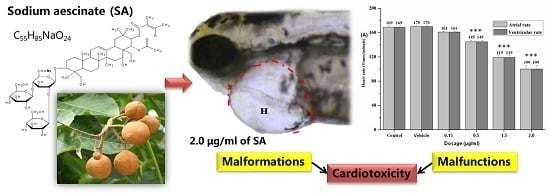
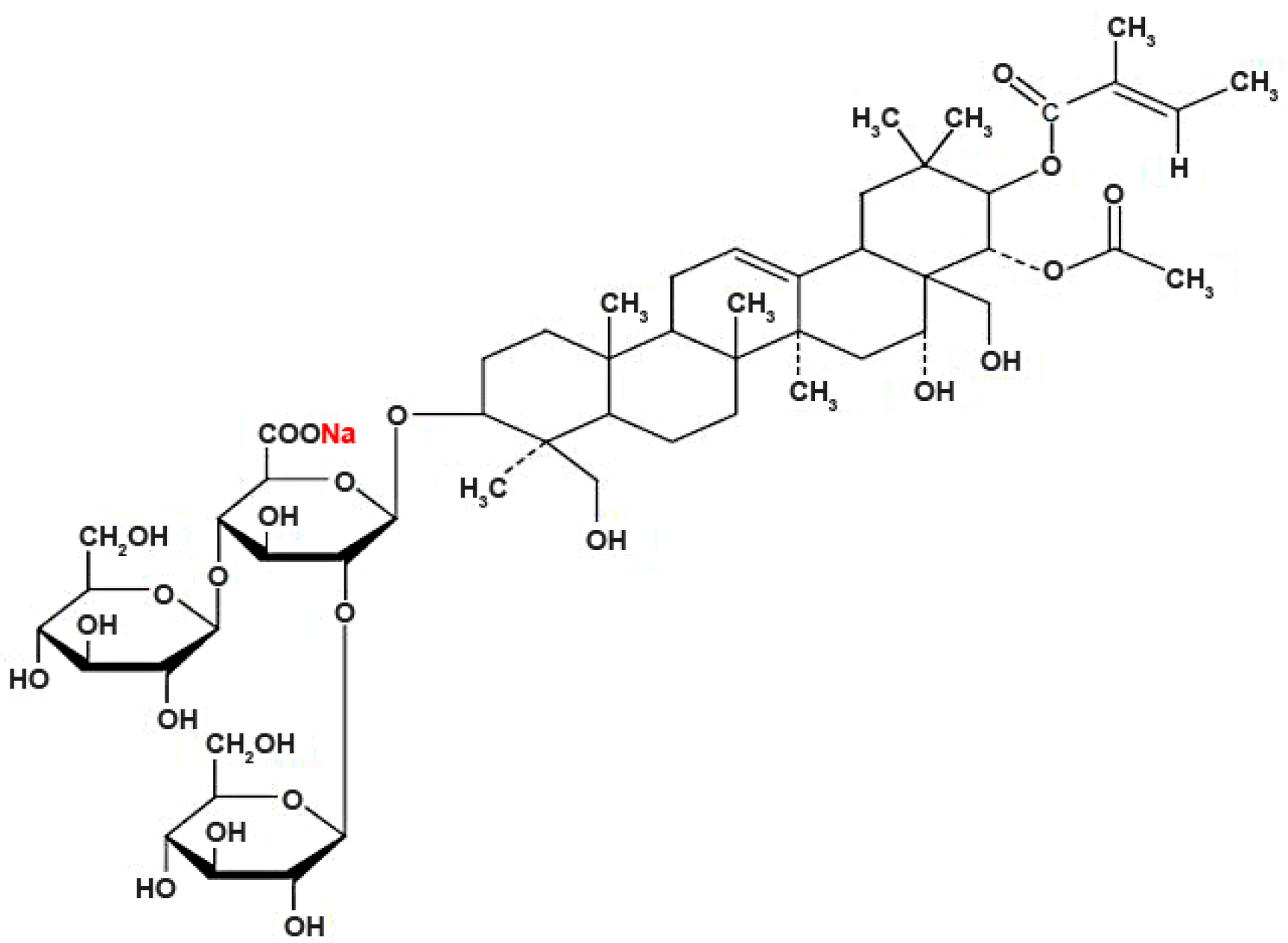
In Vivo Cardiotoxicity Induced by Sodium Aescinate in Zebrafish Larvae
Abstract
:1. Introduction
2. Results
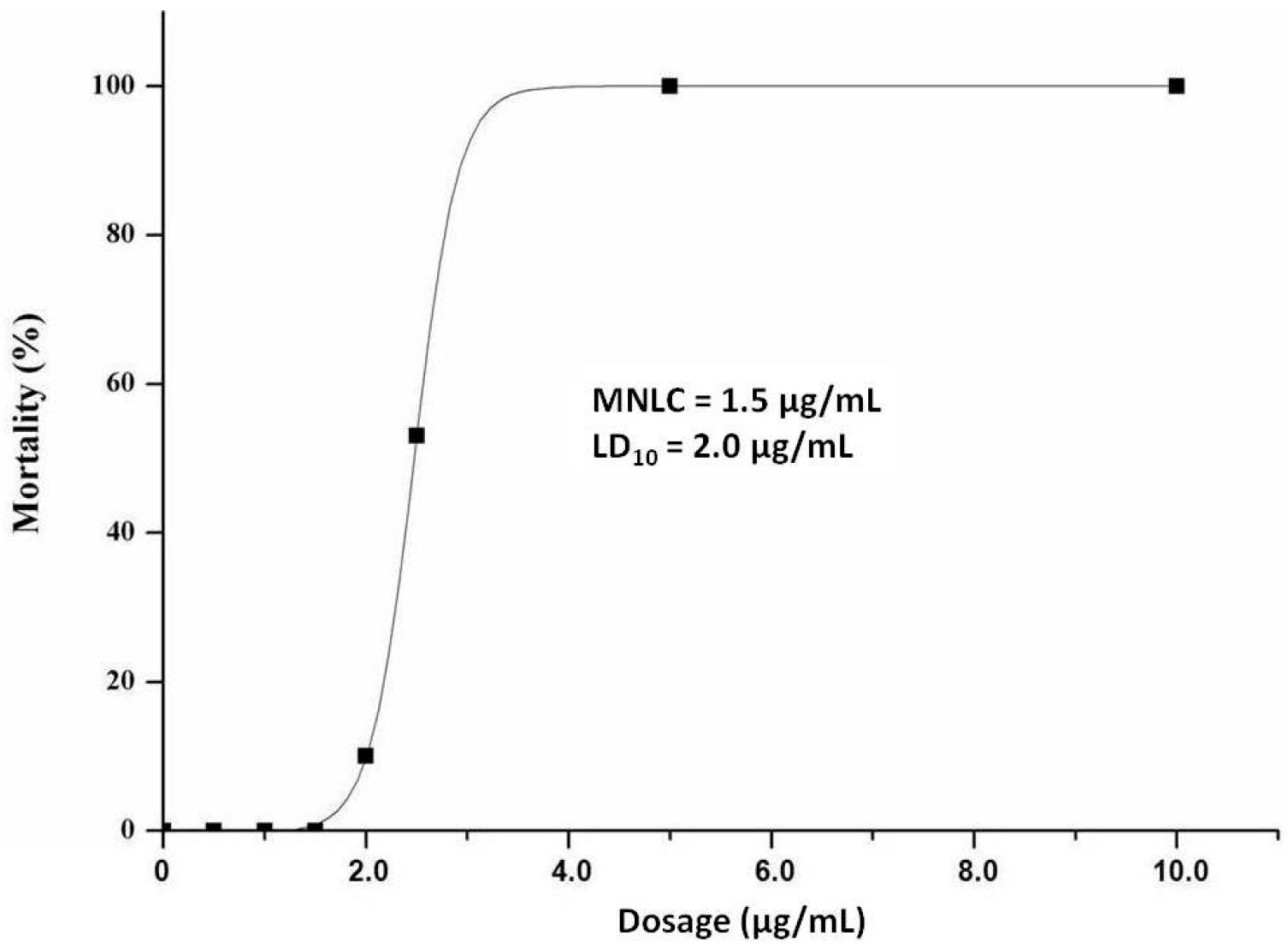
2.1. MNLC and LC10
2.2. Visual Assessment of Cardiotoxicity

2.3. Quantitative Observation of Cardiotoxicity
| Manifestations | Blank Control | Vehicle Control | SA (μg/mL) | |||
|---|---|---|---|---|---|---|
| 0.15 | 0.5 | 1.5 | 2.0 | |||
| Heart malformations | 0 | 0 | 10.0 | 70.0 | 100.0 | 100.0 * |
| Pericardial edema | 0 | 0 | 10.0 | 70.0 | 100.0 | 100.0 * |
| Circulation decrease | 0 | 0 | 3.3 | 36.7 | 53.3 | 66.7 * |
| Circulation absence | 0 | 0 | 0 | 3.3 | 46.7 | 100.0 * |
| Thrombosis | 0 | 0 | 0 | 0 | 0 | 0 |
| Hemorrhage | 0 | 0 | 0 | 0 | 0 | 0 |
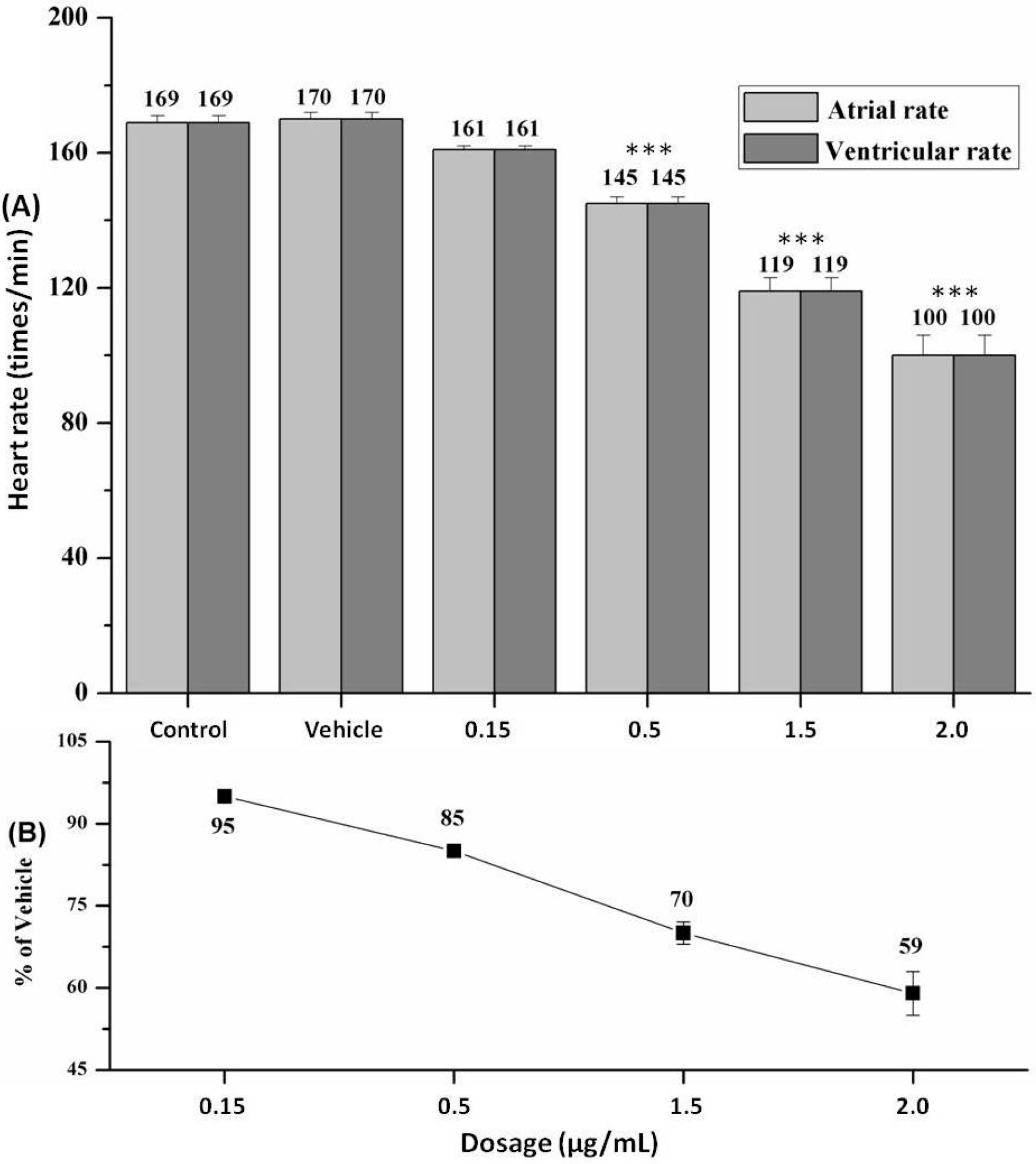
2.4. Heart Rate and Heart Rhythm
3. Discussion
4. Materials and Methods
4.1. Chemical Preparation
4.2. Zebrafish Handling
4.3. Chemical Treatment
4.4. Determination of MNLC and LC10
4.5. Cardiovascular Toxicity Assessment
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sirtori, C.R. Aescin: Pharmacology, pharmacokinetics and therapeutic profile. Pharmacol. Res. 2001, 44, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.; Pincombe, J.; Foster, G. Using horse chestnut seed extract in the treatment of venous leg ulcers: A cost-benefit analysis. Ostomy Wound Manag. 2006, 52, 68–78. [Google Scholar]
- Wang, Y.; Ye, Z.; Hu, X.; Huang, J.; Luo, Z. Morphological changes of the neural cells after blast injury of spinal cord and neuroprotective effects of sodium beta-aescinate in rabbits. Injury 2010, 41, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, T.; Jiang, N.; Ren, R.T.; Li, C.; Li, C.K.; Fu, F.H. Sodium aescinate ameliorate liver injury induced by methyl parathion in rats. Exp. Ther. Med. 2012, 3, 818–822. [Google Scholar] [PubMed]
