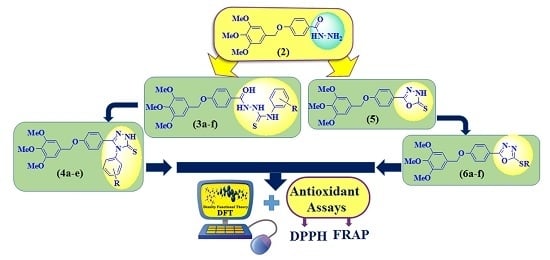
Conjugated Oligo-Aromatic Compounds Bearing a 3,4,5-Trimethoxy Moiety: Investigation of Their Antioxidant Activity Correlated with a DFT Study
Abstract
:1. Introduction
2. Results and Discussion
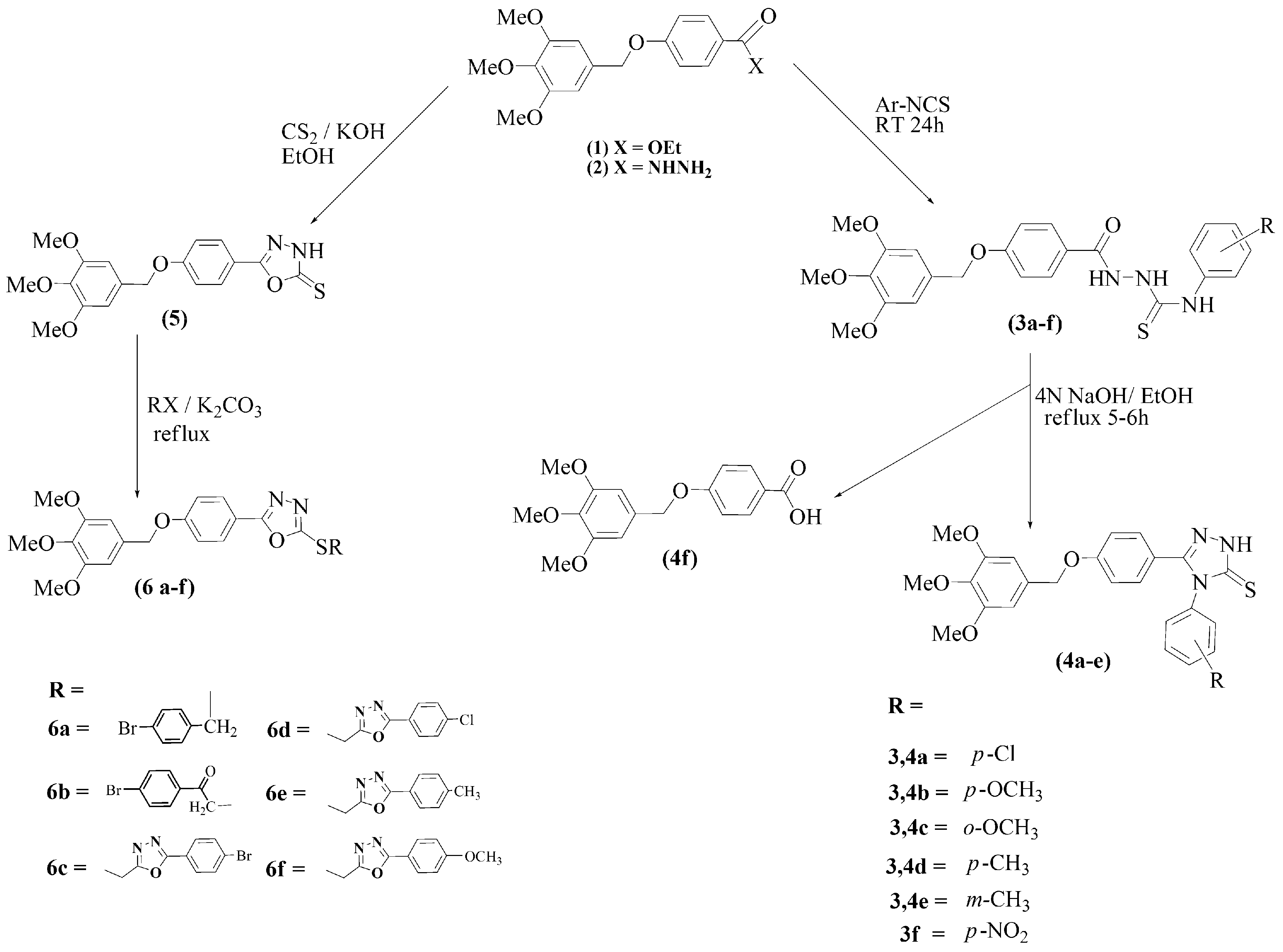
2.1. Synthesis
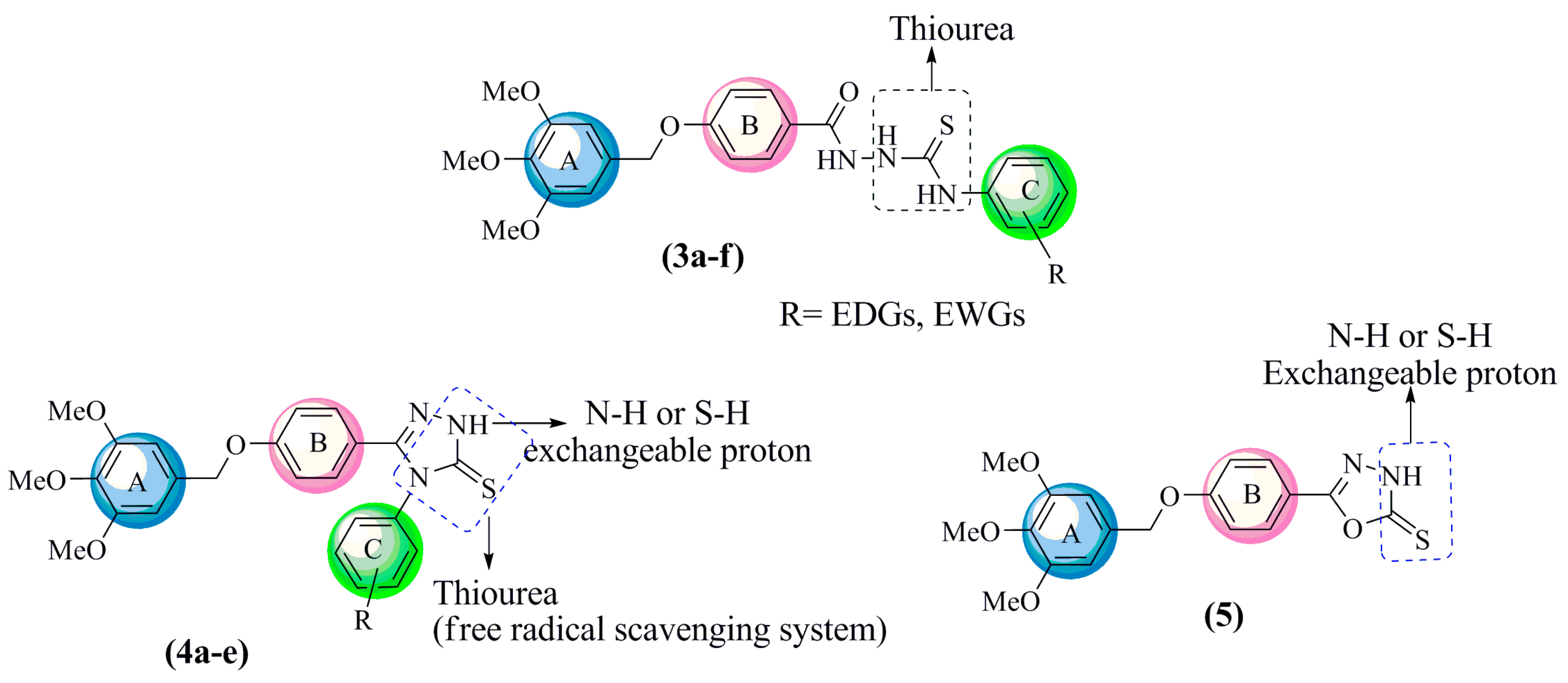
2.2. In Vitro Free Radical Scavenging Activities
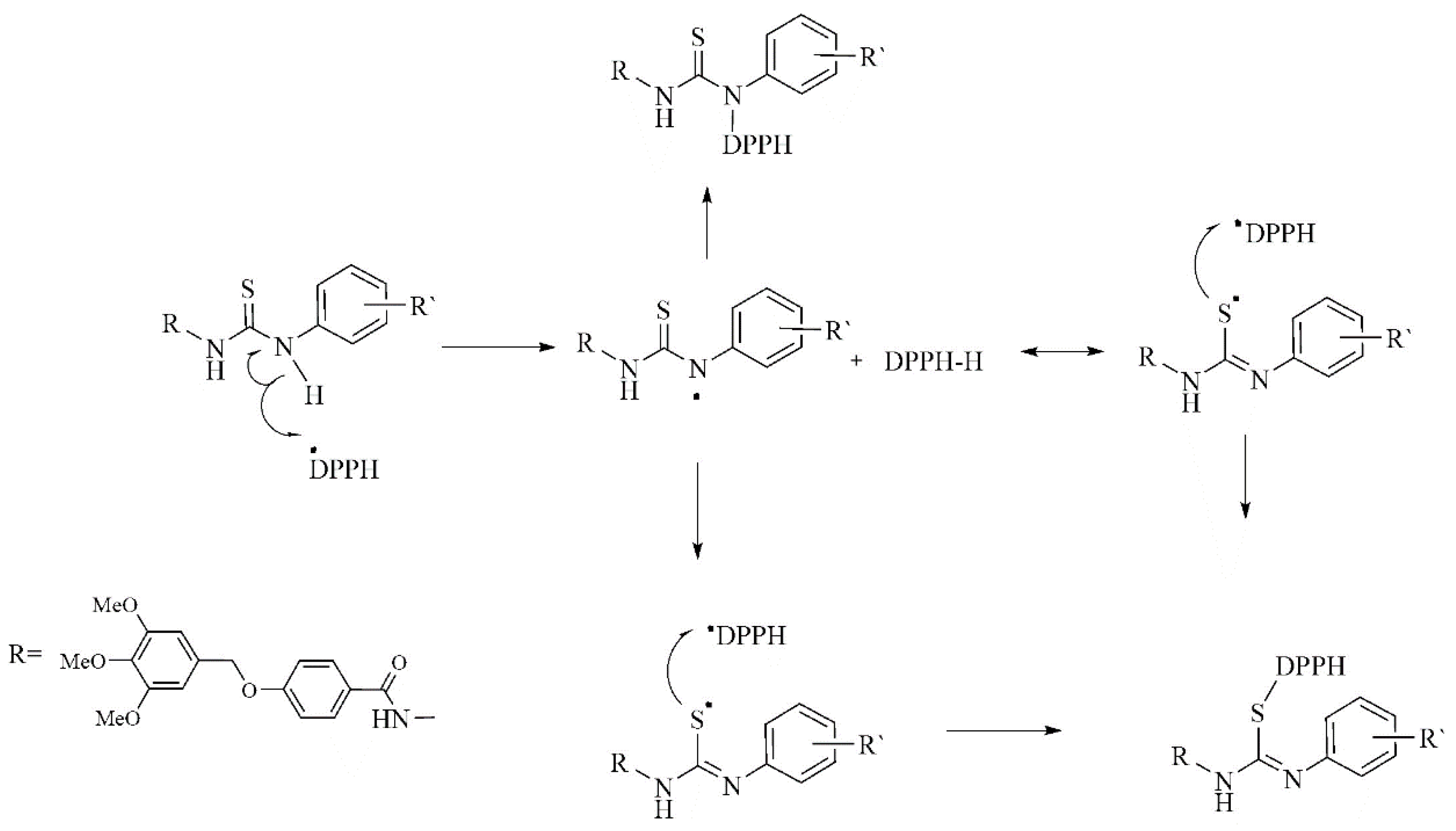
2.2.1. DPPH Free Radical Scavenging Activities
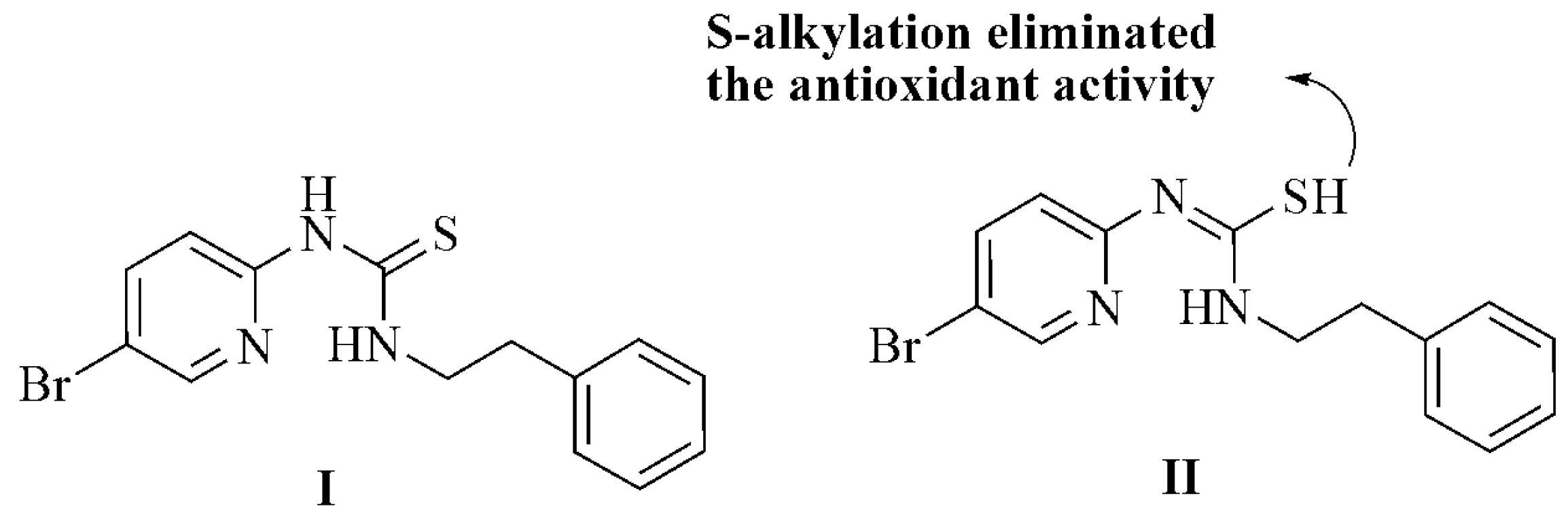
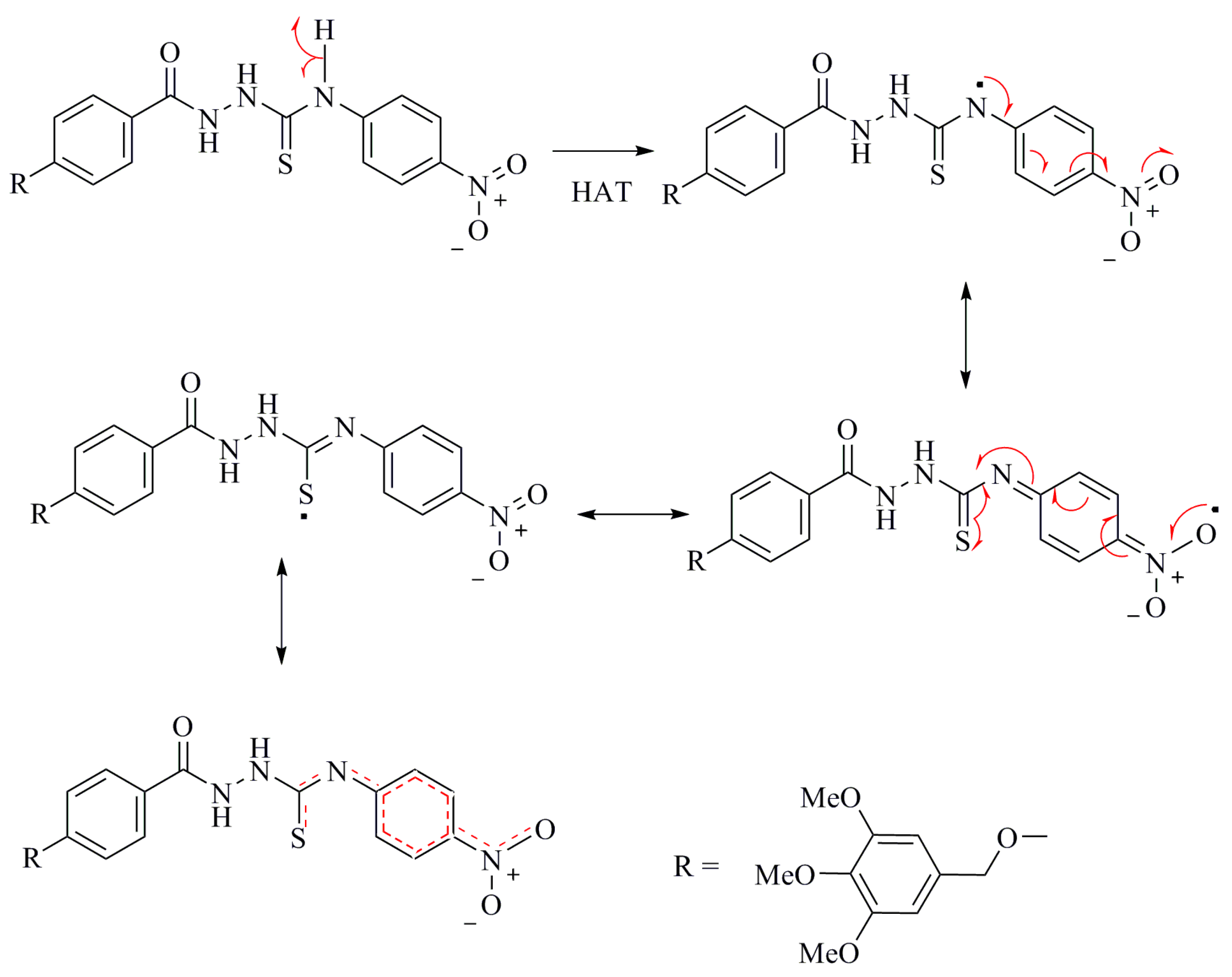
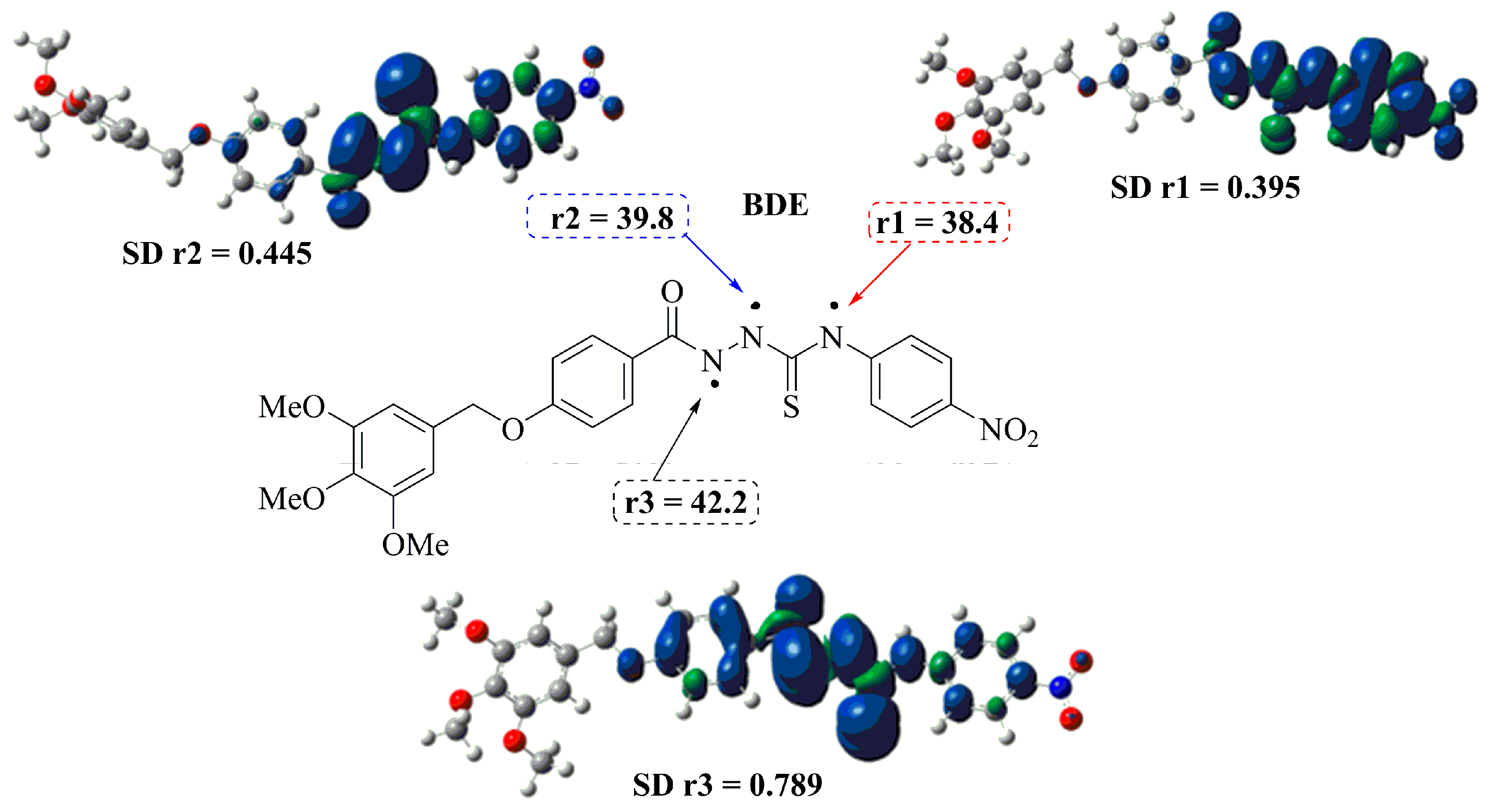
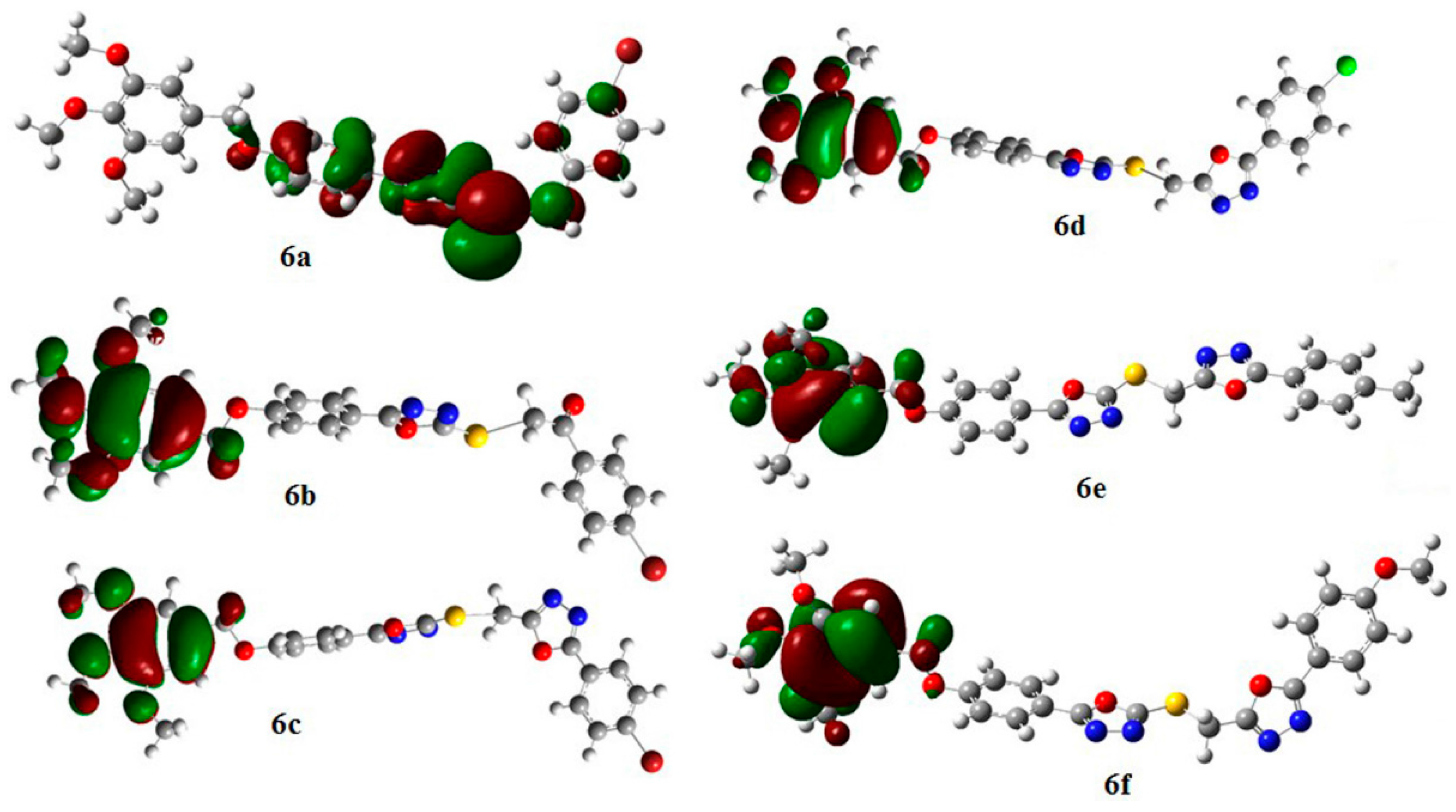
DFT Study of the DPPH Radical Scavenging Activities of Thiosemicarbazide
DPPH Free Radical Scavenging Activities for Oxadiazole
DFT Study of the DPPH Radical Scavenging Activities of Oxadiazole
2.2.2. Ferric Reducing Antioxidant Power (FRAP) Activities
3. Materials and Methods
3.1. General Information
3.2. Synthesis
3.2.1. General Procedure for the Synthesis of N-(4-aryl)-2-(4-(3,4,5-trimethoxybenzyloxy)Benzoyl)hydrazine Carbothioamides (3a–f)
3.2.2. General Procedure for the Synthesis of 4-(4-Aryl)-3-(4-(3,4,5-trimethoxy benzyloxy) Phenyl)-1H-1,2,4-Triazole-5-(4H)-thiones 4a–f
3.2.3. 5-(4-(3,4,5-Trimethoxybenzyloxy)phenyl)-1,3,4-oxadiazole-2-(3H)-thione (5)
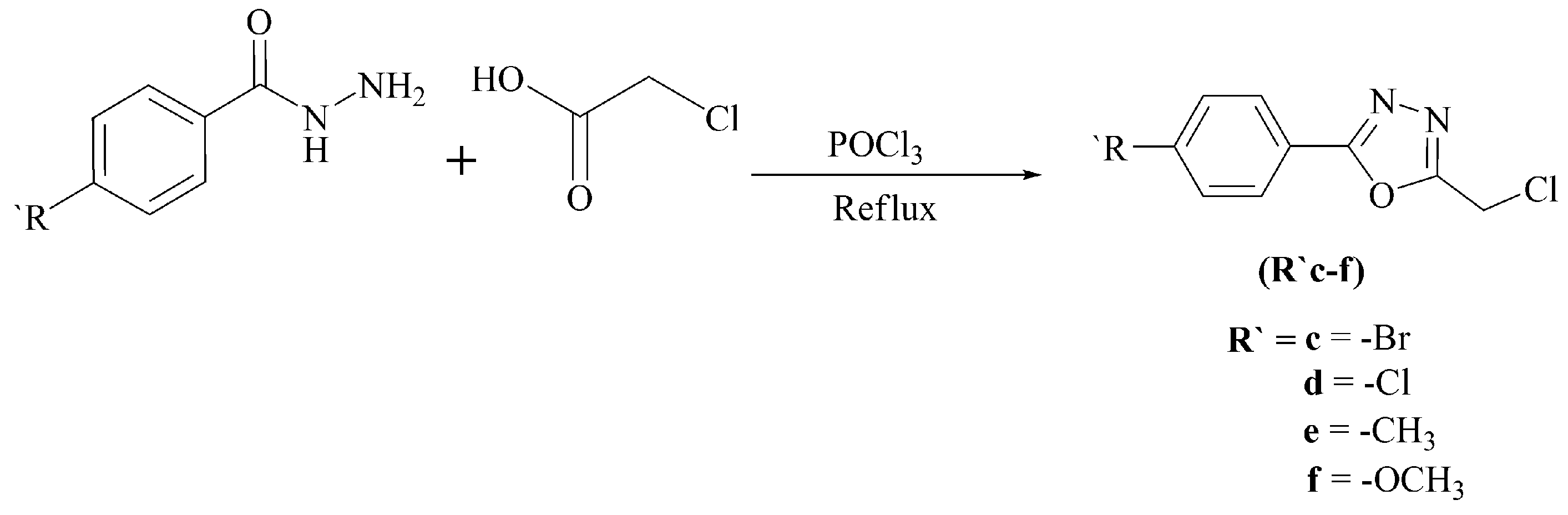
3.2.4. General Procedure for the Synthesis of 5-Aryl-2-(chloromethyl)-1,3,4-oxadiazoles (R’c–f)
3.2.5. General Procedure for the Synthesis of 2-(4-Arylthio)-5-(4-(3,4,5-trimethoxybenzyloxy) Phenyl)-1,3,4-oxadiazoles (6a–f)
3.2.6. In Vitro Antioxidant Activity Assays
DPPH Radical Scavenging Activities
Ferric Reducing Antioxidant Power Activity (FRAP)
Computational Details
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roesslein, M.; Hirsch, C.; Kaiser, J.-P.; Krug, H.F.; Wick, P. Comparability of in vitro tests for bioactive nanoparticles: A common assay to detect reactive oxygen species as an example. Int. J. Mol. Sci. 2013, 14, 24320–24337. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive oxygen species and the central nervous system. In Free Radicals in the Brain; Springer-Verlag: Berlin, Heidelberg, Germany, 1992; pp. 21–40. [Google Scholar]
