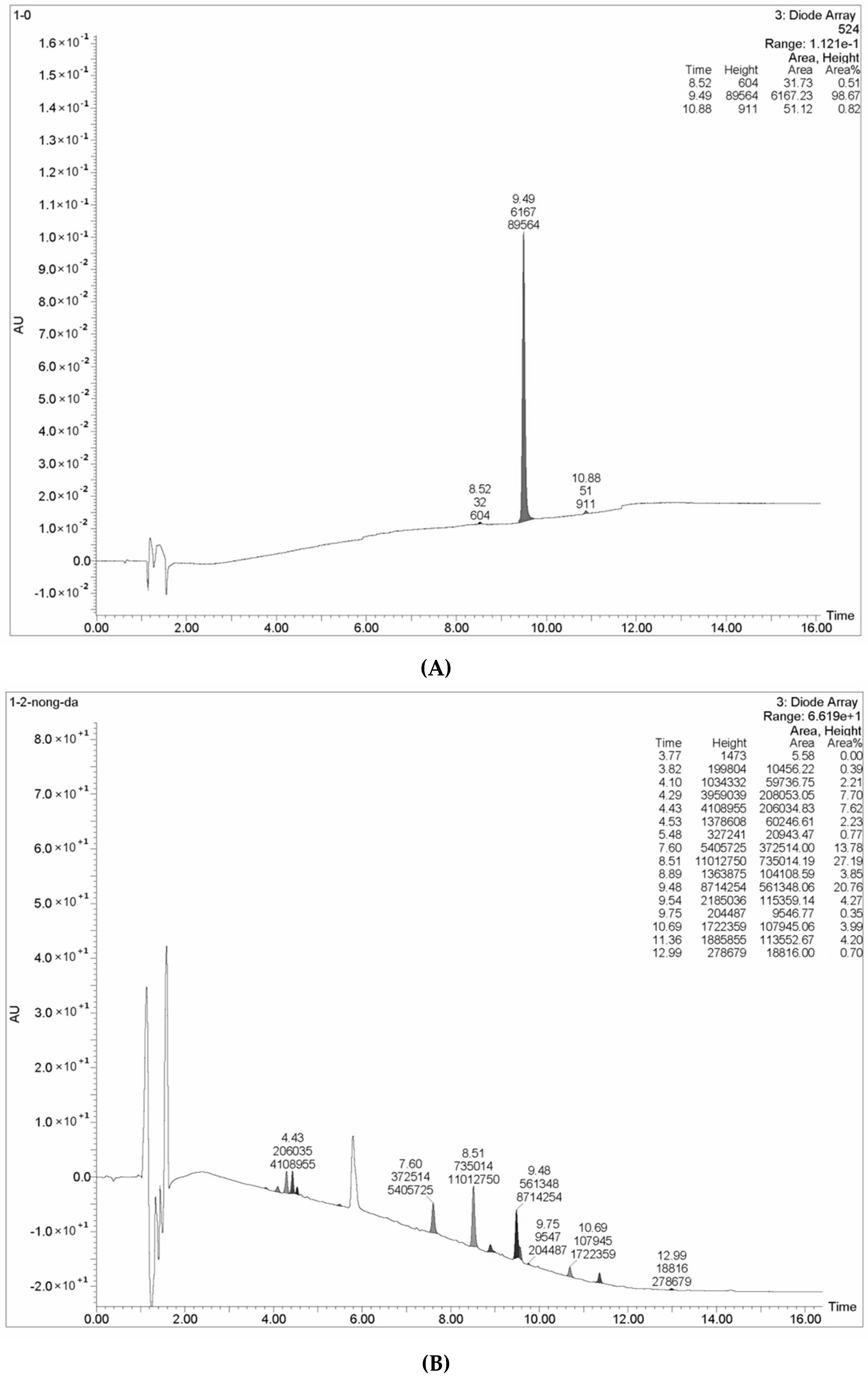
2.3. Purification of L. nuda Laccase
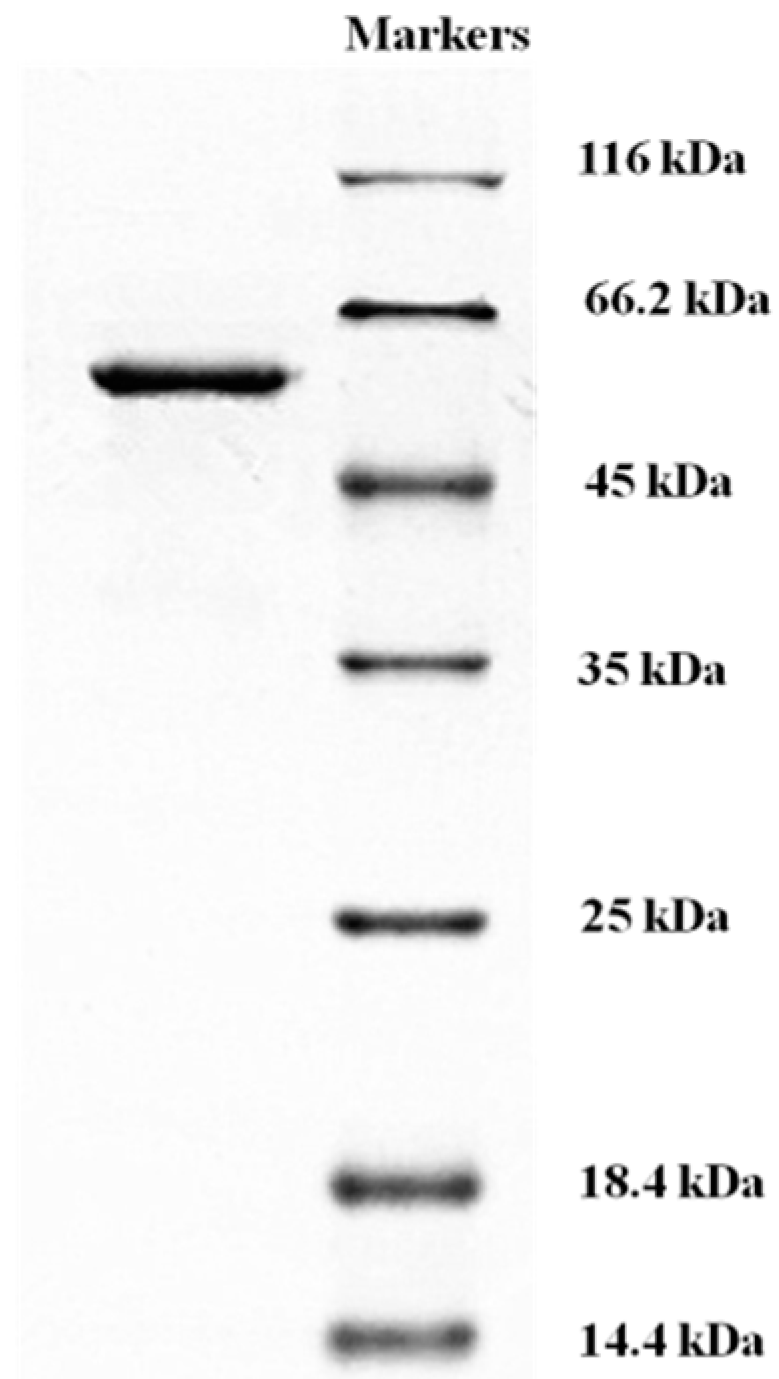
2.4. Determination of Molecular Mass and N-Terminal Amino Acid Sequence of L. nuda Laccase
2.5. Sequence Determination of L. nuda Laccase by Electrospray Ionization Mass-Mass Spectrometry (ESI-MS/MS) and 2D Nano-Liter Liquid Chromatography & Linear Ion Trap Quadrupole Mass Spectrometer (LC-LTQ)
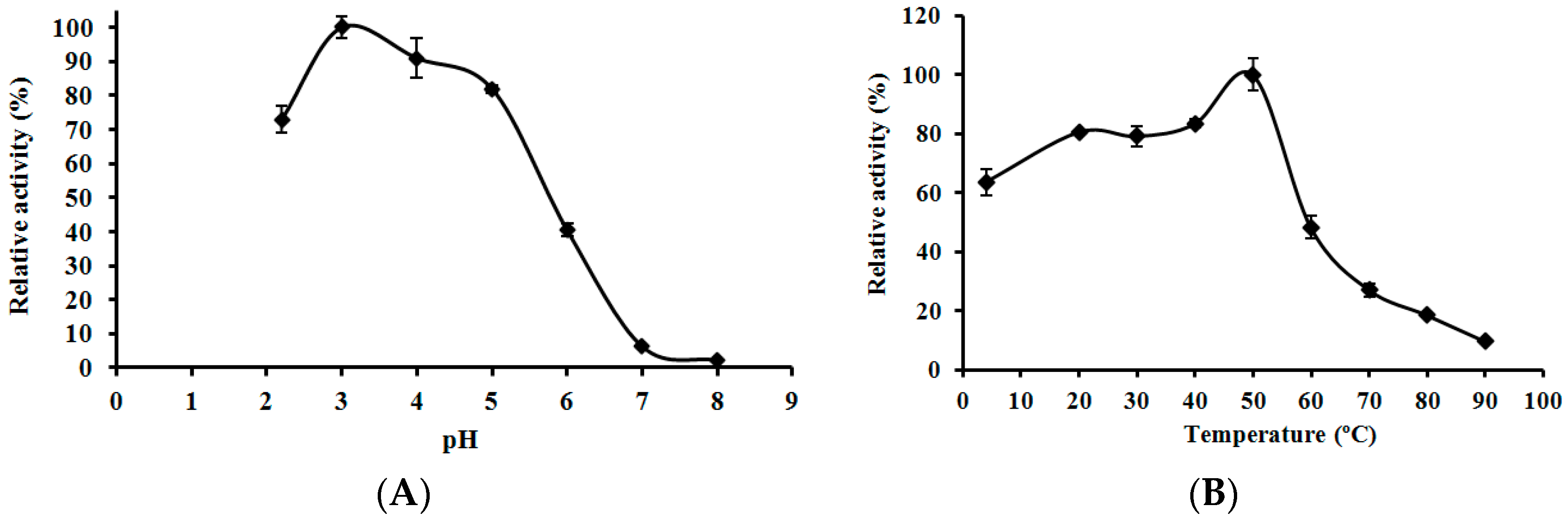
2.6. Effects of pH and Temperature on the Activity of L. nuda Laccase
2.7. Effects of Metal Ions and NaCl on Laccase Activity of L. nuda Laccase
2.8. Determination of L. nuda Laccase for Kinetic Parameters and Type
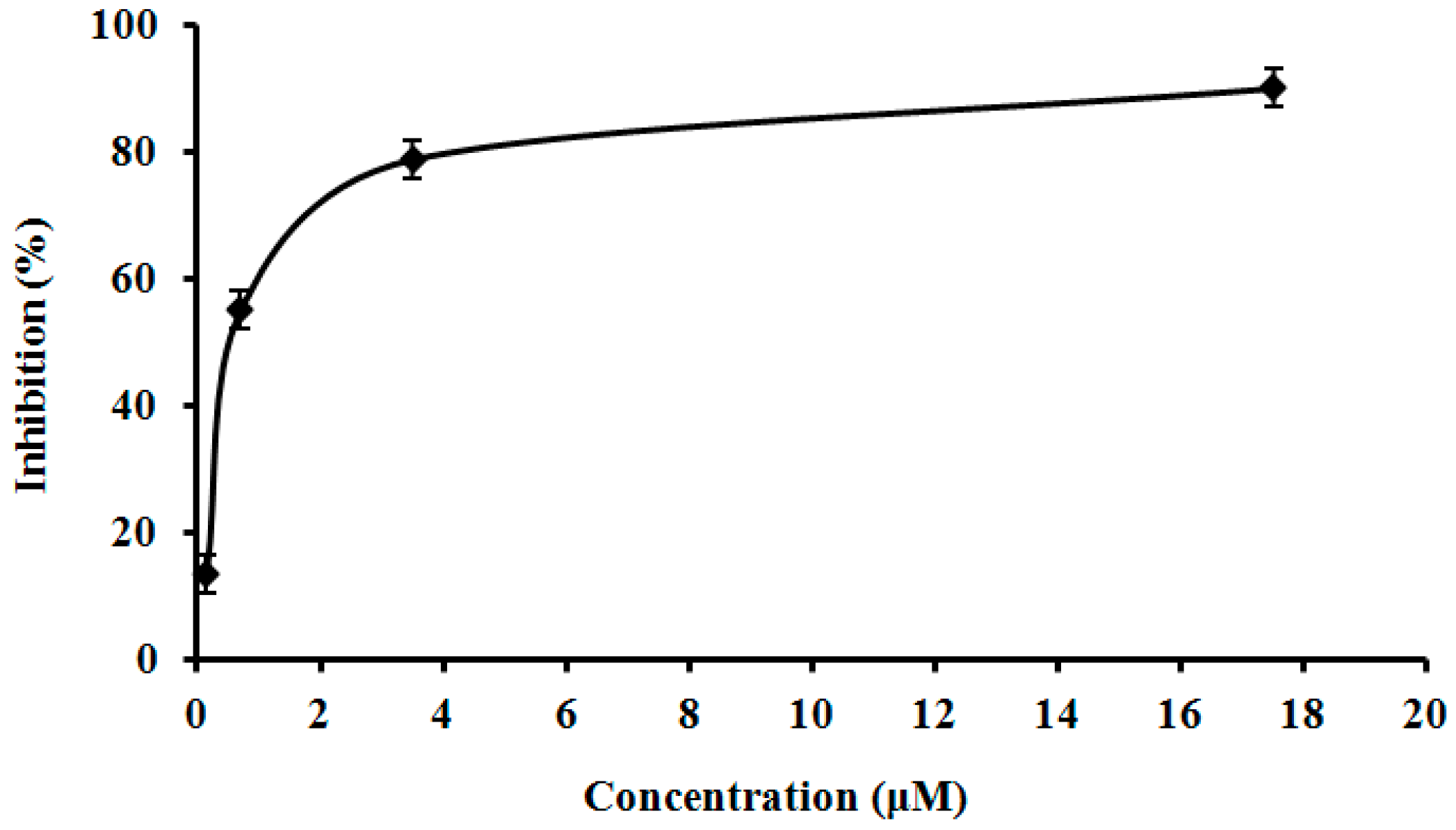
2.9. Assay of L. nuda Laccase for HIV-1 Reverse Transcriptase Inhibitory Activity
2.10. The Decolorizing Ability and Pesticide Degradation Ability of the Isolated Laccase
3. Experimental Section
3.1. Strain and Culture Conditions
3.2. Assay of Laccase Activity
3.3. Effect of Cu2+ Ions on Laccase Production in Liquid Fermentation
3.4. Purification of L. nuda Laccase
3.5. Determination of Laccase Molecular Mass and N-terminal Amino Acid Sequence of L. nuda Laccase
3.6. Sequence Determination of L. nuda Laccase by Electrospray Ionization Mass-Mass Spectrometry (ESI-MS/MS) and 2D Nano-Liter Liquid Chromatography & Linear Ion Trap Quadrupole Mass Spectrometer (LC-LTQ)
3.7. Effects of pH and Temperature on the Activity of L. nuda Laccase
3.8. Effects of Metal Ions and NaCl on the Activity of L. nuda Laccase
3.9. Determination of L. nuda Laccase for Kinetic Parameters and Type
3.10. Assay of L. nuda Laccase for HIV-1 Reverse Transcriptase Inhibitory Activity
3.11. Decolorizing and Pesticide Degrading Abilities of L. nuda Laccase