- Kang, J.; Gong, P.; Ren, Y.B.; Gao, D.N.; Ding, Q.L. Effect of β-sodium aescinate on hypoxia-inducible factor-1α expression in rat brain cortex after cardiopulmonary resuscitation. World J. Emerg. Med. 2013, 4, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, G.P.; Li, L.; Wang, K.J.; Zhang, B.Q.; Hu, S.J. Dual effects of sodium aescinate on vascular tension in rat thoracic aorta. Microvasc. Res. 2010, 79, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Berti, F.; Omini, C.; Longiave, D. The mode of action of aescin and the release of prostaglandins. Prostaglandines 1977, 14, 241–249. [Google Scholar] [CrossRef]
- Guillaume, M.; Padioleau, F. Veinotonic effect, vascular protection, anti-inflammatory and free radical scavenging properties of horse chestnut extract. Arzneimittelforschung 1994, 44, 25–35. [Google Scholar] [PubMed]
- Luo, X.M.; Peng, T.; Wang, L. Pharmaceutical efficacy of Aescin. Foreign Med. Sci. Sec. Pharm. 2002, 29, 168–171. [Google Scholar]
- Wang, Y.K.; Han, J.; Xiong, W.J.; Yuan, Q.Y.; Gu, Y.P.; Li, J.; Zhu, Z.; Zhang, H.; Wang, C.J. Evaluation of in vivo antioxidant and immunity enhancing activities of sodium aescinate injection liquid. Molecules 2012, 17, 10267–10275. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.T.; Macrae, C.A. Systematic approaches to toxicology in the zebrafish. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 433–453. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, L.; Jauhiainen, A.; Kristiansson, E.; Nerman, O.; Larsson, D.G. Evolutionary conservation of human drug targets in organisms used for environmental risk assessments. Environ. Sci. Technol. 2008, 42, 5807–5813. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Patton, E.E.; Zon, L.I. The art and design of genetic screens: Zebrafish. Nat. Rev. Genet. 