- Halliwell, B.; Aeschbach, R.; Löliger, J.; Aruoma, O. The characterization of antioxidants. Food Chem. Toxicol. 1995, 33, 601–617. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Wu, D. How many peroxyl radicals can be scavenged by hydroxyl-substituted Schiff bases in the oxidation of linoleic acid? J. Phys. Org. Chem. 2009, 22, 308–312. [Google Scholar] [CrossRef]
- Hussain, H.H.; Babic, G.; Durst, T.; Wright, J.S.; Flueraru, M.; Chichirau, A.; Chepelev, L.L. Development of novel antioxidants: Design, synthesis, and reactivity. J. Org. Chem. 2003, 68, 7023–7032. [Google Scholar] [CrossRef] [PubMed]
- Mobinikhaledi, A.; Foroughifar, N.; Kalhor, M.; Ebrahimi, S.; Fard, M.B. Synthesis of some symmetrical novel bis-thiosemicarbazides, 1,2,4-triazoles, 1,3,4-thiadiazoles, and their derivatives. Phosphorus Sulfur Silicon 2010, 186, 67–73. [Google Scholar] [CrossRef]
- Hu, K.; Yang, Z.; Pan, S.-S.; Xu, H.; Ren, J. Synthesis and antitumor activity of liquiritigenin thiosemicarbazone derivatives. Eur. J. Med. Chem. 2010, 45, 3453–3458. [Google Scholar] [CrossRef] [PubMed]
- Dolman, S.J.; Gosselin, F.; O’Shea, P.D.; Davies, I.W. Superior reactivity of thiosemicarbazides in the synthesis of 2-amino-1,3,4-oxadiazoles. J. Org. Chem. 2006, 71, 9548–9551. [Google Scholar] [CrossRef] [PubMed]
- Salih Ağırtaş, M.; Cabir, B.; Özdemir, S. Novel metal(II) phthalocyanines with 3,4,5-trimethoxybenzyloxy-substituents: Synthesis, characterization, aggregation behaviour and antioxidant activity. Dyes Pigment. 2013, 96, 152–157. [Google Scholar] [CrossRef]
- Odlo, K.; Fournier-Dit-Chabert, J.; Ducki, S.; Gani, O.A.; Sylte, I.; Hansen, T.V. 1,2,3-triazole analogs of combretastatin A-4 as potential microtubule-binding agents. Bioorg. Med. Chem. 2010, 18, 6874–6885. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jiang, Y.; Tan, C.; Zu, X.; Liu, H.; Cao, D. Synthesis and potent antileukemic activities of 10-Benzyl-9 (10H)-acridinones. Bioorg. Med. Chem. 2008, 16, 8670–8675. [Google Scholar] [CrossRef] [PubMed]