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xu, L.; Zhu, M.; Chen, X.; Wang, H.; Zhang, G. A novel laccase from fresh fruiting bodies of the wild medicinal mushroom Tricholoma matsutake. Acta Biochim. Pol. 2015, 62, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Giardina, P.; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: A never-ending story. Cell. Mol. Life Sci. 2010, 67, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhou, Y.; Xiao, Y.; Xu, Z.; Bian, Y. Cloning, expression and phylogenetic analysis of a divergent laccase multigene family in Auricularia auricula-judae. Microbiol. Res. 2014, 169, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Beccaglia, M.; Anastasi, P.; Grimaldi, E.; Rota, A.; Faustini, M.; Luvoni, G.C. Enhancement with inducers of lacasse production by some strains and application of enzyme to dechlorination of 2,4,5-trichlorophenol. Electr. J. Biotechnol. 2010, 13, 6–7. [Google Scholar]
- Janusz, G.; Czurylo, A.; Frac, M.; Rola, B.; Sulej, J.; Pawlik, A.; Siwulski, M.; Rogalski, J. Laccase production and metabolic diversity among Flammulina velutipes strains. World J. Microbiol. Biotechnol. 2015, 31, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hui, C.; Chen, M.; Ren, A.; Huang, J.; Hong, W.; Zhao, M.; Feng, Z. Cloning and functional analysis of a laccase gene during fruiting body formation in Hypsizygus marmoreus. Microbiol. Res. 2015, 179, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, F.; Jiang, Y.; Wu, G.; Guo, L.; Chen, R.; Chen, B.; Lu, Y.; Dai, Y.; Xie, B. The multigene family of fungal laccases and their expression in the white rot basidiomycete Flammulina velutipes. Gene 2015, 563, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.Z.; Li, P.Q. Cloning and expression of a novel laccase gene from Phytophthora capsici. J. Plant Pathol. 2013, 95, 417–421. [Google Scholar]
