2.1. Experimental Studies
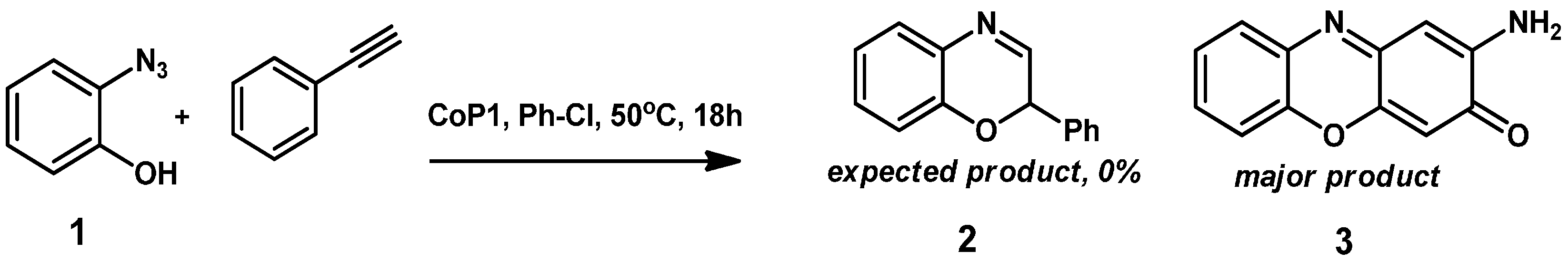
As an initial test reaction,
o-azidophenol
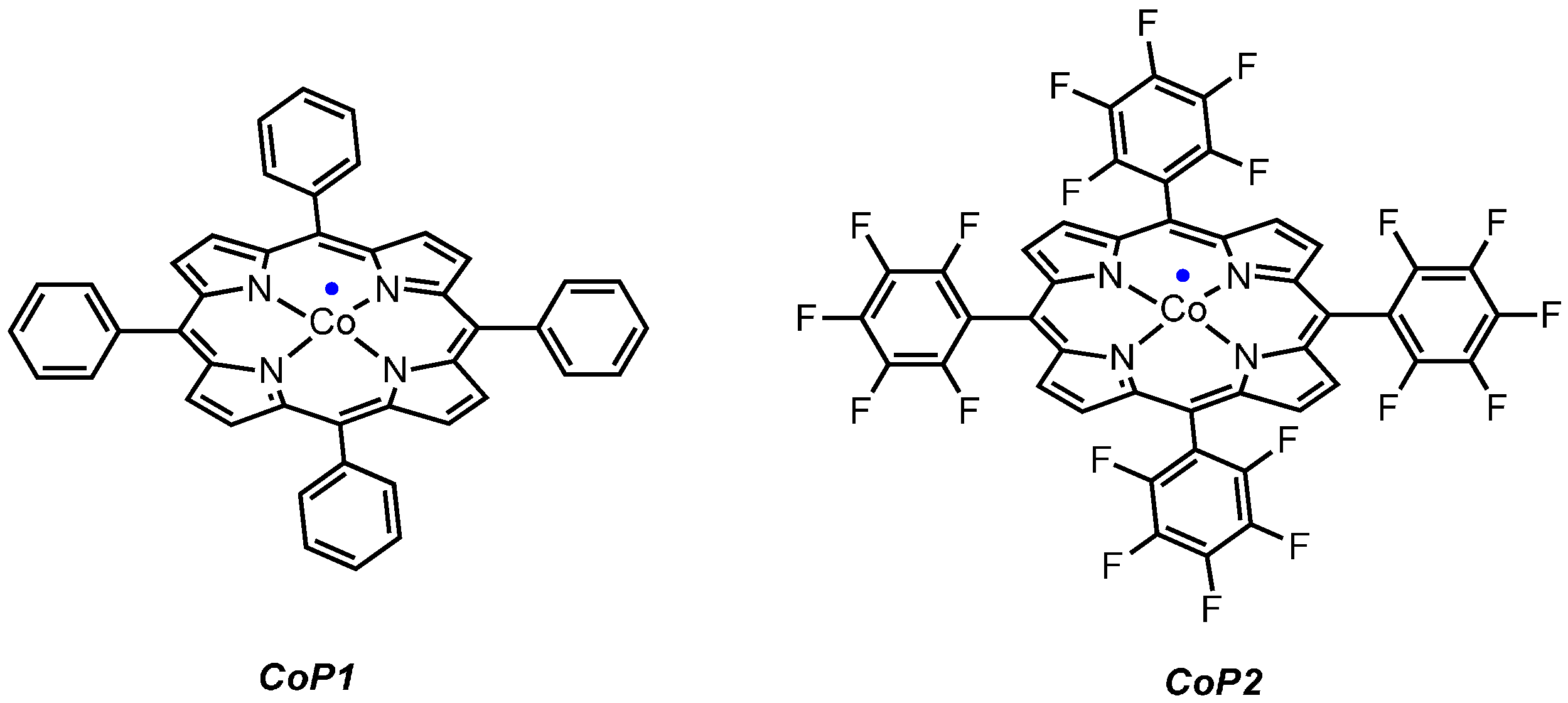
1 was reacted with
CoP1 (
Figure 1) in the presence of phenyl acetylene, expecting formation of benzoxazine
2 as the product (
Scheme 3). However, on analyzing the crude reaction mixture the expected product
2 was not detected. Instead, new
1H-NMR peaks were detected, and the azide was fully consumed. The main product in the reaction was separated from the reaction mixture by column chromatography, and proved to be phenoxizinone
3 (
Scheme 3).
