Sesquiterpenoids from Chinese Agarwood Induced by Artificial Holing
Abstract
:1. Introduction
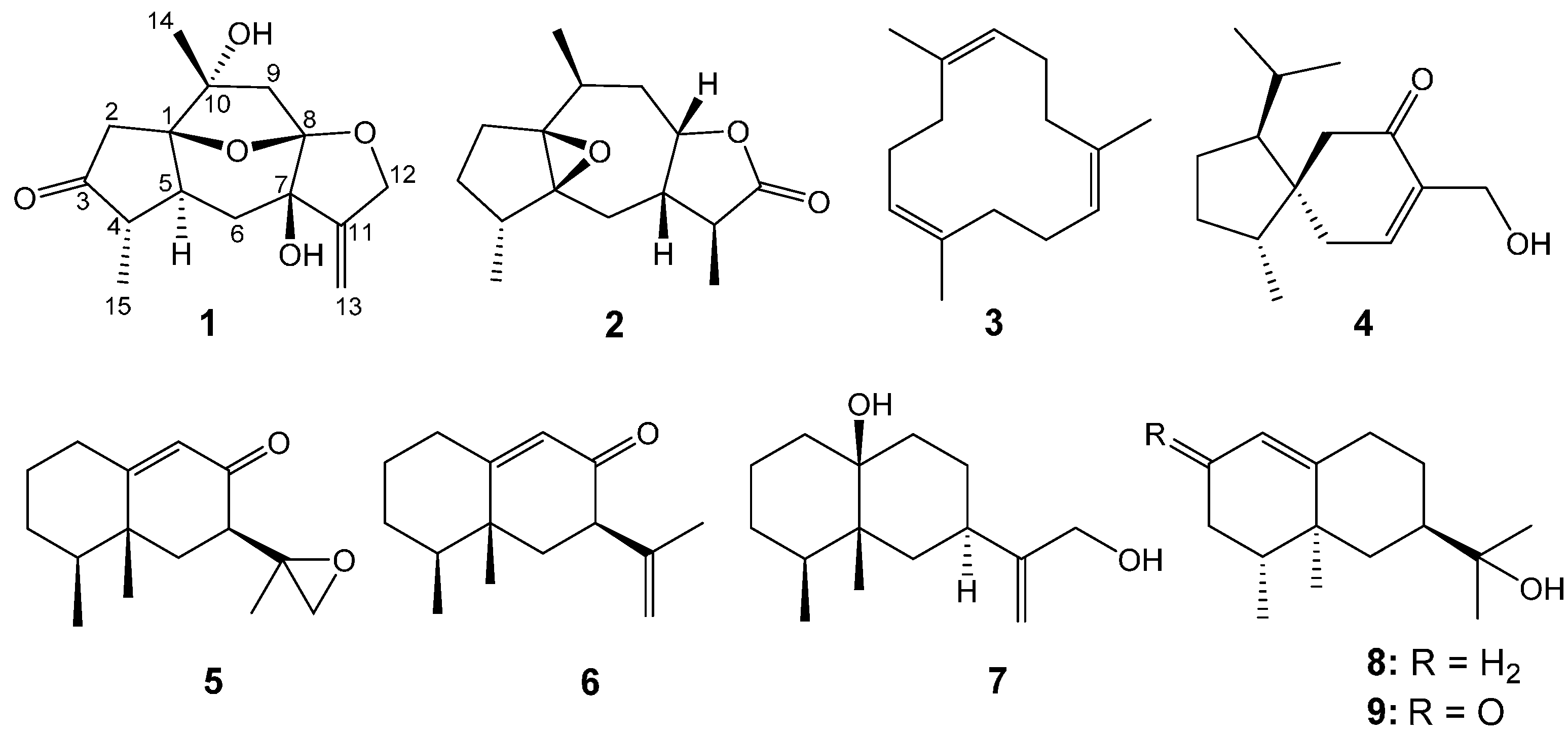
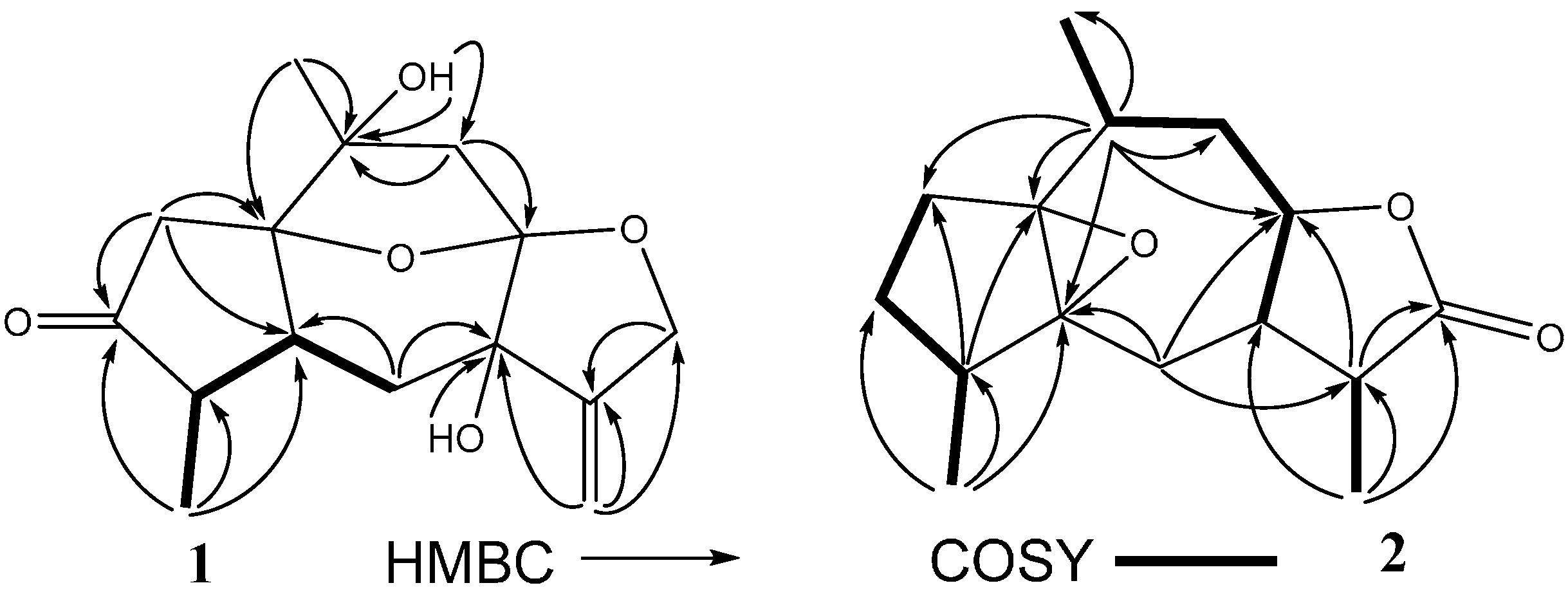
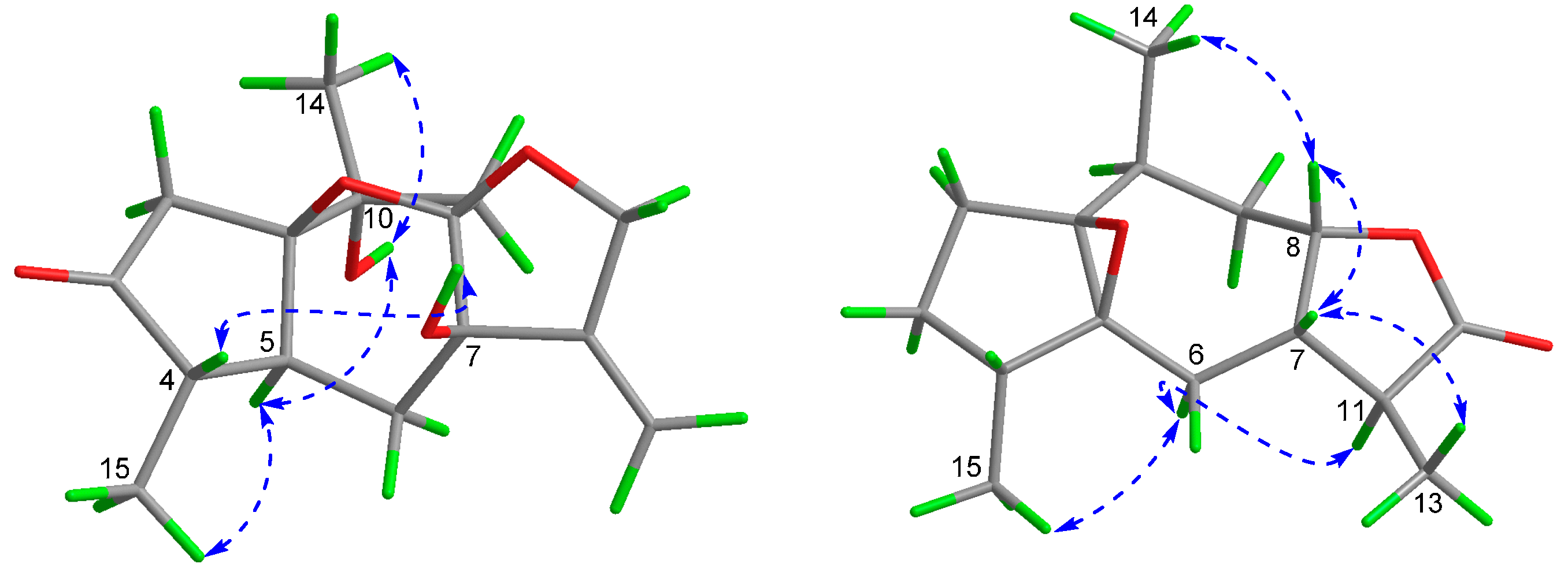
2. Results and Discussion
3. Materials and Methods
3.1. General Imformation
3.2. Plant Material
3.3. Extraction and Isolation
3.4. AChE Inhibition Activity
3.5. Antibacterial Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- China Pharmacopoeia Editorial Board. Pharmacopoeia of the People's Republic of China; China Medical Science and Technology Press: Beijing, China, 2010; p. 172. [Google Scholar]
- Yang, L.; Qiao, L.R.; Xie, D.; Yuan, Y.H.; Chen, N.H.; Dai, J.G.; Guo, S.X. 2-(2-Phenylethyl)chromones from Chinese eaglewood. Phytochemistry 2012, 76, 92–97. [Google Scholar] [PubMed]
- Mei, W.L.; Zuo, W.J.; Yang, D.L.; Dong, W.H.; Dai, H.F. Advances in the Mechanism, artificial agarwood-introduction techniques and chemical constituents of artificial agarwood production. Chin. J. Trop. Crop. 2013, 34, 2513–2520. [Google Scholar]