- Nam, N.-H.; Kim, Y.; You, Y.-J.; Hong, D.-H.; Kim, H.-M.; Ahn, B.-Z. Synthesis and anti-tumor activity of novel combretastatins: Combretocyclopentenones and related analogues. Bioorg. Med. Chem. Lett. 2002, 12, 1955–1958. [Google Scholar] [CrossRef]
- Odlo, K.; Hentzen, J.; Ducki, S.; Gani, O.A.; Sylte, I.; Skrede, M.; Flørenes, V.A.; Hansen, T.V. 1,5-disubstituted 1,2,3-triazoles as cis-restricted analogues of combretastatin A-4: Synthesis, molecular modeling and evaluation as cytotoxic agents and inhibitors of tubulin. Bioorg. Med. Chem. 2008, 16, 4829–4838. [Google Scholar] [CrossRef] [PubMed]
- Medarde, M.; Maya, A.B.; Pérez-Melero, C. Review article naphthalene combretastatin analogues: Synthesis, cytotoxicity and antitubulin activity. J. Enzym. Inhib. Med. Chem. 2004, 19, 521–540. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.Y.-C.; Yu, T.-H.; Lateef, S.K. Removal of pharmaceuticals in secondary wastewater treatment processes in Taiwan. J. Hazard. Mater. 2009, 167, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Gulkowska, A.; Leung, H.W.; So, M.K.; Taniyasu, S.; Yamashita, N.; Yeung, L.W.; Richardson, B.J.; Lei, A.; Giesy, J.P.; Lam, P.K. Removal of antibiotics from wastewater by sewage treatment facilities in Hong Kong and shenzhen, china. Water Res. 2008, 42, 395–403. [Google Scholar] [CrossRef]
- Lindberg, R.H.; Wennberg, P.; Johansson, M.I.; Tysklind, M.; Andersson, B.A. Screening of human antibiotic substances and determination of weekly mass flows in five sewage treatment plants in Sweden. Environ. Sci. Technol. 2005, 39, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Göbel, A.; Thomsen, A.; McArdell, C.S.; Joss, A.; Giger, W. Occurrence and sorption behavior of sulfonamides, macrolides, and trimethoprim in activated sludge treatment. Environ. Sci. Technol. 2005, 39, 3981–3989. [Google Scholar] [CrossRef] [PubMed]