- Shleev, S.; Morozova, O.; Nikitina, O.; Gorshina, E.; Rusinova, T.; Serezhenkov, V.; Burbaev, D.; Gazaryan, I.; Yaropolov, A. Comparison of physico-chemical characteristics of four laccases from different basidiomycetes. Biochimie 2004, 86, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Xu, Z.S.; Wang, F.; Xiong, A.S. Isolation, purification and characterization of two laccases from carrot (Daucus carota L.) and their response to abiotic and metal ions stresses. Protein J. 2015, 34, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Rong, C.B.; Kong, C.; Liu, Y.; Xu, F.; Miao, Q.J.; Wang, S.X.; Wang, H.X.; Zhang, G.Q. A novel laccase with potent antiproliferative and HIV-1 reverse transcriptase inhibitory activities from mycelia of mushroom Coprinus comatus. Biomed. Res. Int. 2014, 417–441. [Google Scholar]
- Kunamneni, A.; Plou, F.J.; Ballesteros, A.; Alcalde, M. Laccases and their applications: A patent review. Recent Pat. Biotechnol. 2008, 2, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.V.S.; Phale, P.S.; Durani, S.; Wangikar, P.P. Combined sequence and structure analysis of the fungal laccase family. Biotechnol. Bioeng. 2003, 83, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Giardina, P.; Palmieri, G.; Scaloni, A.; Fontanella, B.; Faraco, V.; Cennamo, G.; Sannia, G. Protein and gene structure of a blue laccase from Pleurotus ostreatus 1. Biochem. J. 1999, 341, 655–663. [Google Scholar] [PubMed]
- Decker, H.; Terwilliger, N. Cops and robbers: Putative evolution of copper oxygen-binding proteins. J. Exp. Biol. 2000, 203, 1777–1782. [Google Scholar] [PubMed]
- Dan, Z.; Xi, Z.; Daizong, C.; Min, Z. Characterisation of a novel white laccase from the deuteromycete fungus Myrothecium verrucaria NF-05 and its decolourisation of dyes. PLoS ONE 2012, 7, e38817. [Google Scholar]
- Si, J.; Peng, F.; Cui, B. Purification, biochemical characterization and dye decolorization capacity of an alkali-resistant and metal-tolerant laccase from Trametes pubescens. Bioresour. Technol. 2013, 128, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Majeau, J.A.; Brar, S.K.; Tyagi, R.D. Laccases for removal of recalcitrant and emerging pollutants. Bioresour. Technol. 2010, 101, 2331–2350. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.R.; Wang, S.S.; Zhu, M.J.; Ning, Y.J.; Wang, S.N.; Li, B.; Yang, A.Z.; Zhang, G.Q.; Zhao, X.M. An extracellular laccase with potent dye decolorizing ability from white rot fungus Trametes sp. LAC-01. Int. J. Biol. Macromol. 2015, 81, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, M.; Mishra, S.; Sreekrishnan, T.R. Immobilized laccase mediated dye decolorization and transformation pathway of azo dye acid red 27. J. Environ. Health Sci. Eng. 2015. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-J.; Du, F.; Zhang, G.-Q.; Wang, H.-X.; Ng, T.-B. Purification of a laccase exhibiting dye decolorizing ability from an edible mushroom Russula virescens. Int. Biodeterior. Biodegrad. 2013, 82, 33–39. [Google Scholar] [CrossRef]
- Shraddha; Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial sources, production, purification, and potential biotechnological applications. Enzym. Res. 2011, 2011, 217–261. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Staples, R.C. Laccase: New functions for an old enzyme. Phytochemistry 2002, 60, 551–565. [Google Scholar] [CrossRef]
- Rodríguez, C.S.; Toca Herrera, J.L. Industrial and biotechnological applications of laccases: A review. Biotechnol. Adv. 2006, 24, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lin, Q.; Ng, T.B.; Ye, X.; Lin, J. Purification and characterization of a novel laccase from Cerrena sp. HYB07 with dye decolorizing ability. PLoS ONE. 2014, 9, e110834. [Google Scholar] [CrossRef] [PubMed]
- Bourbonnais, R.; Paice, M.G.; Freiermuth, B.; Bodie, E.; Borneman, S. Reactivities of various mediators and laccases with kraft pulp and lignin model compounds. Appl. Environ. Microbiol. 1997, 63, 4627–4632. [Google Scholar] [PubMed]
- Riva, S. Laccases: Blue enzymes for green chemistry. Trends Biotechnol. 2006, 5, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Leticia, P.; Castillo, M.D.P.; John, S.M. Degradation of glyphosate and other pesticides by ligninolytic enzymes. Biodegradation 2009, 20, 751–759. [Google Scholar]