2001, 2, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.T.; Shaw, S.Y.; Peterson, T.A.; Milan, D.J.; Zhong, T.P.; Schreiber, S.L.; MacRae, C.A.; Fishman, M.C. Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat. Biotechnol. 2004, 22, 595–99. [Google Scholar] [CrossRef] [PubMed]
- Gerull, B.; Gramlich, M.; Atherton, J.; McNabb, M.; Trombitás, K.; Sasse-Klaassen, S.; Seidman, J.G.; Seidman, C.; Granzier, H.; Labeit, S.; et al. Mutations of TTN, encoding the giant muscle filament titin, cause familial dilated cardiomyopathy. Nat. Genet. 2002, 30, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Meiler, S.E.; Zhong, T.P.; Mohideen, M.; Crossley, D.A.; Burggren, W.W.; Fishman, M.C. Cardiomyopathy in zebrafish due to mutation in an alternatively spliced exon of titin. Nat. Genet. 2002, 30, 205–209. [Google Scholar] [PubMed]
- Peterson, R.T.; Link, B.A.; Dowling, J.E.; Schreiber, S.L. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc. Natl. Acad. Sci. USA 2000, 97, 12965–12969. [Google Scholar] [CrossRef] [PubMed]
- Khersonsky, S.M.; Jung, D.W.; Kang, T.W.; Walsh, D.P.; Moon, H.S.; Jo, H.; Jacobson, E.M.; Shetty, V.; Neubert, T.A.; Chang, Y.T. Facilitated forward chemical genetics using a tagged triazine library and zebrafish embryo screening. J. Am. Chem. Soc. 2003, 125, 11804–11805. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, H.H.; Cho, K.H. Acute cardiovascular toxicity of sterilizers, PHMG, and PGH: Severe inflammation in human cells and heart failure in zebrafish. Cardiovasc. Toxicol. 2013, 13, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.J.; Jia, Y.F.; Chen, N.; Bian, W.P.; Li, Q.K.; Ma, Y.B.; Chen, Y.L.; Pei, D.S. Zebrafish as a model system to study toxicology. Environ. Toxicol. Chem. 2014, 33, 11–17. [Google Scholar]