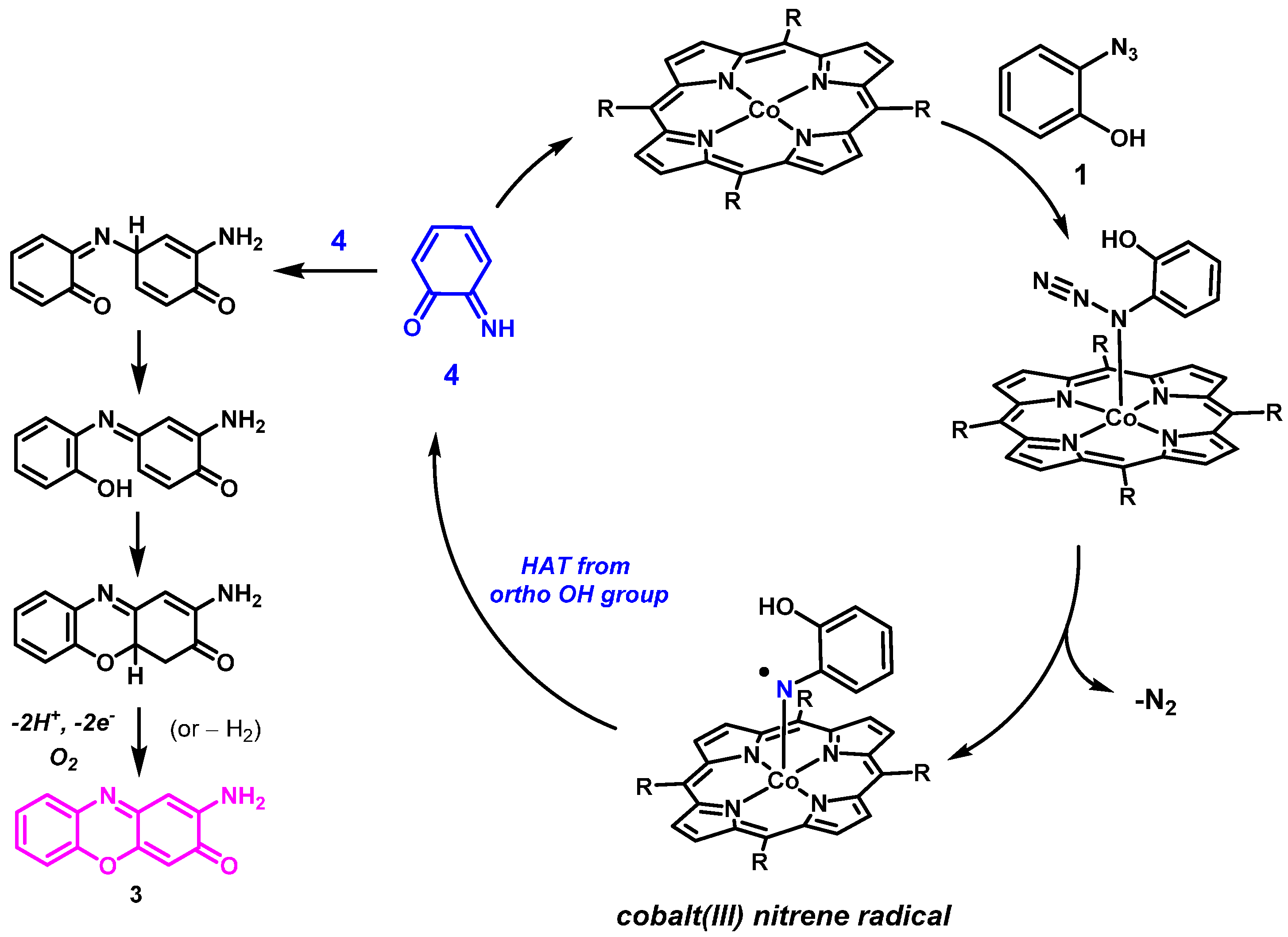
The unexpected formation of product
3 can be rationalized by considering HAT from the hydroxyl group in the
ortho-position of the cobalt(III) nitrenoid intermediate formed on activation of the azide by the catalyst
CoP1. This should result in the formation of the reactive
ortho-imino-quinonoid compound
4 (
Scheme 4). Attack of the imine N atom of
4 on the para position (w.r.t to the carbonyl group) of another molecule of
4, followed by 1,5-sigmatropic migration of a H atom, subsequent deprotonation and oxidation would lead to formation of the phenoxazine product 3. Such a pathway using
ortho-aminophenol (OAP) has been reported before using manganese porphyrins that use H
2O
2 as the external oxidant [
36]. In our case, the deprotonation and oxidation steps probably took place in air during column chromatography.
Compound
3 has anti-inflammatory and immunomodulatory properties and is, therefore, valuable for its medicinal properties. As mentioned before, other reported methods involving metalloporphyrin-catalyzed synthesis of
3 from OAP make use of more powerful oxidants like hydrogen peroxide, and are believed to be formed via different mechanisms [
36].
To see if formation of
3 could be avoided by using an excess of the alkyne, the reaction was also performed in neat alkyne. However, also in this case almost exclusive formation of compound
3 was observed. In addition to that, simple aziridination of alkenes like cyclohexene and styrene were also attempted using
o-azidophenol
1, which in all cases led to formation of only one identifiable and major product, the phenoxizinone
3 (
Table 1).