- Cody, V.; Luft, J.R.; Pangborn, W.; Gangjee, A.; Queener, S.F. Structure determination of tetrahydroquinazoline antifolates in complex with human and pneumocystis carinii dihydrofolate reductase: Correlations between enzyme selectivity and stereochemistry. Acta Cryst. D Biol. Crystallogr. 2004, 60, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Kareem, H.S.; Ariffin, A.; Nordin, N.; Heidelberg, T.; Abdul-Aziz, A.; Kong, K.W.; Yehye, W.A. Correlation of antioxidant activities with theoretical studies for new hydrazone compounds bearing a 3,4,5-trimethoxy benzyl moiety. Eur. J. Med. Chem. 2015, 103, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Siatra-Papastaikoudi, T.; Tsotinis, A.; Raptopoulou, C.; Sambani, C.; Thomou, H. Synthesis of new alkylaminoalkyl thiosemicarbazones of 3-acetylindole and their effect on DNA synthesis and cell proliferation. Eur. J. Med. Chem. 1995, 30, 107–114. [Google Scholar] [CrossRef]
- Tsotinis, A.; Varvaresou, A.; Calogeropoulou, T.; Siatra-Papastaikoudi, T.; Tiligada, A. Synthesis and antimicrobial evaluation of indole containing derivatives of 1,3,4-thiadiazole, 1,2,4-triazole and their open-chain counterparts. Arzneim.-Forsch. 1997, 47, 307–310. [Google Scholar] [CrossRef]
- Shafiee, A.; Naimi, E.; Mansobi, P.; Foroumadi, A.; Shekari, M. Syntheses of substituted-oxazolo-1,3,4-thiadiazoles, 1,3,4-oxadiazoles, and 1,2,4-thiazoles. J. Heterocycl. Chem. 1995, 32, 1235–1239. [Google Scholar] [CrossRef]
- Padmavathi, V.; Reddy, G.D.; Reddy, S.N.; Mahesh, K. Synthesis and biological activity of 2-(bis ((1,3,4-oxadiazolyl/1,3,4-thiadiazolyl) methylthio) methylene) malononitriles. Eur. J. Med. Chem. 2011, 46, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant properties of resveratrol: A structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Bakalbassis, E.G.; Lithoxoidou, A.T.; Vafiadis, A.P. Theoretical insights, in the liquid phase, into the antioxidant mechanism-related parameters in the 2-monosubstituted phenols. J. Phys. Chem. A 2006, 110, 11151–11159. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Le, T.H.; Bui, T.T.T. Antioxidant activities of thiosemicarbazones from substituted benzaldehydes and N-(tetra-O-acetyl-β-d-galactopyranosyl) thiosemicarbazide. Eur. J. Med. Chem. 2013, 60, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, D.N.; Fylaktakidou, K.C.; Litinas, K.E.; Hadjipavlou-Litina, D. Synthesis and biological evaluation of several coumarin-4-carboxamidoxime and 3-(coumarin-4-yl)-1,2,4-oxadiazole derivatives. Eur. J. Med. Chem. 1998, 33, 715–724. [Google Scholar] [CrossRef]