- Bhaskar, L.; Ramakrishna, B.S. Colonic mucosal antioxidant enzymes and lipid peroxide levels in normal subjects and patients with ulcerative colitis. J. Gastroenterol. Hepatol. 1995, 10, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Hou, Y.; Jiang, W.; Wang, R.; Liu, K. Escin: Inhibiting inflammation and promoting gastrointestinal transit to attenuate formation of postoperative adhesions. World J. Surg. 2005, 29, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Montopoli, M.; Froldi, G.; Comelli, M.C.; Prosdocimi, M.; Caparrotta, L. Aescin protection of human vascular endothelial cells exposed to cobalt chloride mimicked hypoxia and inflammatory stimuli. Planta Med. 2007, 73, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Davila, J.C.; Rodriguez, R.J.; Melchert, R.B.; Acosta, D., Jr. Predictive value of in vitro model systems in toxicology. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 63–96. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, L.; Haverkamp, W.; Borggrefe, M.; Breithardt, G. Experimental models of torsade de pointes. Cardiovasc. Res. 1998, 39, 178–193. [Google Scholar] [CrossRef]
- MacRae, C.A.; Fishman, M.C. Zebrafish: The complete cardiovascular compendium. Cold Spring Harb. Symp. Quant. Biol. 2002, 67, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.H.; Webb, S.; Brown, N.A.; Lamers, W.; Moorman, A. Development of the heart: (3) Formation of the ventricular outflow tracts, arterial valves, and intrapericardial arterial trunks. Heart 2003, 89, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Langheinrich, U. Zebrafish: A new model on the pharmaceutical catwalk. BioEssays 2003, 25, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Milan, D.J.; Peterson, T.A.; Ruskin, J.N.; Peterson, R.T.; MacRae, C.A. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation 2003, 107, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [PubMed]
