Synthesis, Molecular Structure Optimization, and Cytotoxicity Assay of a Novel 2-Acetyl-3-amino-5-[(2-oxopropyl)sulfanyl]-4-cyanothiophene
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Compound 4

2.2. Crystal Structure of Compound 4
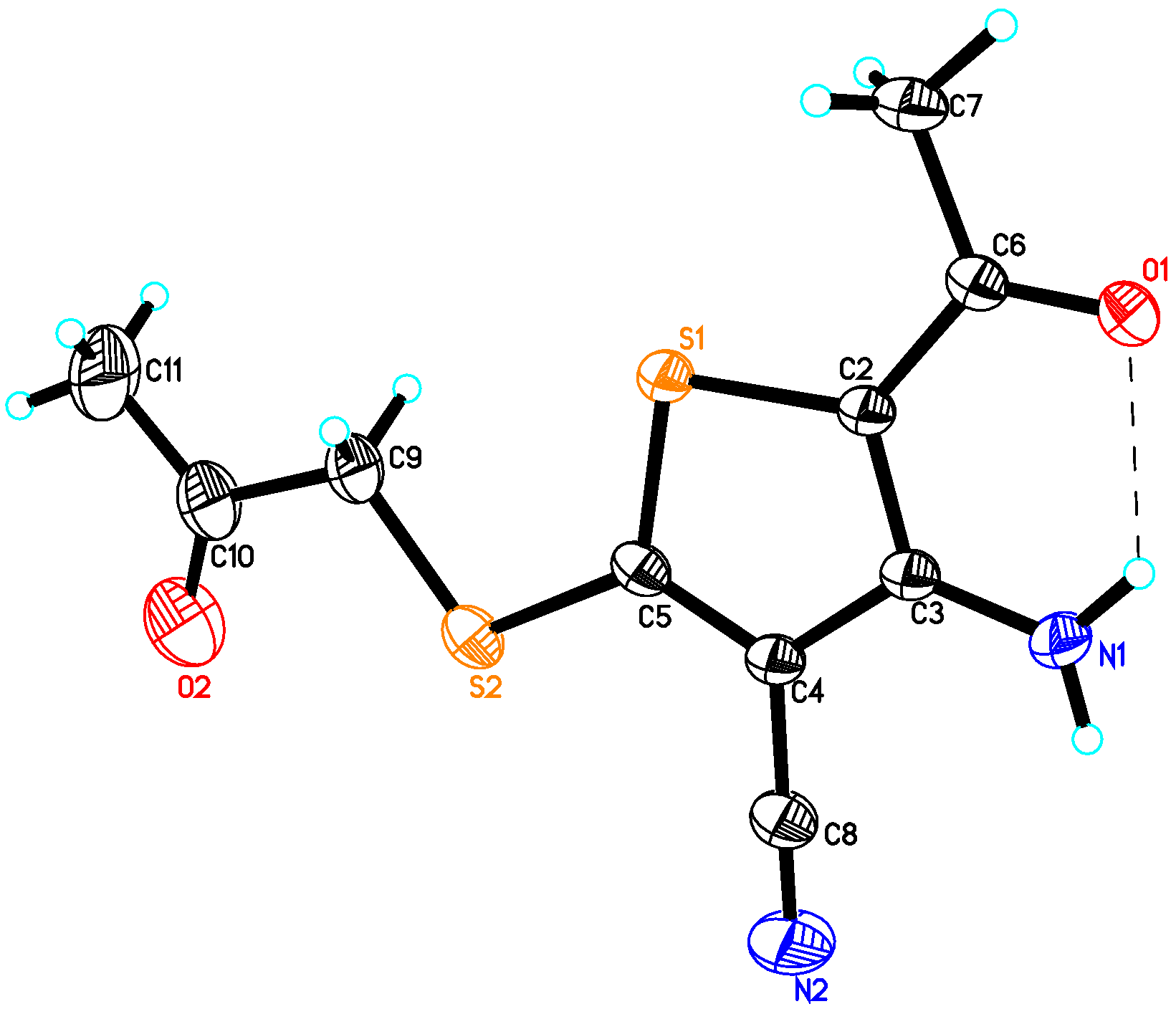
| Empirical formula | C10H10N2O2S2 |
| Formula weight | 254.32 |
| Temperature | 293 (2) K |
| Wave length | 0.71073 Å |
| Crystal system | Orthorhombic |
| space group | Pmna |
| Unit cell dimensions | a = 10.6077(7) Å b = 7.0209(5) Å c = 15.9646(10) Å α = β = σ = 90° |
| Volume | 1188.97(14) Å−3 |
| Z | 4 |
| Calculated density | 1.421 mg/m−3 |
| Absorption coefficient | 0.434 mm−1 |
| F(000) | 528 |
| Crystal size | 0.33 × 0.27 × 0.23 mm |
| Theta range for data collection | 2.31° to 27.50° |
| Limiting indices | −12 ≤ h ≤ 13, −9 ≤ k ≤ 8, −20 ≤ l ≤ 17 |
| Reflections collected/unique | 7832/1477 [R(int) = 0.0266] |
| Data completeness up to theta 27.50° | 100% |
| Absorption correction | Semi-empirical from equivalents |
| Max. and min. transmission | 0.9068 and 0.8701 |
| Refinement method | Full-matrix least-squares on F−2 |
| Data/restraints/parameters | 1477/0/0.8701 |
| Goodness-of-fit on F−2 | 1.048 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0441, wR2 = 0.1151 |
| R indices (all data) | R1 = 0.0534, wR2 = 0.1248 |
| Largest diff. peak and hole | 0.270 and −0.344 e·A−3 |
| Parameter a | Calc. | Exp | Parameter | Calc. | Exp. |
|---|---|---|---|---|---|
| R(1-15) | 1.769 | 1.732 | A(1-18-2) | 125.2 | 123.9 |
| R(1-18) | 1.737 | 1.702 | A(1-18-17) | 111.4 | 112.1 |
| R(2-18) | 1.754 | 1.735 | A(18-2-21) | 100.6 | 100.8 |
| R(2-21) | 1.831 | 1.791 | A(2-18-17) | 123.3 | 124.0 |
| R(3-19) | 1.240 | 1.224 | A(2-21-22) | 110.5 | 109.5 |