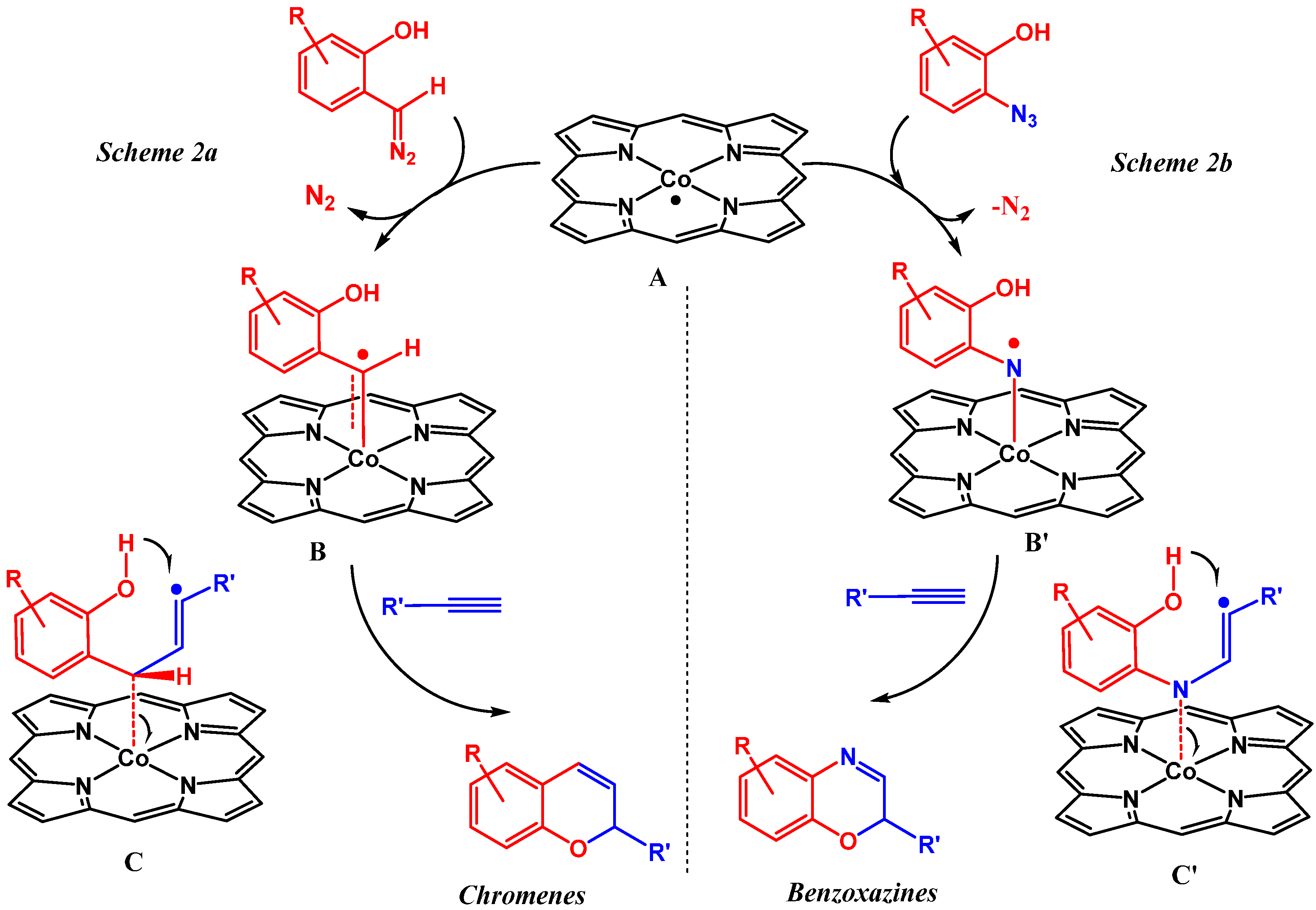
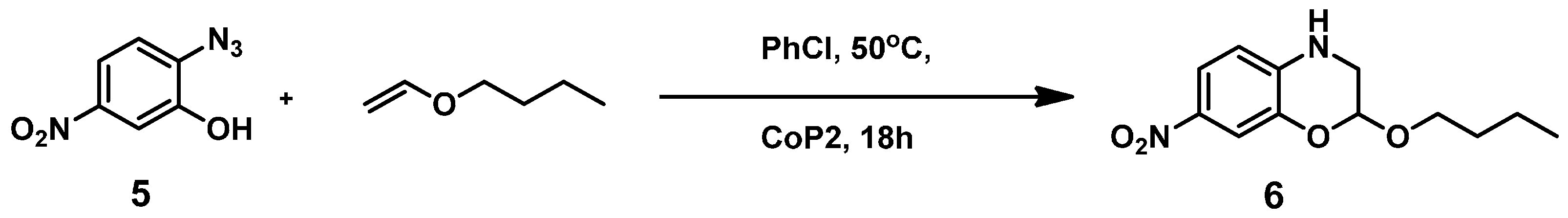
To be able to trap the proposed reactive intermediate
4 with a different substrate we decided to protect the phenyl ring on the 5-position, so that coupling of two iminoquinones to give products like
3 is prevented. As such, 2-azido-5-nitrophenol
5 was synthesized. As a test reaction we tried to trap the corresponding
o-quinone monoamine intermediate with 1-butoxyethene in an Inverse Electron Demand Diels Alder (IEDDA) reaction (
Scheme 5). For this transformation, however, no fruitful results were obtained when using
CoP1 as the catalyst. The crude reaction mixtures showed formation of a mixture of products containing a lot of azide starting material. This is most likely due to the inherent inability of
CoP1 to activate the azide under the applied reaction conditions. However, on switching to
CoP2, the desired transformation could be achieved and product
6 was obtained in 80% yield. Non-catalytic trapping of
o-quinone monoimines has been reported earlier, but the method uses stoichiometric amounts of halogen containing oxidants, which is avoided in the reaction depicted in
Scheme 5 [
37].