- Chen, H.Q.; Wei, J.H.; Yang, J.S.; Zhang, Z.; Yang, Y.; Gao, Z.H.; Sui, C.; Gong, B. Chemical constituents of agarwood originating from the endemic genus Aquilaria plants. Chem. Biodivers. 2012, 9, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S. Review of the chemical constituents isolated from chen-xiang. Nat. Prod. Res. Dev. 1996, 10, 99–103. [Google Scholar]
- Naef, R. The volatile and semi-volatile constituents of agarwood, the infected heartwood of Aquilaria species: A review. Flavour Fragr. J. 2011, 26, 73–87. [Google Scholar] [CrossRef]
- Yang, D.L.; Wang, H.; Guo, Z.K.; Li, W.; Mei, W.L.; Dai, H.F. Fragrant agarofuran and eremophilane sesquiterpenes in agarwood ‘Qi-Nan’ from Aquilaria sinensis. Phytochem. Lett. 2014, 8, 121–125. [Google Scholar] [CrossRef]
- Yang, D.L.; Mei, W.L.; Zeng, Y.B.; Guo, Z.K.; Zhao, Y.X.; Wang, H.; Zuo, W.J.; Dong, W.H.; Wang, Q.H.; Dai, H.F. 2-(2-phenylethyl)chromone derivatives in Chinese Agarwood “Qi-Nan” from Aquilaria sinensis. Planta Med. 2013, 79, 1329–1334. [Google Scholar]