- Wu, Y.Y.; Wang, H.X.; Ng, T.B. A novel metalloprotease from the wild basidiomycete mushroom Lepista nuda. J. Microbiol. Biotechnol. 2011, 21, 256–262. [Google Scholar] [PubMed]
- Riebel, M.; Sabel, A.; Claus, H.; Fronk, P.; Xia, N.; Li, H.; König, H.; Decker, H. Influence of laccase and tyrosinase on the antioxidant capacity of selected phenolic compounds on human cell lines. Molecules 2015, 20, 17194–17207. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, G.; Wang, H.; Ng, T. Purification and characterization of a laccase from the edible wild mushroom Tricholoma mongolicum. J. Microbiol. Biotechnol. 2010, 20, 1069–1076. [Google Scholar] [PubMed]
- Hu, D.D.; Zhang, R.Y.; Zhang, G.Q.; Wang, H.X.; Ng, T.B. A laccase with antiproliferative activity against tumor cells from an edible mushroom, white common Agrocybe cylindracea. Phytomed. Int. J. Phytother. Phytopharmacol. 2011, 18, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Ai, M.Q.; Wang, F.F.; Huang, F. Purification and characterization of a thermostable laccase from Trametes trogii and its ability in modification of kraft lignin. J. Microbiol. Biotechnol. 2015, 25, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Q.; Wang, Y.F.; Zhang, X.Q.; Ng, T.B.; Wang, H.X. Purification and characterization of a novel laccase from the edible mushroom Clitocybe maxima. Process Biochem. 2010, 45, 627–633. [Google Scholar] [CrossRef]
- Hamid, F.; Mohammad Ali, F.; Ahmad Reza, S.; Mojtaba Tabatabaei, Y. Purification and biochemical characterization of extracellular laccase from the ascomycete Paraconiothyrium variabile. Bioresour. Technol. 2011, 102, 1808–1814. [Google Scholar]
- Champagne, P.P.; Nesheim, M.E.; Ramsay, J.A. A mechanism for NaCl inhibition of Reactive Blue 19 decolorization and ABTS oxidation by laccase. Appl. Microbiol. Biotechnol. 2013, 97, 6263–6269. [Google Scholar] [CrossRef] [PubMed]