- McGrath, P.; Li, C.Q. Zebrafish: A predictive model for assessing drug-induced toxicity. Drug Discov. Today 2008, 13, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Xu, Y.Q.; He, J.H.; Yu, H.P.; Huang, C.J.; Gao, J.M.; Dong, Q.X.; Xuan, Y.X.; Li, C.Q. Human cardiotoxic drugs delivered by soaking and microinjection induce cardiovascular toxicity in zebrafish. J. Appl. Toxicol. 2014, 34, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.G.; Milan, D.J.; Grande, E.J.; Rottbauer, W.; MacRae, C.A.; Fishman, M.C. High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nat. Chem. Biol. 2005, 1, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Milan, D.J.; Jones, I.L.; Ellinor, P.T.; MacRae, C.A. In vivo recording of adult zebrafish electrocardiogram and assessment of drug-induced QT prolongation. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H269–H273. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, H.R.; Han, R.X.; Oqani, R.K.; Jin, D. Cardiovascular risk assessment of atypical antipsychotic drugs in a zebrafish model. J. Appl. Toxicol. 2013, 33, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Genschow, E.; Spielmann, H.; Scholz, G.; Seiler, A.; Brown, N.; Piersma, A.; Brady, M.; Clemann, N.; Huuskonen, H.; Paillard, F.; et al. The ECVAM international validation study on in vitro embryotoxicity tests: Results of the definitive phase and evaluation of prediction models. European Centre for the Validation of Alternative Methods. Altern. Lab. Anim. 2002, 30, 151–176. [Google Scholar] [PubMed]
- Ducharme, N.A.; Reif, D.M.; Gustafsson, J.; Bondesson, M. Comparison of toxicity values across zebrafish early life stages and mammalian studies: Implications for chemical testing. Reprod. Toxicol. 2015, 55, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Willett, C.; Fremgen, T. Zebrafish: An animal model for toxicological studies. Curr. Protoc. Toxicol. 2003. [Google Scholar] [CrossRef]