- Ma, Q.Y.; Chen, Y.C.; Huang, S.Z.; Guo, Z.K.; Dai, H.F.; Hua, Y.; Zhao, Y.X. Two new guaiane sesquiterpenoids from Daphne holosericea (Diels) Hamaya. Molecules 2014, 19, 14266–14272. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Pan, J.J.; Krohn, K.J. Determination of the absolute configurations of pharmacological natural products via density functional theory calculations of vibrational circular dichroism: The new cytotoxic iridoid prismatomerin. J. Org. Chem. 2007, 72, 7641–7649. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.K.; Zhu, H.J.; Hong, K.; Wang, Y.; Liu, P.P.; Wang, X.; Peng, X.P.; Zhu, W.M. Novel cyclic hexapeptides from marine-derived fungus, aspergillus sclerotiorum PT06–1. Org. Lett. 2009, 11, 5262–5265. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Tsuneya, T.; Uneyama, K. Fragrant sesquiterpenes from agarwood. Phytochemistry 1993, 33, 1147–1155. [Google Scholar] [CrossRef]
- Tezuka, Y.; Tasaki, M.; Huang, Q.; Hatanaka, Y.; Kikuchi, T. 15-Hydroxyacorenone: New Acorane-Type Sesquiterpene from the Culture Broth of the Mycoparasitic Fungus Trichoderma harzianum. Eur. J. Org. Chem. 1997, 12, 2579–2580. [Google Scholar]
