Insolubilization of Chestnut Shell Pigment for Cu(II) Adsorption from Water
Abstract
:1. Introduction
2. Results and Discussion
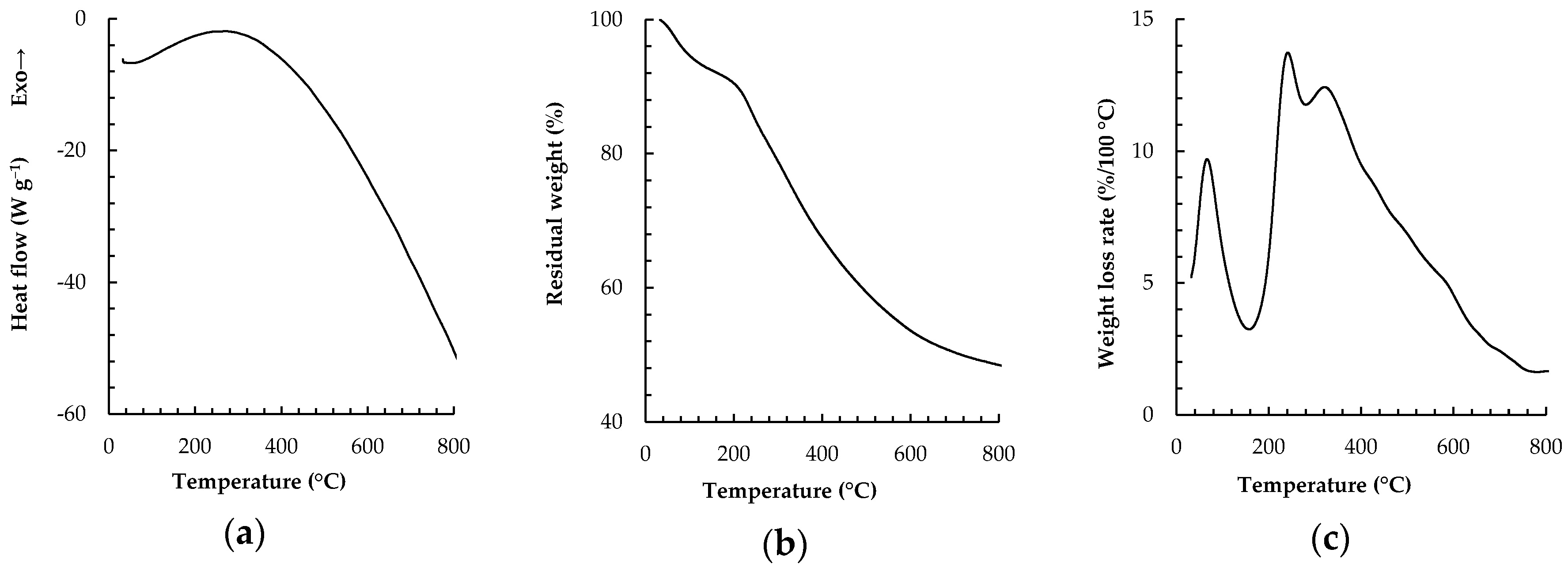
2.1. TDA and DSC of CSP
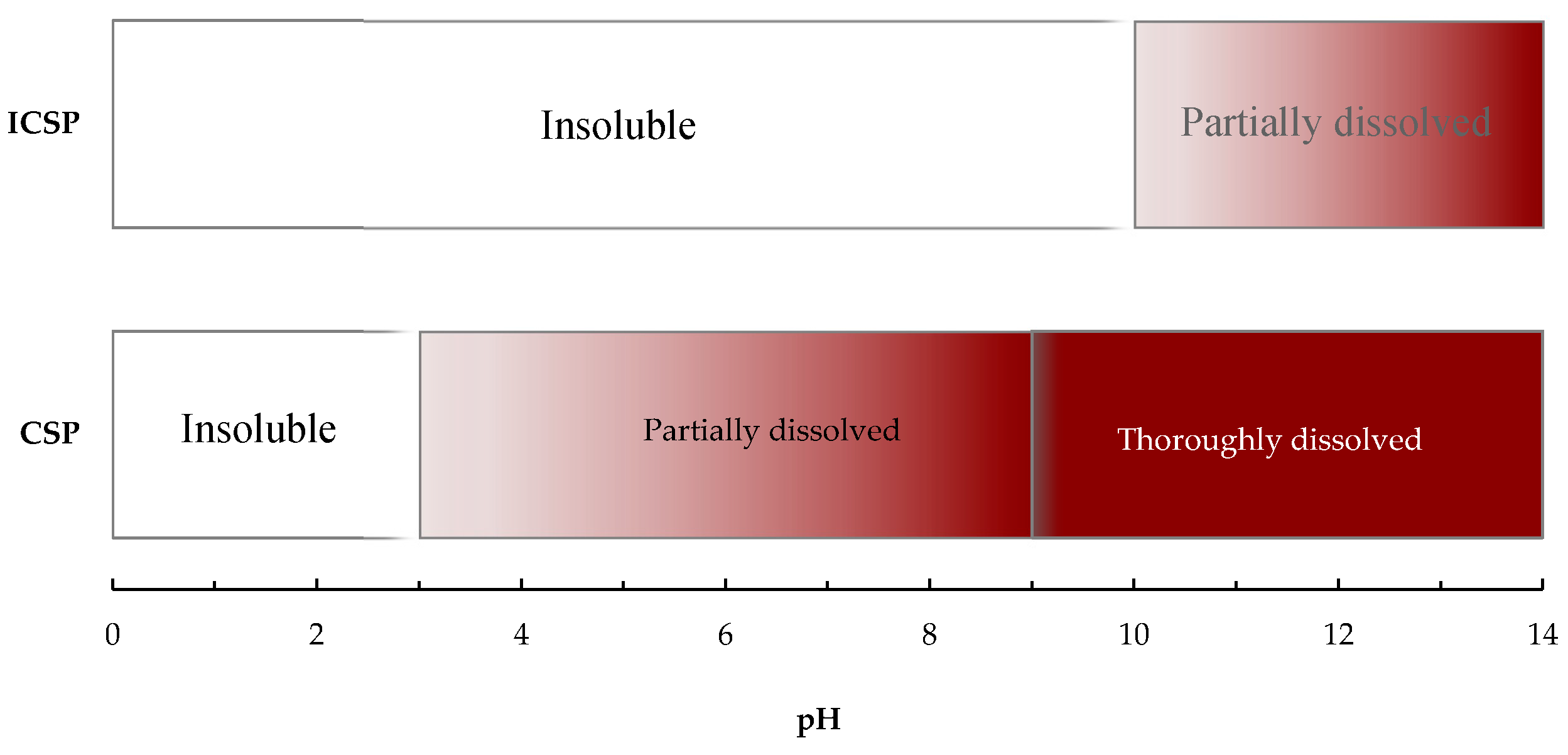
2.2. Solubility of ICSP in the Aqueous Media
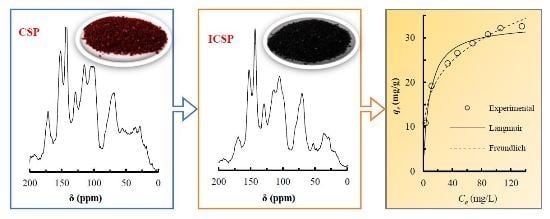
2.3. 13C-NMR of CSP and ICSP
2.4. Elemental Composition of CSP and ICSP
2.5. FT-IR of CSP and ICSP
2.6. Adsorption Kinetics
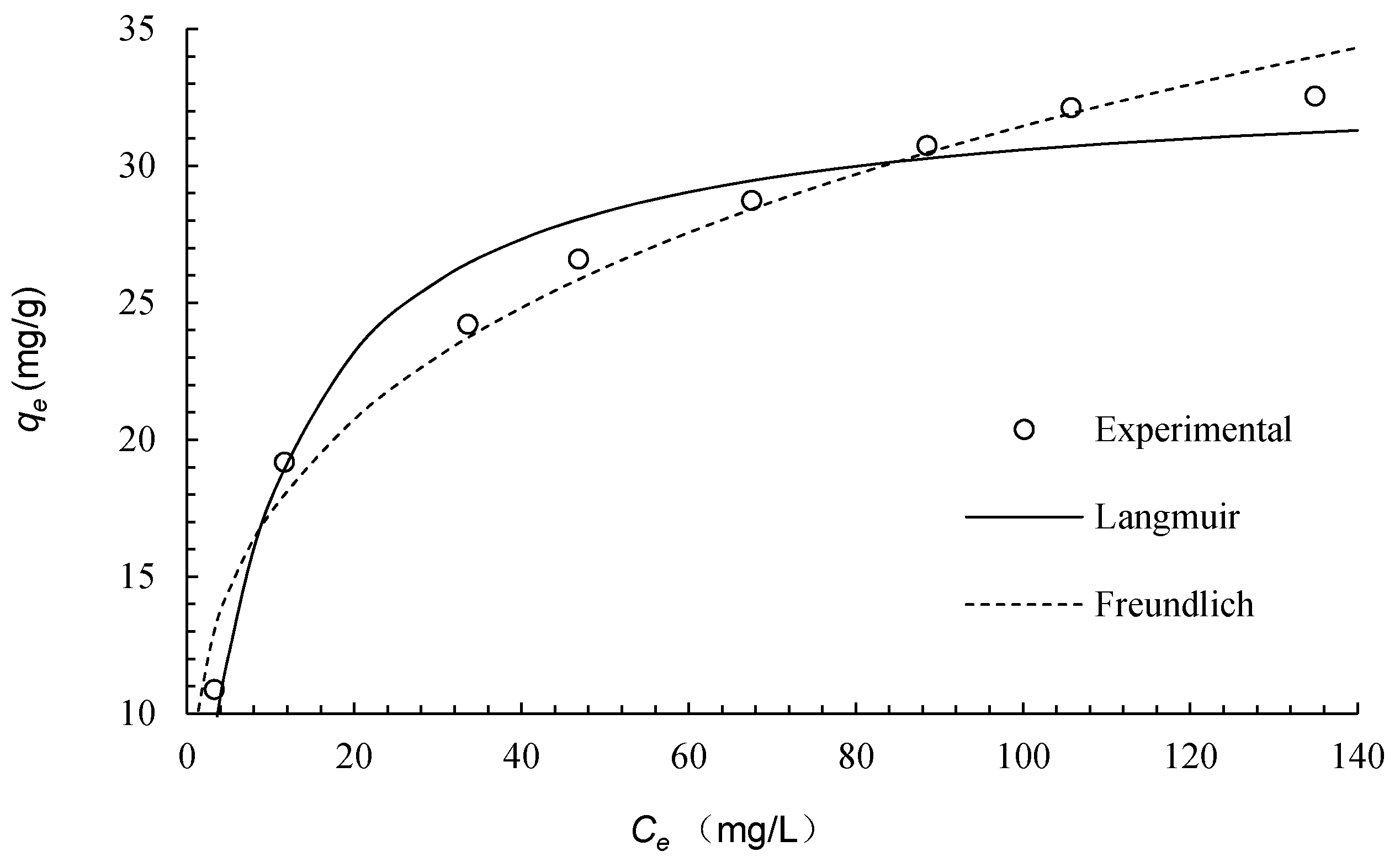
2.7. Adsorption Isotherms
3. Materials and Methods
3.1. Materials
3.2. Preparation and Insolubilization of CSP
3.3. TGA and DSC
3.4. Solubility Test
3.5. 13C-NMR Analysis
3.6. Elemental Analysis
3.7. FT-IR Spectroscopy Analysis
3.8. Adsorption Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stern, B.R. Essentiality and toxicity in copper health risk assessment: Overview, update and regulatory considerations. J. Toxicol. Environ. Health. A 2010, 73, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Plonka, P.M.; Grabacka, M. Melanin synthesis in microorganisms—Biotechnological and medical aspects. Acta Biochim. Pol. 2006, 53, 429–443. [Google Scholar] [PubMed]