Substrate
5 was also tested in reactions with other potential reaction partners, in which we expected to be able to trap the iminoquinone intermediate with different C=C and C≡C bonds (
Table 2). With phenyl acetylene no reaction involving the C≡C bond was observed. Other alkenes also proved unreactive in this process. Apparently, only electron rich alkenes like 1-butoxyethene are suitable reaction partners in this process.
To investigate the generality of the observed HAT reactivity, we decided to explore the reactivity of aryl azide
7, containing an NH
2 substituent in the
ortho-position instead of an OH substituent. The
o-amino phenylazide
7 was synthesized and tested under the same reaction conditions, using
CoP1 as the catalyst (
Scheme 6). In contrast to our expectations, however, azabenzene
8 was obtained as the major product. This observation intrigued us, as formation of aza compounds as products in reaction of azides with cobalt(II) porphyrin catalyzed processes has been reported only once before, and only as a minor side product [
38].
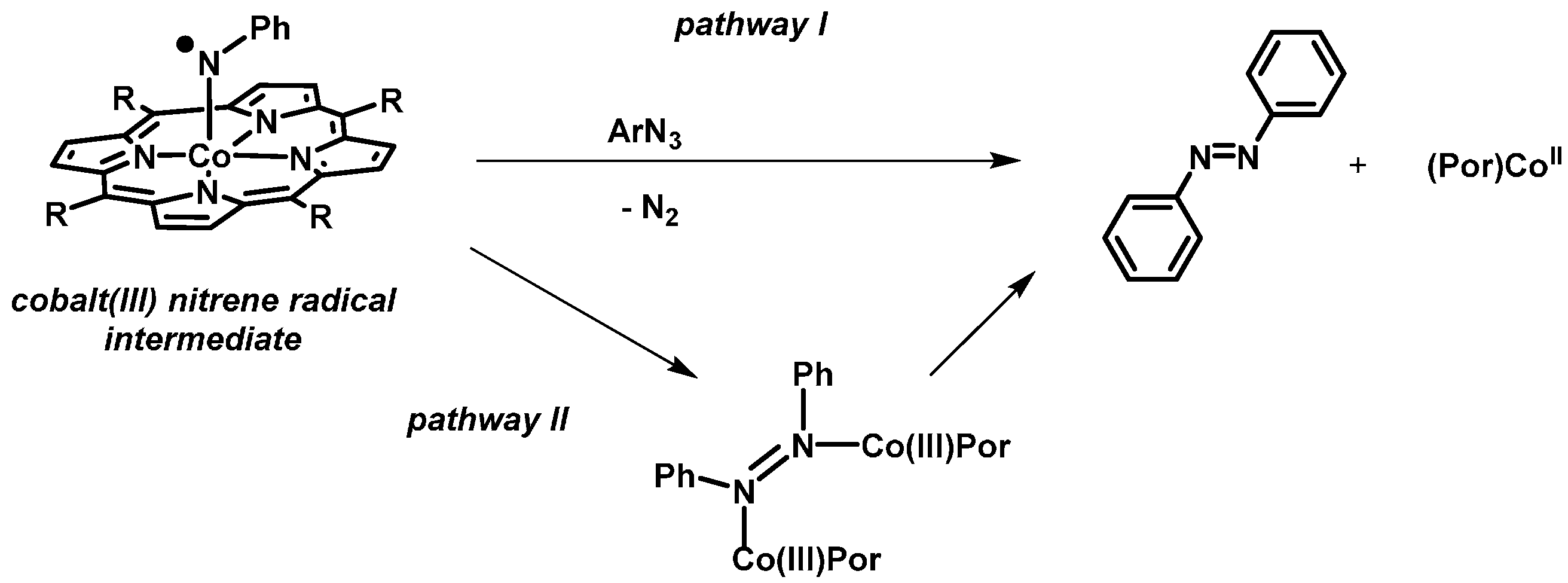
Two mechanistic proposals for formation of aza compounds in cobalt(II) porphyrins catalyzed reactions have been suggested: (I) Attack of the azide starting compound on the nitrene intermediate, producing azabenzene with simultaneous N
2-loss, and (II) dinuclear N-N coupling involving two nitrene complexes (
Scheme 7).
It is not so clear which reaction conditions lead to the formation of azabenzenes and which not. One suggested possibility was that in the presence of a large excess of another reaction partner, the concentration of the nitrene intermediate cannot build-up in a sufficient manner to allow formation of aza compounds via dinuclear N–N coupling [
39]. To check this hypothesis, we repeated the reaction of azide
7 in the presence of a large excess of phenyl acetylene or styrene in refluxing toluene. However, once again the aza compound
8 was obtained as the major product. This led us to believe that there is something unique about the NH
2 substituent in azide
7 that relates to formation of only the aza compound. We speculate that this relates to rapid formation of
o-phenylenediimine (OPDI) undergoing further reactions to form
8.