- Mueangtoom, K.; Kittl, R.; Mann, O.; Haltrich, D.; Ludwig, R. Low pH dye decolorization with ascomycete Lamprospora wrightii laccase. Biotechnol. J. 2010, 5, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Telke, A.A.; Kagalkar, A.N.; Jagtap, U.B.; Desai, N.S.; Bapat, V.A.; Govindwar, S.P. Biochemical characterization of laccase from hairy root culture of Brassica juncea L. and role of redox mediators to enhance its potential for the decolorization of textile dyes. Planta 2011, 234, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Nakade, K.; Nakagawa, Y.; Yano, A.; Sato, T.; Sakamoto, Y. Characterization of an extracellular laccase, PbLac1, purified from Polyporus brumalis. Fungal Biol. 2010, 114, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Telke, A.A.; Ghodake, G.S.; Kalyani, D.C.; Dhanve, R.S.; Govindwar, S.P. Biochemical characteristics of a textile dye degrading extracellular laccase from a Bacillus sp. ADR. Bioresour Technol. 2011, 102, 1752–1766. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Peng, R.; Zhang, Z.; Tian, Y.; Zhao, W.; Xue, Y.; Gao, J.; Yao, Q. Expression, characterization and 2,4,6-trichlorophenol degradation of laccase from Monilinia fructigena. Mol. Biol. Rep. 2012, 39, 3871–3877. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Tian, G.; Zhao, Y.; Zhao, L.; Wang, H.; Ng, T.B. A Fungal α-galactosidase from Tricholoma matsutake with broad substrate specificity and good hydrolytic activity on raffinose family oligosaccharides. Molecules 2015, 20, 13550–13562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tian, G.; Geng, X.; Zhao, Y.; Ng, T.B.; Zhao, L.; Wang, H. Isolation and characterization of a novel lectin from the edible mushroom Stropharia rugosoannulata. Molecules 2014, 19, 19880–19891. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds such as dyes are available from the authors.