- Zdybel, M.; Chodurek, E.; Pilawa, B. Free radicals in ultraviolet irradiated melanins and melanin complexes with Cd(II) and Cu(II)—EPR examination. J. Appl. Biomed. 2015, 13, 131–141. [Google Scholar] [CrossRef]
- Hong, L.; Simon, J.D. Current understanding of the binding sites, capacity, affinity, and biological significance of metals in melanin. J. Phys. Chem. B 2007, 111, 7938–7947. [Google Scholar] [CrossRef] [PubMed]
- Bridelli, M.G.; Crippa, P.R. Theoretical analysis of the adsorption of metal ions to the surface of melanin particles. Adsorption 2008, 14, 101–109. [Google Scholar] [CrossRef]
- Chen, S.G.; Xue, C.H.; Wang, J.F.; Feng, H.; Wang, Y.M.; Ma, Q.; Wang, D.F. Adsorption of Pb(II) and Cd(II) by squid Ommastrephes bartrami melanin. Bioinorg. Chem. Appl. 2009, 2009, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M.; Rome, L. Biosorption of copper by fungal melanin. Appl. Microbiol. Biotechnol. 1988, 29, 610–617. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Faostat. Available online: http://faostat3.fao.org/browse/Q/QC/E (accessed on 19 January 2016).
- Yao, Z.; Qi, J.; Wang, L. Isolation, fractionation and characterization of melanin-like pigments from chestnut (Castanea mollissima) shells. J. Food Sci. 2012, 77, C671–C676. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.-H.; Yao, Z.-Y.; Wang, L.-H. Comparison of antioxidant activities of crude pigments extracted from chestnut shell by ethanol and alkali. Sci. Technol. Food Ind. 2012, 33, 104–107. [Google Scholar]