- Wu, B.; Lee, J.G.; Lim, C.J.; Jia, S.D.; Kwon, S.W.; Hwang, G.S.; Park, J.H. Sesquiterpenoids and 2-(2-phenylethyl)-4H-chromen-4-one (=2-(2-Phenylethyl)-4H-1-benzopyran-4-one) derivatives from Aquilaria malaccensis agarwood. Helv. Chim. Acta 2012, 95, 636–642. [Google Scholar] [CrossRef]
- Arantes, F.S.; Hanson, J.R.; Hitchcock, P.B. The hydroxylation of the sesquiterpenoid valerianol by Mucor plumbeus. Phytochemistry 1999, 52, 1063–1067. [Google Scholar] [CrossRef]
- Savona, G.; Pipzzi, F.; Torre, M.C.D.; Servettaz, O.; Rodriguez, B. A valencane sesquiterpenoid from Teucrium carolipaui. Phytochemistry 1987, 26, 571–572. [Google Scholar] [CrossRef]
- Li, W.; Cai, C.H.; Guo, Z.K.; Wang, H.; Zuo, W.J.; Dong, W.H.; Mei, W.L.; Dai, H.F. Five new eudesmane-type sesquiterpenoids from Chinese agarwood induced by artificial holing. Fitoterapia 2015, 100, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Valentino, A.J.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Xu, S.Y.; Bian, R.L.; Chen, X. Methods of Pharmacology Experiment; People’s Sanitation Press: Beijing, China, 2003; pp. 1651–1653. [Google Scholar]
- Sample Availability: Not available.