- Kopylova, B.; Gasanov, R.; Freidlina, R.K. Radical arylation of thiosemicarbazide and acetone thiosemicarbazone by aryldiazonium borofluorides. Bull. Acad. Sci. Chem. USSR Div. Chem. Sci. 1981, 30, 1059–1062. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ghosh, S.; Misra, A.K.; Bhatia, G.; Khan, M.; Khanna, A. Syntheses and evaluation of glucosyl aryl thiosemicarbazide and glucosyl thiosemicarbazone derivatives as antioxidant and anti-dyslipidemic agents. Bioorg. Med. Chem. Lett. 2009, 19, 386–389. [Google Scholar] [CrossRef]
- Barbuceanu, S.-F.; Ilies, D.C.; Saramet, G.; Uivarosi, V.; Draghici, C.; Radulescu, V. Synthesis and antioxidant activity evaluation of new compounds from hydrazinecarbothioamide and 1,2,4-triazole class containing diarylsulfone and 2,4-difluorophenyl moieties. Int. J. Mol. Sci. 2014, 15, 10908–10925. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T.; Yamaguchi, T.; Fukui, T.; Tomii, K. Chain-breaking fused heterocyclic antioxidants: Antioxidant activities of phenothiazines compared to related compounds. Polym. Degrad. Stab. 1999, 64, 33–38. [Google Scholar] [CrossRef]
- Lucarini, M.; Pedrielli, P.; Pedulli, G.F.; Cabiddu, S.; Fattuoni, C. Bond dissociation energies of OH bonds in substituted phenols from equilibration studies. J. Org. Chem. 1996, 61, 9259–9263. [Google Scholar] [CrossRef]
- Dong, Y.; Venkatachalam, T.; Narla, R.K.; Trieu, V.N.; Sudbeck, E.A.; Uckun, F.M. Antioxidant function of phenethyl-5-bromo-pyridyl thiourea compounds with potent anti-HIV activity. Bioorg. Med. Chem. Lett. 2000, 10, 87–90. [Google Scholar] [CrossRef]
- Yehye, W.A.; Rahman, N.A.; Ariffin, A.; Hamid, S.B.A.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M. Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): A review. Eur. J. Med. Chem. 2015, 101, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xie, M.-Y.; Nie, S.-P.; Li, C.; Wang, Y.-X. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of ganoderma atrum. Food Chem. 2008, 107, 231–241. [Google Scholar] [CrossRef]