- Yu, X.; Huang, K.; Zou, J. Studies on characteristics and application of the pigment from chestnut shell. Chem. Ind. Forest Prod. 1997, 17, 67–72. [Google Scholar]
- Zhou, M.; Su, P.; Qi, J.-H.; Hu, Y.; Yao, Z.-Y. Double-catalyzed base-acid synthesis of chestnut shell pigment resin cross-linked with formaldehyde. Appl. Mech. Mater. 2014, 587, 663–668. [Google Scholar] [CrossRef]
- Su, P.; Zhou, M.; Qi, J.-H.; Kan, H.; Yao, Z.-Y. Synthesis and copper sorption of chestnut-shell-pigment/SiO2 composite. Adv. Mater. Res. 2014, 1035, 53–57. [Google Scholar] [CrossRef]
- He, L.; Wang, X.; Xu, R. Study on the thermal decomposition of the pigment extracted with water from chestnut shell. Food. Mach. 2007, 23, 57–58. [Google Scholar]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetenskapsakad. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.; McKay, G. Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf. Environ. Prot. 1998, 76, 183–191. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Concerning adsorption in solutions. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Duong, D.D. Adsorption Analysis: Equilibria and Kinetics; Imperial College Press: London, UK, 1998. [Google Scholar]
- Ayranci, E.; Hoda, N. Adsorption kinetics and isotherms of pesticides onto activated carbon-cloth. Chemosphere 2005, 60, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, R.; Zhao, Y.; Liu, C.-Z. Separation of chlorogenic acid from honeysuckle crude extracts by macroporous resins. J. Chromatogr. B 2008, 867, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Rengaraj, S.; Kim, Y.; Joo, C.; Choi, K.; Yi, J. Batch adsorptive removal of copper ions in aqueous solutions by ion exchange resins: 1200H and IRN97H. Korean J. Chem. Eng. 2004, 21, 187–194. [Google Scholar] [CrossRef]
- Rengaraj, S.; Yeon, J.-W.; Kim, Y.; Jung, Y.; Ha, Y.-K.; Kim, W.-H. Adsorption characteristics of Cu(II) onto ion exchange resins 252H and 1500H: Kinetics, isotherms and error analysis. J. Hazard. Mater. 2007, 143, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Gezici, O.; Kara, H.; Yanık, S.; Ayyildiz, H.F.; Kucukkolbasi, S. Investigating sorption characteristics of copper ions onto insolubilized humic acid by using a continuously monitored solid phase extraction technique. Colloids Surf. A 2007, 298, 129–138. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.