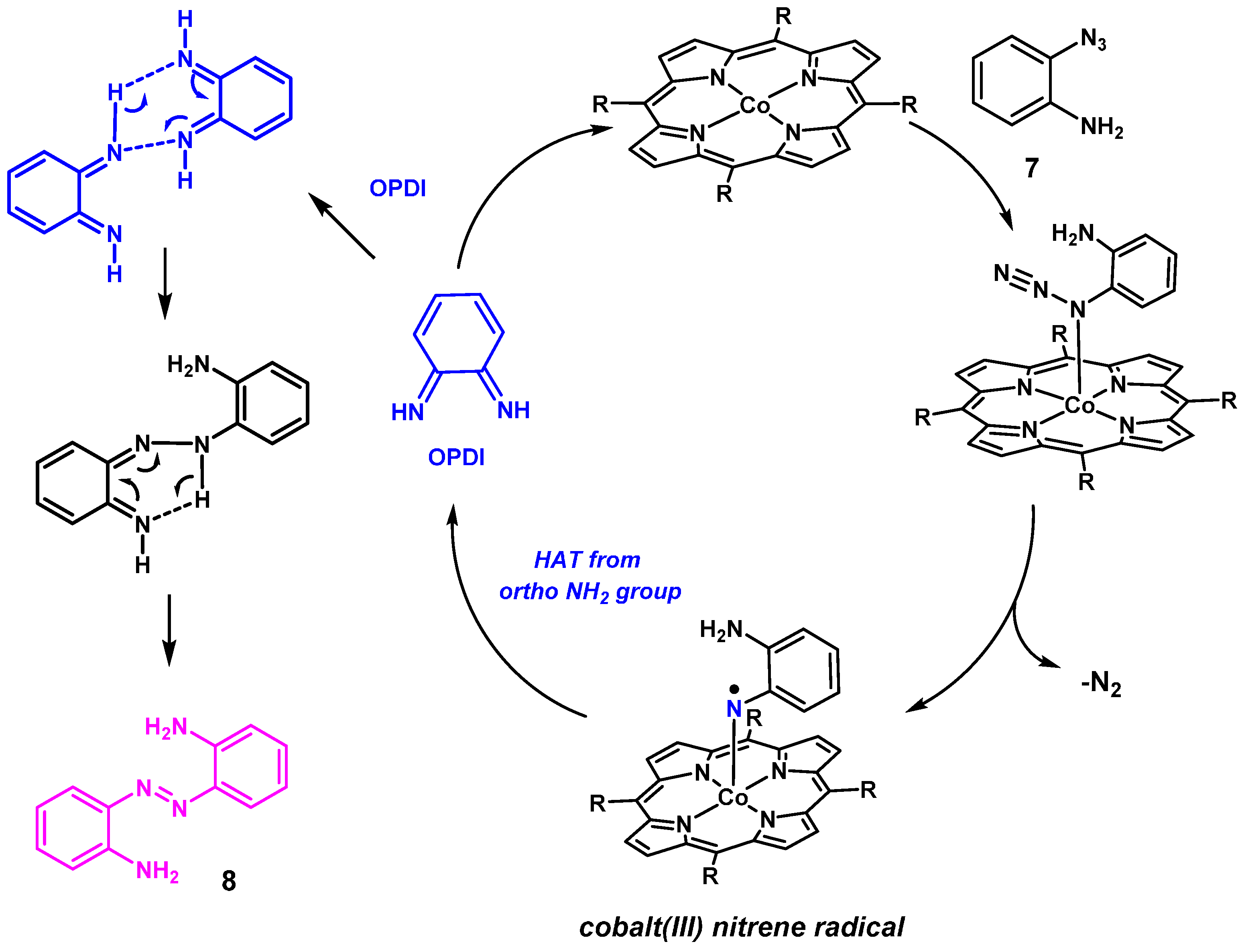
The formation of azabenzene
8 from
ortho-aminophenyl azides via the proposed phenylene diimine intermediate
9 can be reasoned in the mechanism depicted in
Scheme 8. Activation of azide
7 by the cobalt(II) catalysts leads to formation of a reactive nitrene radical intermediate. Subsequent rapid HAT from the ortho-NH
2 group to the nitrene radical moiety in this intermediate produces OPDI. This is followed by intermolecular coupling of two OPDI molecules to form aza compound
8. The ODPI coupling steps could also be catalyzed by trace amounts of orthophenylenediamine (generated by some minor side-reaction), as has been suggested in literature [
40].
It is worth mentioning that this reaction of
ortho-amino substituted phenyl azides to give the azabenzene compounds as the major product is one of the few catalytic examples reported so far to synthesize aza compounds in high yields [
41,
42,
43]. An example involving a ruthenium metallo-radical system proceeds via a free nitrene intermediate and works catalytically only for aryl azides with electron rich substitutents like OMe and OEt [
43]. Another iron based example is limited in the sense that only azides with bulky substitutents like mestiyl groups result in formation of aza compounds [
41]. With trifluoromethyl and methyl substituents dimers of the metal complex are obtained. The other example involves a nickel complex, but this system produces only stoichiometric amounts of aza compounds [
42].
2.2. Computational Studies
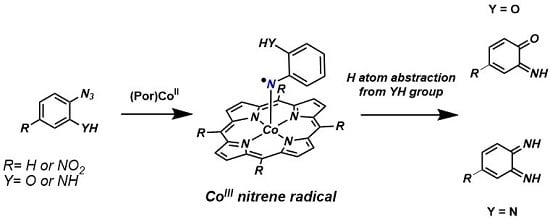
To estimate the reaction barriers for the assumed facile HAT process from the ortho-YH substituent (Y = O, NH) to the nitrene moiety, we investigated this process with DFT for both the OH and the NH2 substituents.