- Queiroz, A.N.; Gomes, B.A.; Moraes, W.M.; Borges, R.S. A theoretical antioxidant pharmacophore for resveratrol. Eur. J. Med. Chem. 2009, 44, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, T.; Ohkatsu, Y. Effect of para-substituents of phenolic antioxidants. Polym. Degrad. Stab. 2001, 71, 445–452. [Google Scholar] [CrossRef]
- Jorgensen, L.V.; Cornett, C.; Justesen, U.; Skibsted, L.H.; Dragsted, L.O. Two-electron electrochemical oxidation of quercetin and kaempferol changes only the flavonoid C-ring. Free Radic. Res. 1998, 29, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Lucarini, M.; Mugnaini, V.; Pedulli, G.F. Antioxidant activity of o-bisphenols: The role of intramolecular hydrogen bonding. J. Org. Chem. 2003, 68, 5198–5204. [Google Scholar] [CrossRef] [PubMed]
- Kus, C.; Ayhan-Kilcigil, G.; Eke, B.C. Synthesis and antioxidant properties of some novel benzimidazole derivatives on lipid peroxidation in the rat liver. Arch. Pharmacal Res. 2004, 27, 156–163. [Google Scholar] [CrossRef]
- Ariffin, A.; Rahman, N.A.; Yehye, W.A.; Alhadi, A.A.; Kadir, F.A. Pass-assisted design, synthesis and antioxidant evaluation of new butylated hydroxytoluene derivatives. Eur. J. Med. Chem. 2014, 87, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Velkov, Z.; Balabanova, E.; Tadjer, A. Radical scavenging activity prediction of o-coumaric acid thioamide. J. Mol. Struct. Theochem 2007, 821, 133–138. [Google Scholar] [CrossRef]
- Ozdem, S.; Alicigüzel, Y.; Ozdem, S.; Karayalcin, U. Effects of propylthiouracil treatment on antioxidant activities in blood of toxic multinodular goiter patients. Pharmacology 2000, 61, 31–36. [Google Scholar] [PubMed]
- Indira Priyadarsini, K.; Maity, D.K.; Naik, G.; Sudheer Kumar, M.; Unnikrishnan, M.; Satav, J.; Mohan, H. Role of phenolic OH and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Boone, C.W.; Simic, M.G. H-atom transfer is a preferred antioxidant mechanism of curcumin. J. Am. Chem. Soc. 1999, 121, 9677–9681. [Google Scholar] [CrossRef]