| No. | 1 c | 2 b,c | 3 b,c | ||||
|---|---|---|---|---|---|---|---|
| δC a | δH a | δH (in DMSO) | δC | δH | δC | δH | |
| 1 | 89.5 | 73.8 | 135.4 | ||||
| 2 | 45.4 | 2.42 d (18.5), 2.17 d (18.5) | 2.40 d (18.5), 2.05 d (18.5) | 29.6 | 2.04 m, 1.74 m | 125.2 | 5.12 t (6.6) |
| 3 | 219.0 | 26.4 | 1.65 m, 1.14 m | 26.6 | 2.04 m | ||
| 4 | 48.6 | 2.36 dq (2.0, 7.3) | 2.16 dq (1.6, 7.0) | 39.6 | 2.21 d (1.7) | 32.4 | 2.04 m |
| 5 | 44.7 | 2.12 m | 2.00 m | 72.4 | 135.4 | ||
| 6 | 28.2 | 2.33 br d (14.8), 2.26 dd (7.3, 14.8 ) | 2.17 br d (14.1), 2.09 dd (7.4, 14.1) | 28.0 | 2.26 m, 1.87 dd (8.5, 12.3) | 125.2 | 5.12 t (6.6) |
| 7 | 75.1 | 47.2 | 2.01 m | 26.6 | 2.04 m | ||
| 8 | 112.9 | 80.7 | 3.89 ddd (11.3, 10.2, 3.0) | 32.4 | 2.04 m | ||
| 9 | 43.3 | 2.53 d (13.8), 1.97 d (13.8) | 2.39 d (13.4), 1.77 d (13.4) | 35.2 | 1.98 m, 1.93 dd (12.9, 3.9) | 135.4 | |
| 10 | 77.1 | 30.3 | 2.59 m | 125.2 | 5.12 t (6.6) | ||
| 11 | 152.5 | 42.1 | 2.29 m | 26.6 | 2.04 m | ||
| 12 | 71.3 | 4.62 dt (12.8, 2.4), 4.42 dt (12.8, 2.4) | 4.49 dt (12.6, 2.2), 4.26 dt (12.6, 2.2) | 178.9 | 32.4 | 2.04 m | |
| 13 | 105.8 | 5.28 br t (2.4), 5.07 br t (2.4) | 5.18 br t (2.2), 4.97 br t (2.2) | 12.8 | 1.23 d (7.0) | 23.6 | 1.68 s |
| 14 | 28.6 | 1.35 s | 1.23 s | 17.0 | 1.21 d (7.3) | 23.6 | 1.68 s |
| 15 | 12.4 | 1.06 d (7.3) | 0.96 d (7.0) | 16.8 | 0.95 d (7.2) | 23.6 | 1.68 s |
| 7-OH | 5.16 s | ||||||
| 10-OH | 5.00 s | ||||||
| Compound | Percentage of Inhibition | Compound | Percentage of Inhibition |
|---|---|---|---|
| 1 | 21.1 ± 0.8 | 6 | 70.7 ± 0.6 |
| 2 | 13.3 ± 0.9 | 7–9 | <10 |
| 3 | <10 | EtOAc | 18.5 ± 0.9 |
| 4 | 14.7 ± 0.9 | Tacrine a | 73.3 ± 0.8 |
| 5 | 17.8 ± 0.6 |
| Compound | S. aureus | R. solanacearum |
|---|---|---|
| 2, 3 | – | – |
| 4 | 12.35 ± 0.21 | 16.90 ± 0.09 |
| 5 | 9.87 ± 0.14 | 9.02 ± 0.25 |
| 6 | – | 8.07 ± 0.16 |
| 7 | – | – |
| 8 | 10.10 ± 0.12 | 8.86 ± 0.13 |
| 9 | – | – |
| Kanamycin sulfate a | 24.06 ± 0.29 | 30.64 ± 0.13 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Liao, G.; Dong, W.-H.; Kong, F.-D.; Wang, P.; Wang, H.; Mei, W.-L.; Dai, H.-F. Sesquiterpenoids from Chinese Agarwood Induced by Artificial Holing. Molecules 2016, 21, 274. https://doi.org/10.3390/molecules21030274
Li W, Liao G, Dong W-H, Kong F-D, Wang P, Wang H, Mei W-L, Dai H-F. Sesquiterpenoids from Chinese Agarwood Induced by Artificial Holing. Molecules. 2016; 21(3):274. https://doi.org/10.3390/molecules21030274
Chicago/Turabian StyleLi, Wei, Ge Liao, Wen-Hua Dong, Fan-Dong Kong, Pei Wang, Hao Wang, Wen-Li Mei, and Hao-Fu Dai. 2016. "Sesquiterpenoids from Chinese Agarwood Induced by Artificial Holing" Molecules 21, no. 3: 274. https://doi.org/10.3390/molecules21030274
APA StyleLi, W., Liao, G., Dong, W.-H., Kong, F.-D., Wang, P., Wang, H., Mei, W.-L., & Dai, H.-F. (2016). Sesquiterpenoids from Chinese Agarwood Induced by Artificial Holing. Molecules, 21(3), 274. https://doi.org/10.3390/molecules21030274