| R(4-23) | 1.213 | 1.203 | A(2-21-23) | 109.0 | 110.9 |
| R(5-16) | 1.346 | 1.332 | A(2-21-26) | 110.5 | 109.5 |
| R(8-20) | 1.166 | 1.122 | A(3-19-9) | 120.4 | 120.5 |
| R(9-19) | 1.518 | 1.504 | A(3-19-15) | 120.4 | 120.5 |
| R(12-23) | 1.515 | 1.470 | A(4-23-12) | 122.9 | 123.4 |
| R(15-16) | 1.400 | 1.411 | A(4-23-21) | 121.2 | 120.8 |
| R(15-19) | 1.444 | 1.424 | A(5-16-15) | 124.1 | 124.9 |
| R(16-17) | 1.444 | 1.430 | A(5-16-17) | 123.6 | 124.5 |
| R(17-18) | 1.390 | 1.375 | A(8-20-17) | 176.9 | 179.4 |
| R(17-20) | 1.419 | 1.426 | A(9-19-15) | 119.1 | 118.9 |
| R(21-23) | 1.527 | 1.492 | A(12-23-21) | 115.9 | 115.8 |
| A(15-1-18) | 92.3 | 92.4 | A(16-15-19) | 124.8 | 125.3 |
| A(1-15-16) | 110.6 | 111.3 | A(15-16-17) | 112.3 | 110.6 |
| A(1-15-19) | 124.6 | 123.4 | A(16-17-18) | 113.4 | 113.6 |
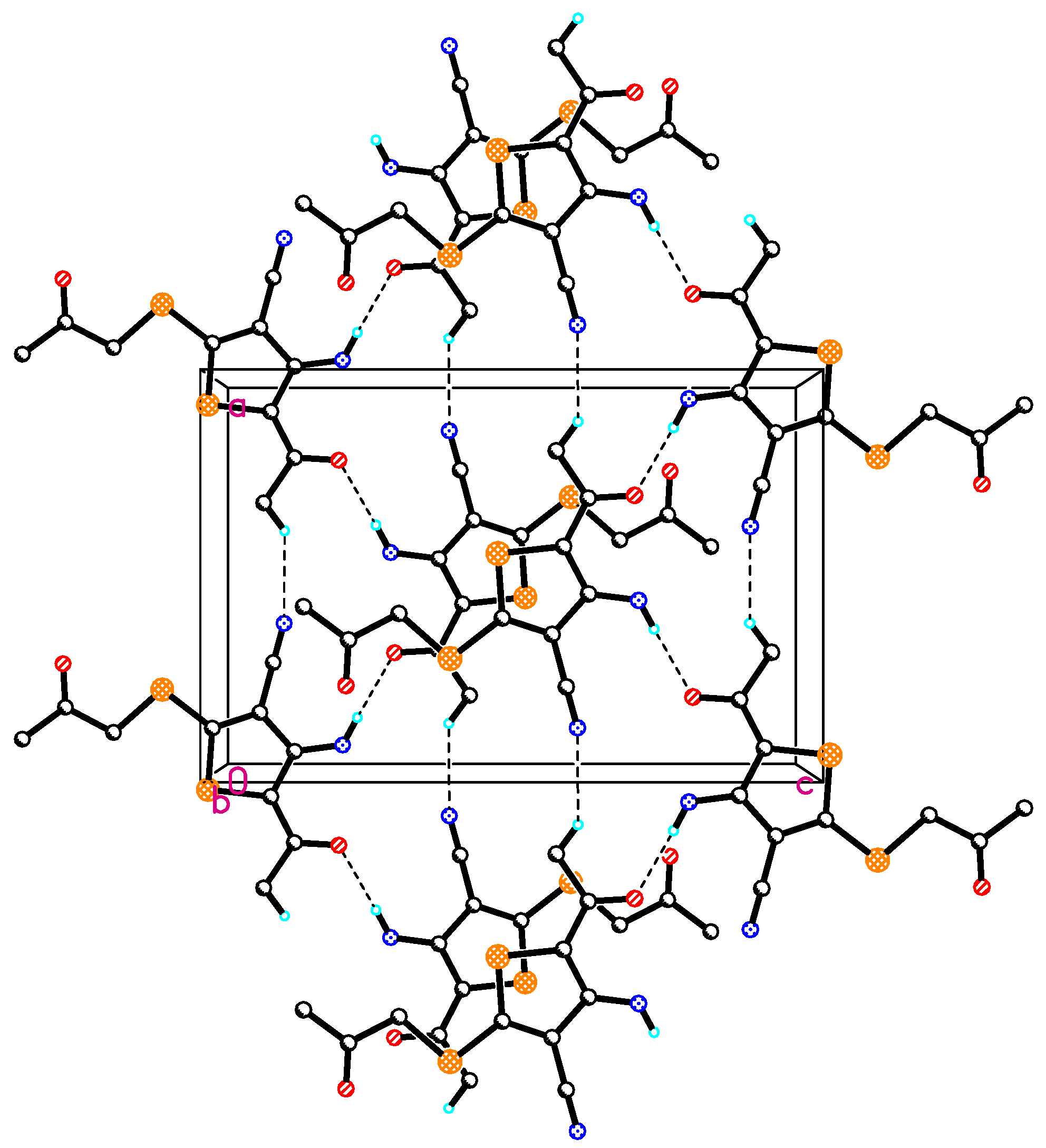
| D | H | A | D-H | H···A | D···A | D-H···A |
|---|---|---|---|---|---|---|
| N1 | H1 | O1 | 0.84(4) | 2.13(4) | 2.765(4) | 132(4) |
| N1 | H2 | O1 a | 0.89(4) | 2.03(4) | 2.916(4) | 179(3) |
| C7 | H7A | N2 b | 0.8600 | 2.5400 | 3.359(5) | 158.00 |
2.3. Optimized Molecular Geometry

2.4. Natural Atomic Charge
| Atom a | NAC | Atom | NAC |
|---|---|---|---|
| S1 | 0.4539 | H14 | 0.2587 |
| S2 | 0.4278 | C15 | −0.3936 |
| O3 | −0.6092 | C16 | 0.2516 |
| O4 | −0.5191 | C17 | −0.2642 |
| N5 | −0.8023 | C18 | −0.3495 |
| H6 | 0.4562 | C19 | 0.5238 |
| H7 | 0.4287 | C20 | 0.2624 |
| N8 | −0.2969 | C21 | −0.7120 |
| C9 | −0.7706 | H22 | 0.2746 |
| H10 | 0.2659 | C23 | 0.5892 |
| H11 | 0.2511 | H24 | 0.2511 |
| C12 | −0.7809 | H25 | 0.2587 |
| H13 | 0.2701 | H26 | 0.2746 |
2.5. Molecular Electrostatic Potential

2.6. Nonlinear Optical Properties
2.7. Frontier Molecular Orbitals

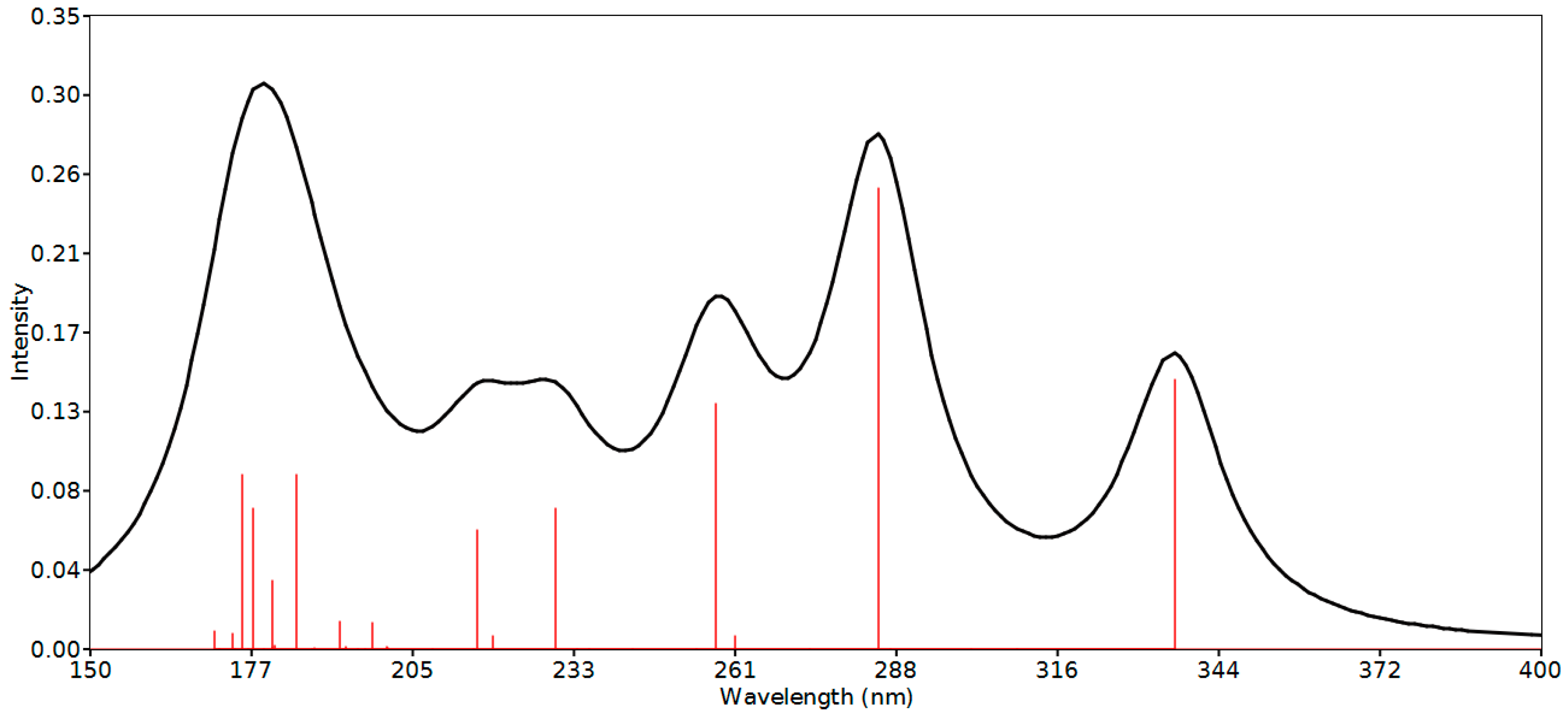