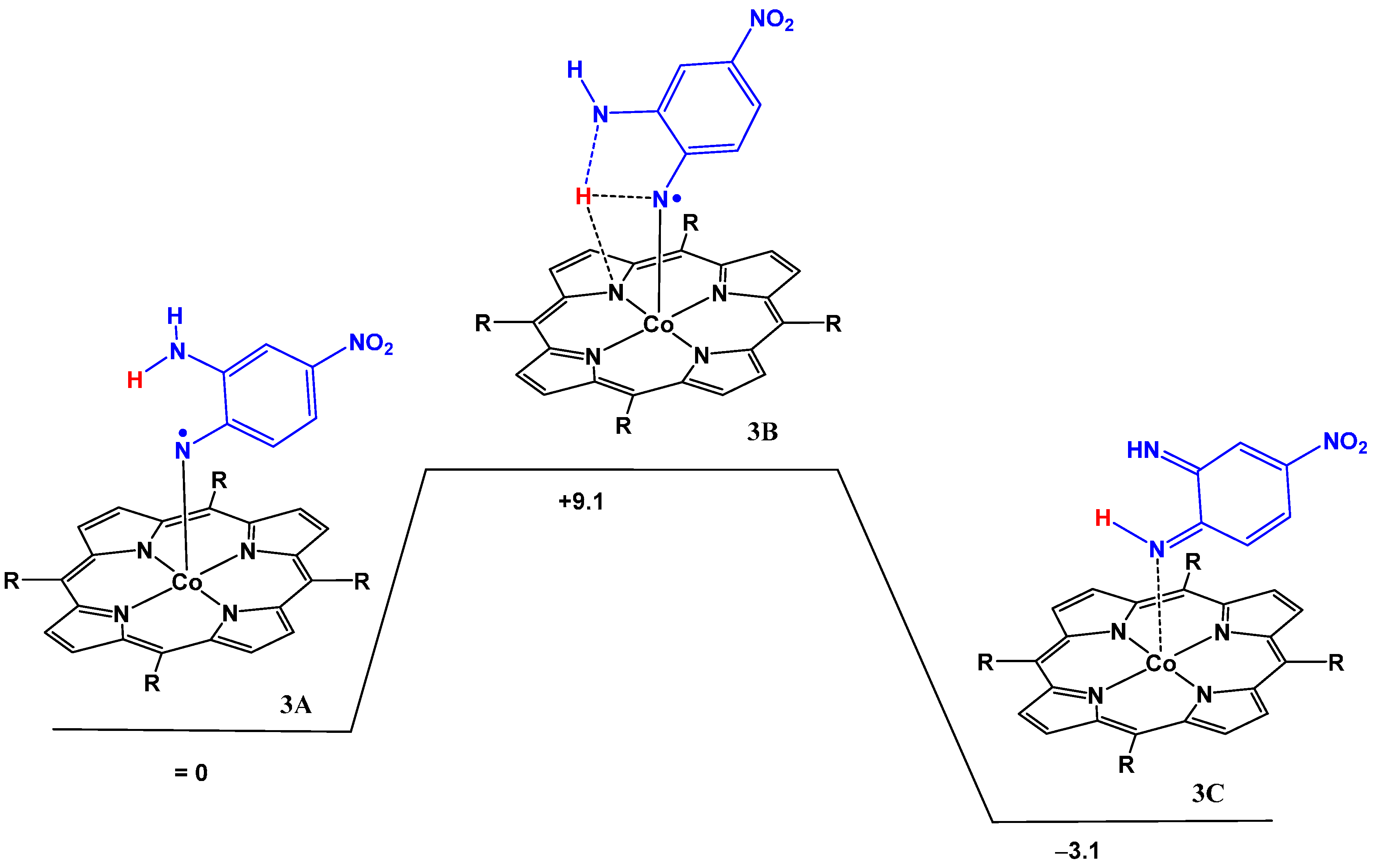
Starting from the cobalt(III) nitrene radical species the intramolecular HAT reaction of the cobalt(III)nitrene radical of the azide
5 was found to proceed via a 6-membered transition state, further stabilized by a hydrogen bonding interaction between the transferred hydrogen atom and a pyrrole nitrogen atom of the porphyrin (
Figure 2, Computational details in
Table S1). The barrier is very low (+1.0 kcal·mol
−1), thus explaining the experimental observations. Overall, the HAT process is exergonic by −10.8 kcal·mol
−1.