| δ (ppm) | Molecular Fragments | Percentage (%) | |
|---|---|---|---|
| CSP | ICSP | ||
| 0–47 | saturated alkyl carbons (CH, CH2, and CH3) | 12.0 | 9.9 |
| 47–60 | Carbons of amino groups and/or –O–CH3 moieties | 4.8 | 2.9 |
| 60–111 | C–O groups of carbohydrates, alcohols, and ethers | 31.2 | 36.4 |
| 111–148 | Carbons of aromatic rings | 30.6 | 32.4 |
| 148–164 | Car–O of phenols | 11.2 | 11.7 |
| 164–188 | Carboxyl carbon atoms | 7.2 | 4.9 |
| 188–220 | Carbonyl carbons (quinone, aldehyde and ketone) | 3.0 | 1.8 |
| Samples | Elemental Composition (wt %) | Molar Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | C | H | S | O | N/C | H/C | S/C | O/C | |
| CSP | 1.39 | 52.0 | 5.54 | 0.53 | 40.6 | 0.023 | 1.28 | 0.004 | 0.586 |
| ICSP | 1.04 | 51.5 | 4.64 | 0.39 | 42.5 | 0.017 | 1.08 | 0.003 | 0.618 |
| qe,exp (mg·g−1) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| qe,cal (mg·g−1) | k1 (10−3 min−1) | R2 | qe,cal (mg·g−1) | k2 (10−3 g·mg−1·min−1) | R2 | |
| 32.6 | 29.1 | 44.0 | 0.917 | 31.7 | 2.10 | 0.979 |
| Langmuir | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|
| qm (mg·g−1) | KL (L·mg−1) | R2 | 1/n | KF (L1/n·mg1−1/n·g−1) | R2 |
| 33.2 | 0.116 | 0.985 | 0.259 | 9.56 | 0.991 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Z.-Y.; Qi, J.-H.; Hu, Y.; Wang, Y. Insolubilization of Chestnut Shell Pigment for Cu(II) Adsorption from Water. Molecules 2016, 21, 405. https://doi.org/10.3390/molecules21040405
Yao Z-Y, Qi J-H, Hu Y, Wang Y. Insolubilization of Chestnut Shell Pigment for Cu(II) Adsorption from Water. Molecules. 2016; 21(4):405. https://doi.org/10.3390/molecules21040405
Chicago/Turabian StyleYao, Zeng-Yu, Jian-Hua Qi, Yong Hu, and Ying Wang. 2016. "Insolubilization of Chestnut Shell Pigment for Cu(II) Adsorption from Water" Molecules 21, no. 4: 405. https://doi.org/10.3390/molecules21040405
APA StyleYao, Z.-Y., Qi, J.-H., Hu, Y., & Wang, Y. (2016). Insolubilization of Chestnut Shell Pigment for Cu(II) Adsorption from Water. Molecules, 21(4), 405. https://doi.org/10.3390/molecules21040405