2.8. Natural Bond Orbital (NBO) Analysis
| Donor NBO (i) | Acceptor NBO (j) | E(2) kcal/mol |
|---|---|---|
| BD(2)C15-C16 | BD*(2)O3-C19 | 31.02 |
| BD(2)C15-C16 | BD*(2)C17-C18 | 11.99 |
| BD(2)C17-C18 | BD*(3)N8-C20 | 18.60 |
| BD(2)C17-C18 | BD*(2)C15-C16 | 18.69 |
| BD(3)N8-C20 | BD*(2)C17-C18 | 9.01 |
| LP(2)S1 | BD*(2)C15-C16 | 13.30 |
| LP(2)S1 | BD*(2)C17-C18 | 24.70 |
| LP(2)S2 | BD*(2)C17-C18 | 23.97 |
| LP(2)O3 | BD*(1)N5-H6 | 6.63 |
| LP(2)O3 | BD*(1)C 9-C19 | 19.04 |
| LP(2)O3 | BD*(1)C15-C19 | 15.68 |
| LP(2)O4 | BD*(1)C12-C23 | 20.65 |
| LP(2)O4 | BD*(1)C21-C23 | 22.82 |
| LP(1)N5 | BD*(2)C15-C16 | 63.23 |
| LP(1)N8 | BD*(1)C17-C20 | 12.82 |
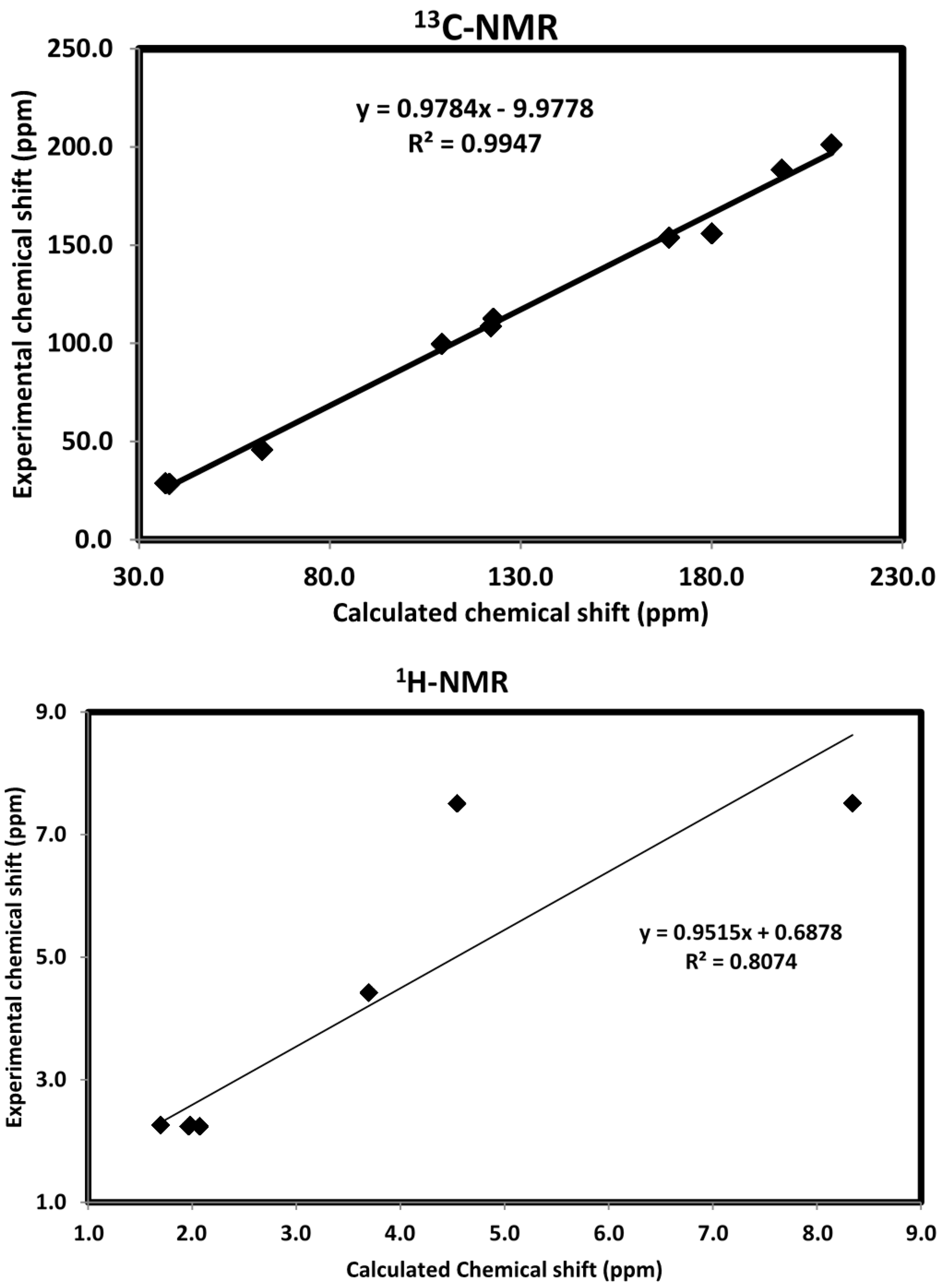
2.9. NMR Spectra
2.10. Biological Activity Evaluation
| Compound | Cytotoxic Activity (PC-3 Cell line) IC50 ± SEM [μM] | Cytotoxic Activity (Hella Cell line) IC50 ± SEM [μM] |
|---|---|---|
| 4 | >30 | >30 |
| Standard | Doxorubicin 0.912 ± 0.12 | Soxorubicin 0.306 ± 0.155 |
3. Materials and Methods
3.1. General Information
3.2. Preparation of 2-Acetyl-3-amino-5-[(2-oxopropyl)sulfanyl]-4-cyanothiophene (4)
3.3. Crystal Structure Determination
3.4. Computational Details
3.5. Cytotoxicity Activity by Using MTT Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arroyo, S.; Salas-Puig, J. An open study of tiagabine in partial epilepsy. Rev. Neurol. 2001, 32, 1041–1046. [Google Scholar] [PubMed]
- Noguchi, H.; Kitazumi, K.; Mori, M.; Shiba, T. Electroencephalographic properties of zaleplon, a non-benzodiazepine sedative/hypnotic, in rats. J. Pharm. Sci. 2004, 94, 246–251. [Google Scholar] [CrossRef]