| Fraction | Total Protein (mg) | Specific Laccase Activity (U/mg) | Total laccase ACTIVITY (U) | Recovery of Laccase Activity (%) | Purification Fold |
|---|---|---|---|---|---|
| Crude | 1307.6 | 0.9 | 1149.4 | 100.0 | 1.0 |
| D3 | 241.2 | 3.3 | 802.1 | 69.8 | 3.8 |
| Q2 | 25.3 | 26.2 | 662.8 | 57.7 | 29.8 |
| CM2 | 9.2 | 50.5 | 464.8 | 40.4 | 57.4 |
| SU3 | 2.5 | 163.3 | 408.3 | 35.5 | 185.8 |
| Accession Number | Fungus | N-Terminal Amino Acid Sequence |
|---|---|---|
| This study | Lepista nuda | 1 20 |
| AFN10626.1 | Hypsizygus marmoreus | 20 S M V 38 |
| AFD97049.1 | Coprinus comatus | 22 N F A 41 |
| AFD97050.1 | Coprinus comatus | 19 V R I A 38 |
| AFV15785.1 | Leucoagaricus gongylophorus | 26 T S M Y 44 |
| AAR13230.1 | Panus rudis | 1 V T D N A 20 |
| AAW28937.1 | Trametes sp. 420 | 25 V T N A N 44 |
| ADD14077.1 | Pleurotus eryngii | 24 I M Y E V 43 |
| AAR82932.1 | Pleurotus ostreatus | 24 T G M Y E V 43 |
| AAR03582.1 | Volvariella volvacea | 19 V T E Q D E A 38 |
| BAJ12090.1 | Lentinuda edodes | 20 V T V F Q 39 |
| AAR21094.1 | Pleurotus ostreatus | 24 T G N M Y E V S 43 |
| Metal Ions | Relative Laccase Activity (% of Control) | |||
|---|---|---|---|---|
| 10 mM | 5 mM | 2.5 mM | 1.25 mM | |
| Fe2+ | 8.4 ± 3.2 | 14.8 ± 0.5 | 20.6 ± 0.5 | 28.5 ± 1.2 |
| K+ | 85.1 ± 2.1 | 87.7 ± 3.9 | 92.1 ± 3.9 | 94.1 ± 3.1 |
| Hg2+ | 41.1 ± 1.7 | 58.9 ± 3.8 | 64.3 ± 2.7 | 68.9 ± 2.3 |
| Mg2+ | 91.1 ± 3.7 | 101.2 ± 4.2 | 108.9 ± 3.9 | 105.9 ± 3.6 |
| Pb2+ | 72.0 ± 3.1 | 77.2 ± 2.9 | 78.4 ± 2.5 | 71.7 ± 2.6 |
| Zn2+ | 35.1 ± 2.1 | 53.0 ± 2.5 | 65.7 ± 2.6 | 67.6 ± 2.2 |
| Ca2+ | 90.3 ± 4.3 | 100.2 ± 3.6 | 105.2 ± 0.9 | 109.3 ± 3.6 |
| Cd2+ | 58.6 ± 2.1 | 60.7 ± 2.3 | 83.7 ± 3.1 | 75.4 ± 1.3 |
| Mn2+ | 81.9 ± 4.7 | 83.7 ± 3.2 | 81.7 ± 3.2 | 91.5 ± 2.7 |
| Al3+ | 60.8 ± 1.4 | 67.8 ± 2.1 | 70.4 ± 2.6 | 84.4 ± 2.9 |
| Fe3+ | 16.1 ± 0.9 | 1.9 ± 0.3 | 6.29 ± 2.2 | 14.8 ± 0.7 |
| Dyes | λmax | Concentration | Decolorization (%) | |||
|---|---|---|---|---|---|---|
| (nm) | (mg/L) | 6 h | 12 h | 24 h | 96 h | |
| Methyl red | 524 | 250.0 | 82.3 | 81.5 | 83.2 | 83.5 |
| Methyl orange | 460 | 12.5 | 67.9 | 70.3 | 71.1 | 74.3 |
| Eriochrome black T | 540 | 125.0 | 81.0 | 81.1 | 83.4 | 84.9 |