- Benayahoum, A.; Amira-Guebailia, H.; Houache, O. Homolytic and heterolytic OH bond cleavage in trans-resveratrol and some phenanthrene analogs: A theoretical study. Comput. Theor. Chem. 2014, 1037, 1–9. [Google Scholar] [CrossRef]
- Mikulski, D.; Górniak, R.; Molski, M. A theoretical study of the structure–radical scavenging activity of trans-resveratrol analogues and cis-resveratrol in gas phase and water environment. Eur. J. Med. Chem. 2010, 45, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.; Strain, J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. Period 1999, 299, 15–27. [Google Scholar]
- Benzie, I.F.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, P.; Xuan, L.-N.; Fu, X.-Y.; Jing, F.; Li, S.; Liu, Y.-M.; Chen, B.-Q. Synthesis and antitumor activities of novel hybrid molecules containing 1,3,4-oxadiazole and 1,3,4-thiadiazole bearing Schiff base moiety. Bioorganic Med. Chem. Lett. 2014, 24, 5154–5156. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xue, Y.; An, L.; Zheng, Y.; Dou, Y.; Zhang, L.; Liu, Y. Theoretical study on the structural and antioxidant properties of some recently synthesised 2,4,5-trimethoxy chalcones. Food Chem. 2015, 171, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Ünver, Y.; Sancak, K.; Celik, F.; Birinci, E.; Küçük, M.; Soylu, S.; Burnaz, N.A. New thiophene-1,2,4-triazole-5 (3)-ones: Highly bioactive thiosemicarbazides, structures of Schiff bases and triazole–thiols. Eur. J. Med. Chem. 2014, 84, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Shenvi, S.; Kumar, K.; Hatti, K.S.; Rijesh, K.; Diwakar, L.; Reddy, G.C. Synthesis, anticancer and antioxidant activities of 2,4,5-trimethoxy chalcones and analogues from asaronaldehyde: Structure–activity relationship. Eur. J. Med. Chem. 2013, 62, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Henry, D.J.; Varano, A.; Yarovsky, I. Performance of numerical basis set DFT for aluminum clusters. J. Phys. Chem. A 2008, 112, 9835–9844. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Sample Availability: Samples of the compounds 3a–6f are available from the authors.