- Lankau, H.J.; Unverferth, K.; Grunwald, C.; Hartenhauer, H.; Heinecke, K.; Bernoster, K.; Dost, R.; Egerland, U.; Rundfeldt, C. New GBA-modulating 1,2,4-oxadiazole derivatives and their anticonvulsant activity. Eur. J. Med. Chem. 2007, 42, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, J.R.; Puthucode, R.N.; Smith, J.M.; Hetherington, M.; Quail, J.W.; Pugazhenthi, U.; Lechler, T.; Stables, J. (Aroyloxy)aryl semicarbazones and related compounds: A novel class of anticonvulsant agents possessing high activity in the maximal electroshock screen. J. Med. Chem. 1996, 39, 3984–3997. [Google Scholar] [CrossRef] [PubMed]
- Ragavendran, J.; Sriram, D.; Patil, S.; Reddy, I.V.; Bharathwajan, N.; Stables, J.; Yogeeswari, P. Design and synthesis of anticonvulsants from a combined phthalimide-GABA-anilide and hydrazone pharmacophore. Eur. J. Med. Chem. 2007, 42, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Polivka, Z.; Holubek, J.; Svatek, E.; Metys, J.; Protiva, M. Potential hypnotics and anxiolytics: Synthesis of 2-bromo-4-(2-chlorophenyl)-9-[4-(2-methoxyethyl)-piperazino]–6H-thieno[3,2,4-triazolo[4,3-a]-1,4-diazepine and of some related compounds. Collect. Czech. Chem. Commun. 1984, 49, 621–636. [Google Scholar] [CrossRef]
- Yogeeswari, P.; Thirumurugan, R.; Kavya, R.; Samuel, J.S.; Stables, J.; Siram, D. 3-Chloro-2-methylphenyl-substituted semicarbazones: Synthesis and anticonvulsant activity. Eur. J. Med. Chem. 2004, 39, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Gunizkuculguzel, S.; Mazi, A.; Sahin, F.; Qzturk, S.; Stables, J. Synthesis and biological activities of diflunisal hydrazide-hydrazones. Eur. J. Med. Chem. 2003, 38, 1005–1013. [Google Scholar]
- Thirumurugan, R.; Sriram, D.; Saxena, A.; Stables, J.; Yogeeswari, P. 2,4-Dimethoxyphenylsemicarbazones with anticonvulsant activity against three animal models of seizures: Synthesis and pharmacological evaluation. Bioorg. Med. Chem. 2006, 14, 3106–3012. [Google Scholar] [CrossRef] [PubMed]