| Coomassie brilliant blue | 549 | 25.0 | 80.7 | 86.0 | 87.5 | 86.6 |
| Crystal violet | 584 | 5.0 | 21.1 | 22.0 | 28.0 | 32.2 |
| Bromophenol blue | 590 | 25.0 | 88.7 | 90.0 | 90.3 | 91.1 |
| Malachite green | 614 | 6.3 | 83.0 | 86.7 | 86.0 | 86.8 |
| Reactive brilliant orange | 492 | 50.0 | 0.3 | 1.6 | 1.9 | 1.4 |
| Reactive red | 546 | 50.0 | 0.4 | 0.5 | 0.6 | 0.9 |
| Reactive black | 585 | 50.0 | 4.8 | 3.3 | 4.8 | 4.9 |
| Reactive blue R | 592 | 125.0 | 71.9 | 71.0 | 72.3 | 72.5 |
| Reactive brilliant blue | 605 | 100.0 | 95.7 | 94.9 | 95.8 | 95.0 |
| Reactive jade blue | 627 | 50.0 | 64.0 | 66.8 | 70.7 | 75.4 |
| Indigo carmine | 609 | 25.0 | 51.4 | 66.3 | 78.3 | 80.4 |
| Pesticides | λmax | Concentration | |Changes of OD (%)| | |||
|---|---|---|---|---|---|---|
| (nm) | (mg/L or w/w) | 3 h | 6 h | 12 h | 24 h | |
| glyphosate | 279 | 1.0 mg/L | 16.4 | 15.8 | 18.6 | 24.1 |
| pyrimethanil | 268 | 0.3 mg/L | 21.9 | 40.1 | 51.6 | 55.3 |
| quizalofop-P | 224 | 0.03% | 136.0 | 154.7 | 170.5 | 198.5 |
| chlortoluron | 248 | 1.0% | 224.0 | 234.9 | 248.0 | 240.6 |
| diuron | 266 | 0.03% | 17.0 | 23.2 | 29.6 | 41.1 |
| alachlor | 203 | 0.01% | 235.4 | 327.8 | 464.5 | 564.4 |
| prometryn | 266 | 0.03% | 28.1 | 34.5 | 30.1 | 34.6 |
| simazine | 267 | 0.05% | 26.2 | 25.6 | 31.8 | 37.6 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Zhang, G.; Meng, L.; Wang, H.; Gao, K.; Ng, T. Purification and Characterization of a White Laccase with Pronounced Dye Decolorizing Ability and HIV-1 Reverse Transcriptase Inhibitory Activity from Lepista nuda. Molecules 2016, 21, 415. https://doi.org/10.3390/molecules21040415
Zhu M, Zhang G, Meng L, Wang H, Gao K, Ng T. Purification and Characterization of a White Laccase with Pronounced Dye Decolorizing Ability and HIV-1 Reverse Transcriptase Inhibitory Activity from Lepista nuda. Molecules. 2016; 21(4):415. https://doi.org/10.3390/molecules21040415
Chicago/Turabian StyleZhu, Mengjuan, Guoqing Zhang, Li Meng, Hexiang Wang, Kexiang Gao, and Tb Ng. 2016. "Purification and Characterization of a White Laccase with Pronounced Dye Decolorizing Ability and HIV-1 Reverse Transcriptase Inhibitory Activity from Lepista nuda" Molecules 21, no. 4: 415. https://doi.org/10.3390/molecules21040415