| Compounds | DPPH IC50 (µg/mL) a | Radical Scavenging Maximum Inhibition % b | FRAP µM c |
|---|---|---|---|
| 3a | 42 ± 1 | 74 ± 1.7 | 1140 ± 33 |
| 3b | 38 ± 1 | 76 ± 3 | 2476 ± 64 |
| 3c | 43 ± 4 | 87 ± 2.1 | 1566 ± 26 |
| 3d | 32 ± 1 | 85 ± 2.6 | 1905 ± 42 |
| 3e | 52 ± 2 | 82 ± 2.1 | 2519 ± 103 |
| 3f | 29 ± 2 | 89 ± 1.9 | 838 ± 38 |
| 4a | 154 ± 11 | 46 ± 1.6 | 629 ± 13 |
| 4b | 162 ± 4 | 45 ± 1 | 189 ± 18 |
| 4c | 274 ± 3 | 34 ± 2 | 634 ± 22 |
| 4d | 157 ± 3 | 31 ± 1.8 | 213 ± 29 |
| 4e | 389 ± 2 | 46 ± 1.3 | 365 ± 25 |
| 4f | 201 ± 14 | 34 ± 2.1 | 370 ± 10 |
| 5 | 45 ± 2 | 73 ± 1.6 | 1688 ± 12 |
| 6a | NA | 17 ± 0.9 | 158 ± 32 |
| 6b | NA | 26 ± 1.4 | 238 ± 27 |
| 6c | NA | 24 ± 3.1 | 256 ± 15 |
| 6d | NA | 28 ± 1.2 | 193 ± 14 |
| 6e | NA | 32 ± 2.3 | 268 ± 13 |
| 6f | NA | 38 ± 2.1 | 157 ± 17 |
| AA* | 42 ± 2 | 90 ± 1.4 | 2231 ± 87 |
| BHT | 119 ± 2 | 51 ± 1 | 1274 ± 44 |
| Compound | BDE (kcal/mol) | Spin Density |
|---|---|---|
| 3f-r1 | 38.4 | 0.395 |
| 3f-r2 | 39.8 | 0.445 |
| 3f-r3 | 42.2 | 0.789 |
| Compound | BDE (kcal/mol) | Spin Density | |
|---|---|---|---|
 | 6a-r1 | 290.0 | 0.985 |
| 6a-r2 | 278.2 | 0.983 | |
 | 6b-r1 | 274.5 | 0.921 |
| 6b-r2 | 275.9 | 0.979 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kareem, H.S.; Nordin, N.; Heidelberg, T.; Abdul-Aziz, A.; Ariffin, A. Conjugated Oligo-Aromatic Compounds Bearing a 3,4,5-Trimethoxy Moiety: Investigation of Their Antioxidant Activity Correlated with a DFT Study. Molecules 2016, 21, 224. https://doi.org/10.3390/molecules21020224
Kareem HS, Nordin N, Heidelberg T, Abdul-Aziz A, Ariffin A. Conjugated Oligo-Aromatic Compounds Bearing a 3,4,5-Trimethoxy Moiety: Investigation of Their Antioxidant Activity Correlated with a DFT Study. Molecules. 2016; 21(2):224. https://doi.org/10.3390/molecules21020224
Chicago/Turabian StyleKareem, Huda. S., Nurdiana Nordin, Thorsten Heidelberg, Azlina Abdul-Aziz, and Azhar Ariffin. 2016. "Conjugated Oligo-Aromatic Compounds Bearing a 3,4,5-Trimethoxy Moiety: Investigation of Their Antioxidant Activity Correlated with a DFT Study" Molecules 21, no. 2: 224. https://doi.org/10.3390/molecules21020224