- Riaz, N.; Anis, I.; Rehman, A.; Malik, A.; Ahmed, Z.; Muhammad, P.; Shujaat, S.; Ur-Rahman, A. Emodinol, β-Glucuronidase, inhibiting triterpine from Paeonia emodi. Nat. Prod. Res. 2003, 17, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Shank, R.P.; Doose, D.R.; Streeter, A.J.; Bialer, M. Plasma and whole blood pharmacokinetics of topiramate: The role of carbonic anhydrase. Epilepsy Res. 2005, 63, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, V.U.; Khan, A.; Farooq, U.; Kousar, F.; Khan, S.S.; Nawaz, S.A.; Abbasi, M.A.; Choudhary, M.I. Three new cholinesterase-inhibiting cis-clerodane diterpenoids from Otostegia limbata. Chem. Pharm. Bull. 2005, 53, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, V.U.; Abbasi, M.A.; Hussain, H.; Akhtar, M.N.; Farooq, U.; Fatima, N.; Choudhary, M.I. Phenolic glycosides from Symplocos racemosa: Natural inhibitors of phosphodiesterase I. Phytochemistry 2003, 63, 217–220. [Google Scholar] [CrossRef]
- Khan, I.; Ali, S.; Hameed, N.; Rama, M.; Hussain, A.; Wadood, R.; Uddin, Z.; Ul-Haq, A.; Khan, S.; Ali, M. Synthesis, antioxidant activities and urease inhibition of some new 1,2,4-triazole and 1,3,4-thiadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 5200–5027. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.B.; Afza, N.; Malik, A.; Ul-Haq, A.; Perveen, S.; Ahmad, I.; Ejaz, A.; Choudhary, M.I. Xanthine oxidase inhibiting flavonol glycoside from Amberboa ramose. Nat. Prod. Res. 2006, 20, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Cannell, R.; Kellam, S.; Owsianka, A.; Walker, J. Results of a large screen of microalgae for the production of protease inhibitors. Planta Med. 1988, 54, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Mabkhot, Y.N.; Aldawsari, F.D.; Al-Showiman, S.S.; Barakat, A.; Soliman, S.M.; Choudhary, M.I.; Yousuf, S.; Mubarak, M.S.; Hadda, T.B. Novel enaminone derived from thieno[2,3-b]thiene: Synthesis, X-ray crystal structure, HOMO, LUMO, NBO analyses and biological activity. Chem. Cent. J. 2015, 19. [Google Scholar] [CrossRef] [PubMed]
- Mabkhot, Y.N.; Aldawsari, F.D.; Al-Showiman, S.S.; Barakat, A.; Hadda, T.B.; Mubarak, M.S.; Sehrish, N.; Ul-Haq, Z.; Rauf, A. Synthesis, bioactivity, molecular docking and POM analyses of novel substituted thieno[2,3-b]thiophenes and related congeners. Molecules 2015, 20, 1824–1841. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Sen, K. Molecular Electrostatic Potentials, Concepts and Applications; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Scrocco, E.; Tomasi, J. Electronic molecular structure, reactivity and intermolecular forces: Aneuristic interpretation by means of electrostatic molecular potentials. Adv. Quantum Chem. 1978, 11, 115–193. [Google Scholar]
- Gnanasekaran, P.; Madhavan, J. Synthesis, structural, FT-IR and non-linear optical studies of pure and lanthanum doped l-arginine acetate single crystals. Asian J. Chem. 2010, 22, 109–114. [Google Scholar]
- Geskin, V.M.; Lambert, C.; Bredas, J.L. Origin of high second- and third-order nonlinear optical response in ammonia/borate diphenylpolyene zwitter ions: The remarkable role of polarized aromatic groups. J. Am. Chem. Soc. 2003, 125, 15651–15658. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.S. Observing high second harmonic generation and control of molecular alignment in one dimension. Cyclobutenediones as a promising new acceptor for nonlinear optical materials. ACS Symp. Ser. 1991, 455, 331–342. [Google Scholar]