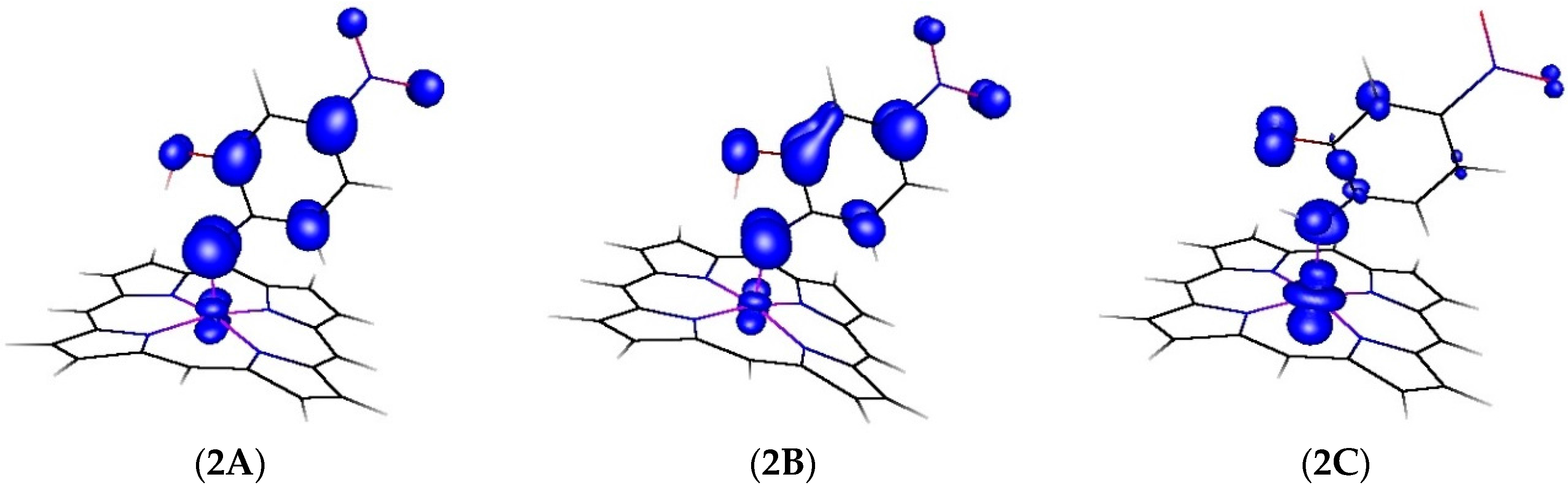
We further evaluated the changes in spin density distribution (see
Figure 3 and
Table 3) during the HAT process (see also
Figure 2). The spin density distribution of the transition state is very similar to that in the initial cobalt(III) nitrene radical, but after the HAT barrier and transfer of the hydrogen atom from the –OH group, most of the spin density moves back to cobalt (
Figure 3 and
Table 3). Simultaneously, the bond length of the Co-N bond elongates from 1.818 Å in the nitrene radical to 1.923 Å in the imide as depicted in
Table 4.
The almost barrierless abstraction of a neighboring hydrogen atom by the reactive cobalt(III)
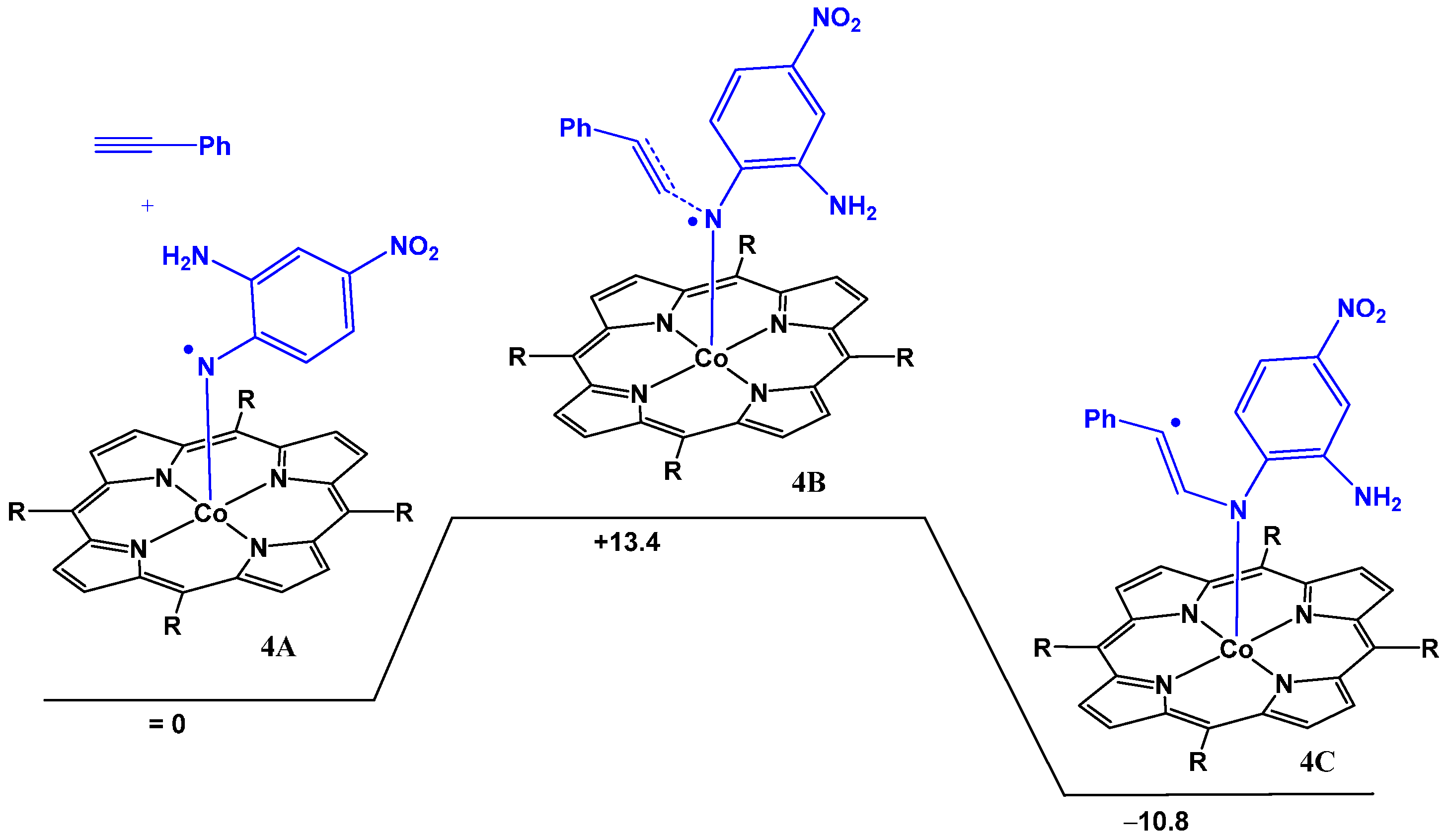
nitrene radical thus prevents any intermolecular coupling reactions of the nitrene moiety with exogenous substrates. A similar process was also calculated for the NH
2 substituted azide, and once again the barrier for intramolecular HAT between the NH
2 group and the nitrene moiety was found to be low (see
Figure 4). The free energy required to reach the transition state is only +9.1 kcal·mol
−1 implying that this intramolecular process is fast, even at room temperature. The overall transformation is exergonic by −3.1 kcal·mol
−1.