- Hill, A.; Ball, J.; Jones, M.; Dodd, A.; Mesens, N.; Vanparys, P. Implementation of Zebrafish toxicity testing between in vitro and in vivo models to advance candidate selection. In Proceedings of the 29th Annual Meeting of the American College of Toxicology, Tucson, AZ, USA, 9–12 November 2008. Abstract #108.
- He, J.H.; Guo, S.Y.; Zhu, F.; Zhu, J.J.; Chen, Y.X.; Huang, C.J.; Gao, J.M.; Dong, Q.X.; Xuan, Y.X.; Li, C.Q. A zebrafish phenotypic assay for assessing drug-induced hepatotoxicity. J. Pharmacol. Toxicol. 2013, 67, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compound sodium aescinate are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Jin, W.; Li, H.; Liu, H.; Huang, Y.; Shan, X.; Li, C.; Shan, L.; Efferth, T. In Vivo Cardiotoxicity Induced by Sodium Aescinate in Zebrafish Larvae. Molecules 2016, 21, 190. https://doi.org/10.3390/molecules21030190
Liang J, Jin W, Li H, Liu H, Huang Y, Shan X, Li C, Shan L, Efferth T. In Vivo Cardiotoxicity Induced by Sodium Aescinate in Zebrafish Larvae. Molecules. 2016; 21(3):190. https://doi.org/10.3390/molecules21030190
Chicago/Turabian StyleLiang, Jinfeng, Wangdong Jin, Hongwen Li, Hongcui Liu, Yanfeng Huang, Xiaowen Shan, Chunqi Li, Letian Shan, and Thomas Efferth. 2016. "In Vivo Cardiotoxicity Induced by Sodium Aescinate in Zebrafish Larvae" Molecules 21, no. 3: 190. https://doi.org/10.3390/molecules21030190
APA StyleLiang, J., Jin, W., Li, H., Liu, H., Huang, Y., Shan, X., Li, C., Shan, L., & Efferth, T. (2016). In Vivo Cardiotoxicity Induced by Sodium Aescinate in Zebrafish Larvae. Molecules, 21(3), 190. https://doi.org/10.3390/molecules21030190