- Fukui, K.; Yonezawa, T.; Shingu, H.J. A molecular orbital theory of reactivity in aromatic hydrocarbons. J. Chem. Phys. 1952, 20, 722–725. [Google Scholar] [CrossRef]
- Padmaja, L.; Ravikumar, C.; Sajan, D.; Joe, I.H.; Jayakumar, V.S.; Pettit, G.R.; Neilsen, F.O. Density functional study on the structural conformations and intramolecular charge transfer from the vibrational spectra of the anticancer drug combretastatin A2. J. Raman Spectrosc. 2009, 40, 419–428. [Google Scholar] [CrossRef]
- Ravikumar, C.; Joe, I.H.; Jayakumar, V.S. Charge transfer interactions and nonlinear optical properties of push-pull chromophore benzaldehyde phenylhydrazone: A vibrational approach. Chem. Phys. Lett. 2008, 460, 552–558. [Google Scholar] [CrossRef]
- Sebastian, S.; Sundaraganesan, N. The spectroscopic (FT-IR, FT-IR gas phase, FT-Raman and UV) and NBO analysis of 4-hydroxypiperidine by density functional method. Spectrochim. Acta Part. A 2010, 75, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Joe, I.H.; Kostova, I.; Ravikumar, C.; Amalanathan, M.; Pinzaru, S.C. Theoritical and vibrational spectral investigation of sodium salt of acenocoumarol. J. Raman Spectrosc. 2009, 40, 1033–1038. [Google Scholar] [CrossRef]
- Osmiałowski, B.; Kolehmainen, E.; Gawinecki, R. GIAO/DFT calculated chemical shifts of tautomeric species. 2-Phenacylpyridines and (Z)-2-(2-hydroxy-2-phenylvinyl)pyridines. Magn. Res. Chem. 2001, 39, 334–340. [Google Scholar] [CrossRef]
- SHELXTL-PC, Version 5.1; Siemens Analytical Instruments, Inc.: Madison, WI, USA, 1997.
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Gaussian-03, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2004.
- Gauss View, Version 4.1; Semichem Inc.: Shawnee Mission, KS, USA, 2007.