The computed changes in the spin density distributions are once again in line with the HAT process, and similar to those computed for HAT from the OH substituent (See
Figure 5 and
Table 5). During the HAT process the spin population shifts from the nitrene radical in
3A to cobalt in
3C, and after the HAT process the spin density is mostly concentrated at the cobalt atom. The bond distance analysis of the relevant bonds are shown in
Table 6. Here the Co-N bond in the final structure elongates from 1.8521 Å in the transition state
3B to 1.9604 Å in the final structure
3C. Interestingly, the adduct remains coordinated to the cobalt complex, as is evident from the bond distances.
While the barrier for the HAT process depicted in
Figure 4 is low, it is not barrierless. Hence, to exclude the possibility of intermolecular coupling of the nitrene radical to phenyl acetylene (see
Scheme 2b) being in competition with the intramolecular HAT process (
Figure 4), we decided to make a direct computational comparison of the two processes. Attack of the porphyrin cobalt(III) nitrene radical on the alkyne to form the γ-radical species
4C (
C’ in
Scheme 2b) was computed at the same DFT level (see
Figure 6). The latter process is exergonic (−10.8 kcal·mol
−1), but has a computed barrier of +13.4 kcal·mol
−1. This barrier is almost 4 kcal·mol
−1 higher than the barrier for intramolecular HAT (see
Figure 4), and hence this process cannot efficiently compete with the intramolecular HAT reaction. Formation of the OPDI intermediate by HAT is expected under all reaction conditions.
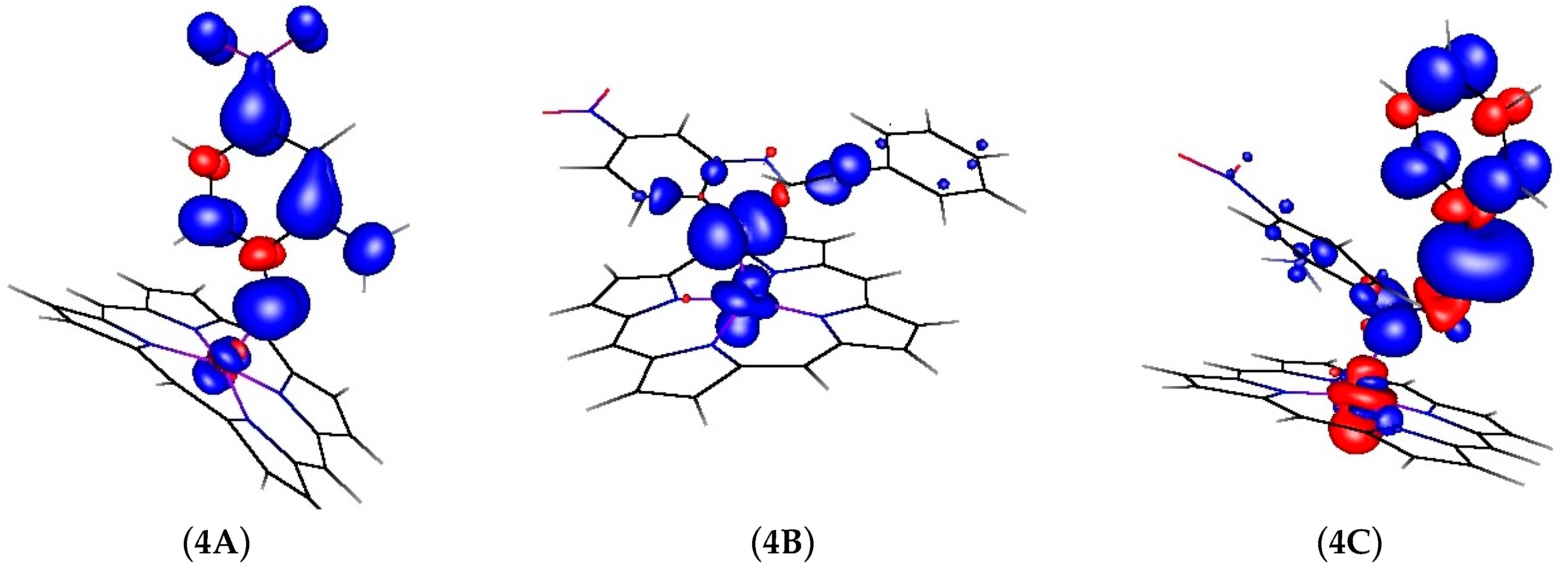
The spin density distribution changes were once again calculated for the structures
4A,
4B and
4C. Interestingly, in contrast to the structures shown in
Figure 3 and
Figure 5, the spin density in structure
4C is strongly delocalized over the carbon atoms of the γ-alkyl radical and the adjacent phenyl ring, with barely any spin density at the cobalt atom (see
Figure 7).
















 (~10%)
(~10%)



 (80%)
(80%)