- Chemcraft. Lite Version Build 08. Available online: http://www.chemcraftprog.com/ (accessed on 1 April 2005).
- Natural Bond Orbital (NBO), Version 3.1; University of Wisconsin: Madison, WI, USA, 1998.
- Sample Availability: Samples of compound 4 are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mabkhot, Y.N.; Aldawsari, F.D.; Al-Showiman, S.S.; Barakat, A.; Soliman, S.M.; Choudhary, M.I.; Yousuf, S.; Ben Hadda, T.; Mubarak, M.S. Synthesis, Molecular Structure Optimization, and Cytotoxicity Assay of a Novel 2-Acetyl-3-amino-5-[(2-oxopropyl)sulfanyl]-4-cyanothiophene. Molecules 2016, 21, 214. https://doi.org/10.3390/molecules21020214
Mabkhot YN, Aldawsari FD, Al-Showiman SS, Barakat A, Soliman SM, Choudhary MI, Yousuf S, Ben Hadda T, Mubarak MS. Synthesis, Molecular Structure Optimization, and Cytotoxicity Assay of a Novel 2-Acetyl-3-amino-5-[(2-oxopropyl)sulfanyl]-4-cyanothiophene. Molecules. 2016; 21(2):214. https://doi.org/10.3390/molecules21020214
Chicago/Turabian StyleMabkhot, Yahia N., Fahad D. Aldawsari, Salim S. Al-Showiman, Assem Barakat, Saied M. Soliman, Muhammad I. Choudhary, Sammer Yousuf, Taibi Ben Hadda, and Mohammad S. Mubarak. 2016. "Synthesis, Molecular Structure Optimization, and Cytotoxicity Assay of a Novel 2-Acetyl-3-amino-5-[(2-oxopropyl)sulfanyl]-4-cyanothiophene" Molecules 21, no. 2: 214. https://doi.org/10.3390/molecules21020214
APA StyleMabkhot, Y. N., Aldawsari, F. D., Al-Showiman, S. S., Barakat, A., Soliman, S. M., Choudhary, M. I., Yousuf, S., Ben Hadda, T., & Mubarak, M. S. (2016). Synthesis, Molecular Structure Optimization, and Cytotoxicity Assay of a Novel 2-Acetyl-3-amino-5-[(2-oxopropyl)sulfanyl]-4-cyanothiophene. Molecules, 21(2), 214. https://doi.org/10.3390/molecules21020214









